Jacques Monod, born 100 years ago, was one of the main founders of molecular biology. A quotation of Roger Stanier seems to me of immediate relevance to the topic of this meeting on “Chance and necessity in evolution,” dedicated to the works of Jacques Monod:
“Jacques Monod, one of the great scientists of the twentieth century, will always have an honored place among the leaders of the second major revolution in the history of biology, which occurred almost exactly 100 years after the Darwinian one. Few of the protagonists were more conscious than Monod of the connections between the two revolutions, of the way in which he and his contemporaries had extended and deepened Darwin’s concepts.” (Stanier 1977).
I first heard of Jacques Monod in 1948, at the time of one of the greatest scientific scandals of the 20th century, the Lyssenko affair. Lyssenko was a Russian agronomist who rejected the science of genetics, whereas giving heredity of acquired characters the major role in evolution. He considered Mendel’s principles incompatible with dialectic materialism. He succeeded not only to ruin the Soviet agriculture but also to eliminate its best geneticists. At that time, I was a young student in science at the University of Budapest; teaching of genetics was not allowed. A friend who had gotten hold of a newspaper called “Combat,” directed by Albert Camus and dated September 19, 1948, showed me the article by a certain Dr Monod, the title of which was “The victory of Lyssenko has no scientific character.” To me this was a revelation. My decision was made: One day I would meet this Dr Monod. And 10 years later, after my thesis work was finished, I found myself working in his laboratory! From that time on I had the immense privilege of working with him on a day-to-day basis up until his death on May 31, 1976.
Jacques Monod was born in Paris on February 9, 1910. During the years of the First World War, the Monod family, which was of Swiss Huguenot origin, fled to Switzerland to live with their cousins. In 1918, they moved to Cannes where Jacques was to remain until 1928. Lucien Monod, his father, was a painter, a rather audacious choice for someone from a puritanical family that counted among its members professors, civil servants, pastors, and doctors (fig. 1). Jacques Monod’s mother, Charlotte Todd McGregor, daughter of a Scottish pastor, who had emigrated to the United States, was an American. In the Monod family reigned a stimulating intellectual, artistic, and musical atmosphere. As a boy, Jacques learnt the cello that he would practice even later.
FIG. 1.—
Portrait of J. Monod by his father, Lucien Monod, 1940.
After completing his secondary education in Cannes, Monod went to Paris in 1928 to study biology at the Sorbonne (the Paris University). In 1931, he obtained his bachelor’s degree (Licence) in science. At the same time, he created a Bach choral group, “La cantate” and was seriously tempted for a time to make a career as a conductor. He made his first research experience, after having obtained a grant, in Strasbourg in the laboratory of the zoologist Edouard Chatton, where he worked on ciliates. Back to Paris in 1932, at the Sorbonne in the “Laboratory of evolution of organized beings” he continued research on protists with more or less success. His true initiation to biology came from scientists he had met at the marine biology research station in Roscoff: Georges Tessier, from whom he learned biometrics, André Lwoff, and Boris Ephrussi, who introduced him into the world of microbiology and genetics and Louis Rapkine, who taught him the importance of chemical and molecular descriptions of living beings (Monod 1966).
After several research projects on different protists, he decided in 1934 to join a scientific expedition in Greenland on Commander Charcot’s boat, the “Pourquoi pas?” to study the natural history of this region (fig. 2). In 1936, he was about to take part in a new expedition to Greenland. But Boris Ephrussi, who was to spend a year in T.H. Morgan’s group at the California Institute of Technology, persuaded Jacques Monod to go off with him to learn genetics, and he obtained for him a Rockefeller grant. Thus, Jacques Monod set off for Pasadena. That same year, the Pourquoi pas? was shipwrecked in a storm off the coast of Greenland and its entire crew perished. Genetics saved the life of Jacques Monod. Much to the regret of Ephrussi, he spent most of his time directing orchestras and choral groups and even got to the point where he was about to sign a contract as head of the local orchestra. Even upon returning to Paris, he continued to hesitate between music and science. He divided his time between his laboratory at the Sorbonne, where he worked as an assistant, and music. He played the cello in a quartet and continued to direct the Bach choral group.
FIG. 2.—

Aboard on Pourquoi pas? 1934.
Starting in 1937, still at the Sorbonne, Jacques Monod began to work on bacterial growth using Escherichia coli as a model. From the very beginning of his research, he made an important discovery, the phenomenon of “diauxy,” a biphasic growth observed when the medium contained a mixture of two sugars, one of them being glucose and the second one lactose or maltose, for instance. His interpretation of the diauxic growth phenomenon was that glucose (the first sugar used by the bacterium) inhibited the formation of an enzyme necessary for assimilating the second sugar; the latency period between the two growth phases corresponded to the “induction time” of that enzyme (Monod 1941). The concept that would later come to be known as induced enzyme synthesis was born.
In 1941, Jacques Monod obtained his science doctorate for a thesis entitled “Research on the growth of bacterial cultures” (Monod 1942). The jury appreciated neither the importance nor the originality of this fundamental work at the time. Indeed, André Lwoff later recounted how, following Monod’s defense of his thesis, the director of the laboratory in which Monod was working told Lwoff that “Monod’s work is of no interest to the Sorbonne.” Alas, it was the sad truth.
Jacques Monod was a man of moral commitments. In 1942, while World War II was devastating Europe and France was occupied by the Germans, Monod entered the underground movement. At the beginning of 1943, he joined one of the most active armed resistance groups and later he became Chief of the national staff, a position in which his three predecessors had disappeared. It was Jacques Monod who, several days before the arrival of the allied forces into Paris, drafted the appeal to Paris citizens to mount the barricades. And later, following the liberation of Paris, he joined the First Army as a member of the staff of General de Lattre de Tassigny (fig. 3). It was during this period that Jacques Monod first entered into contact with American officers and was able to read some American scientific publications. This was how he discovered, in a traveling library of the US army, the article on the spontaneous nature of bacterial mutations (Luria and Delbrück 1943) and the historic publication by Avery, MacLeod, and McCarthy, who identified the transforming principle as being DNA (Avery et al. 1944).
FIG. 3.—

In Alsace 1944. General de Lattre de Tassigny accompanied by J. Monod and J. Kessel decorating a young soldier who will be killed a few days later.
Once the war ended, Jacques Monod returned to Paris—this time as head of a laboratory in André Lwoff’s department at the Institut Pasteur. This department was located in a veritable attic, where Monod’s working space consisted of several small rooms with wooden workbenches, which he initially shared with his technician Madeleine Jolit and an Italian biochemist, Annamaria Torriani. Gradually, the tiny group expanded with the arrival of Alvin Pappenheimer and his student, Melvin Cohn, an immunologist, as well as Germaine Cohen-Bazire and David Hogness. Within this small group, Jacques Monod devoted most of his time to studying “enzymatic adaptation,” choosing as a model β-galactosidase. One of the questions that had to be first answered was whether the enzyme was made de novo after induction or from precursor subunits, as postulated earlier. Using for the first time isotope labeling, they showed that the enzyme was made from amino acids de novo after induction at a maximum rate (Hogness et al. 1955). This led Monod to formulate a new parameter, differential rate of enzyme synthesis, ΔZ/ΔB, called later “Monod plot” (Z stays for β-galactosidase and B for bacteria). Next, they decided to synthesize a number of lactose analogs; some of them (i.e., some thiogalactosides) turned out to be excellent inducers, without being hydrolyzed by the enzyme; they were called gratuitous inducers. Others were shown to be substrates without any inducing activity. As Melvin Cohn noted, “the existence of nonsubstrate inducers had a profound philosophical impact, for, like Ionesco, Monod had created a theatre of absurd. A bacterium growing on succinate was producing a useless enzyme, β-galactosidase, in response to a substrate it could not metabolize” (Cohn 1976). Monod liked the allusion and his immediate answer was: “Each of science’s conquests is a victory of the absurd.” Nevertheless, gratuitous inducers became important tools in biological research.
It was around this period that Monod decided to drop the rather Lamarckian term « enzymatic adaptation » and instead use “induced enzyme synthesis,” which was formulated, as Melvin Cohn recalls, in an encyclical issued in by the Adaptive Enzyme’s College of Cardinals: Monod, Pollock, Spiegelman, and Stanier (Cohn et al. 1953).
From the very beginning, Monod was interested in the study of bacterial growth, which was already the subject of his doctoral thesis. Later, considering bacterial growth as a method for the study of bacterial physiology and biochemistry, he defined its quantitative aspects, such as growth phases, growth rates, and growth constants (Monod 1949). He also made an important experimental and theoretical contribution to the methodology of continuous bacterial growth, the bacteria being maintained indefinitely at constant rate in a chemical and physiological stable state (Monod 1950). The experimental potentialities of the method are wide; besides the possibility of changing growth rates without modifying either the composition of the medium or the temperature, it provides a means, currently used today, to select specific mutants. Melvin Cohn recounted the birth of the first device, called “bactogène,” designed for continuous cultures. For a given experiment, Mel had to dilute the bacterial cultures every hour to keep them growing continuously. He then wrote: “I decided one evening to simply set up an automatic system for feeding the removal of culture. Since I had a liter of culture, which I diluted, with a liter of medium every hour, I simply fed in a liter per hour of fresh medium and siphoned off a liter of culture per hour continuously. To my surprise, the bacteria could not keep up and the density of the culture fell. In fact, to maintain it I could not feed more than 690 ml/hour. As I was wrestling with this paradox, obviously upset, Jacques sat down with me and asked if I had any idea why I could not feed more than 690 ml/hour when I expected 1,000 ml/hour. ‘It may sound wild to you, Jacques, but I think I have discovered that bacteria, like men, have a biological need for rest.’ He smiled patiently and said, ‘You have discovered that the ln 2 = 0,69. Think about that’. The next day, both he and I had the detailed theory of continuous culture. We named the thing the bactogène (Cohn 1976).
To better understand the nature of enzymatic induction, Jacques Monod realized that, first of all, he would have to study the relationships between gene and enzyme. From 1946 on, he isolated lactose+ and lactose− mutants of E. coli. Later, among a number of mutants that had been isolated, several seemed to be lactose− and were yet able to synthesize β-galactosidase. The explanation for such mysterious mutants, referred to as “cryptic,” was found in 1956: such mutants were lacking a specific protein, which, in wild-type bacteria, had the ability to accumulate galactosides. This protein was named “galactoside permease” and the gene, which commanded it, was called “y,” distinct from the gene for β-galactosidase, referred to as “z” (Rickenberg et al. 1956). The two proteins were induced at the same time by the β-galactosides. A new category of enzymes, a “pump” responsible for accumulation of small molecules into the bacteria, was born. Several years later, a galactoside transacetylase (coded by the a gene) and induced at the same time as β-galactosidase and permease, was discovered, thus facilitating later studies on the genetic determinism of induction (Zabin et al. 1962). The physiological function of transacetylase remains unknown to this day.
Studies on galactoside permease and acetylase led to another highly important discovery. Mutants were found in which the three proteins, β-galactosidase, permease, and transacetylase, were simultaneously constitutive, that is, synthesized even in the absence of inducer. The constitutive mutations defined a genetic factor, which could exist in two forms: i+ corresponding to inducibility and i− corresponding to constitutivity. Genetic analysis revealed that the i gene is closely linked to the z, y, and a genes (Jacob and Monod 1959).
In 1953, Jacques Monod was made head of a new department (fig. 4), called Cellular Biochemistry and at about the same time François Jacob and Elie Wollman, in Lwoff’s laboratory, elucidated the mechanisms of bacterial conjugation and gene transfer, thus providing new and powerful tools to attack the problem of genetic regulation (Jacob and Wollman 1956). This was undertaken by Jacques Monod and François Jacob during a long and fruitful collaboration and was carried out with the well-known success.
FIG. 4.—

Monod in his new Department, around 1958.
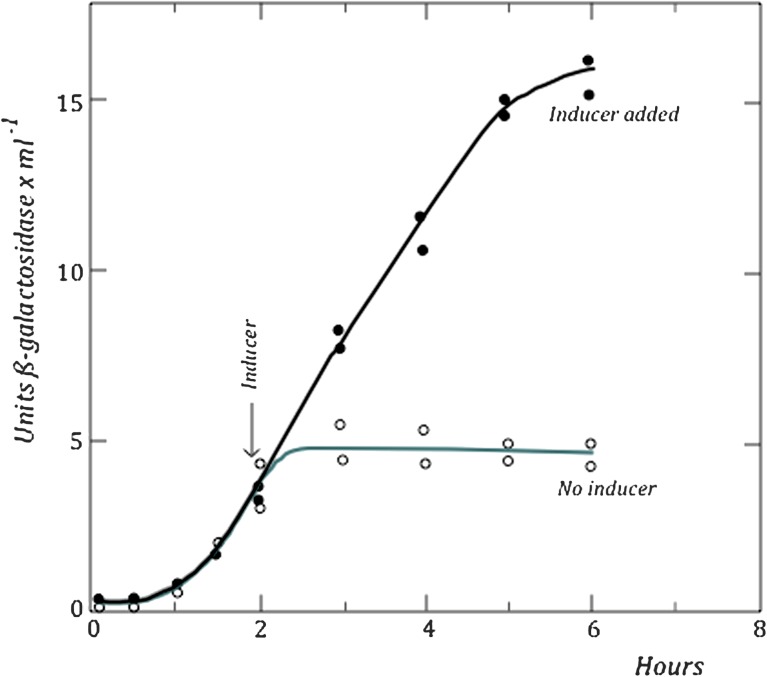
In 1957, a crucial experiment, which marked the beginning of a new scientific era later to become known as molecular biology, was carried out by Jacques Monod, François Jacob, and an American scientist, Arthur Pardee, who was spending his sabbatical year in Paris in Monod’s laboratory at the Institut Pasteur. This experiment involved measuring the synthesis of β-galactosidase in zygotes resulting from the conjugation of male bacteria carrying the z+ and i+ genes with females, carrying z− and i− genes. In the absence of inducer, none of the parents are able to synthesize the enzyme: the male because of the absence of inducer and the female because of a defective z gene. Crossing the two strains, enzyme synthesis began within a few minutes after the z+ gene entered the recipient, but after an hour or so, enzyme synthesis stopped. When inducer was added, enzyme synthesis resumed, suggesting that the transferred i+ gene was becoming gradually expressed and the zygote became phenotypically inducible (fig. 5). That experiment remains a landmark and is generally referred to by the initials of the three scientists who performed it: PaJaMo, or in scientific jargon, just simply “pajama” (Pardee et al. 1959).
FIG. 5.—
The PaJaMo experiment (from Pardee et al. 1959.).
The PaJaMo experiment was the point of departure for proposing a model of negative regulation: the i+ gene produces a substance called “repressor” that blocks the expression of the z gene. A previous hypothesis that the inducer acts by provoking enzyme synthesis had to be abandoned. Rather, it acts by “inhibiting an inhibitor” of enzyme synthesis.
Two other concepts of utmost importance came out of those experiments: that of messenger RNA and that of the operon. The model of genetic regulation (the operon model) proposed by Jacques Monod and François Jacob in a series of articles that have since become classic can be summed up as follows (Jacob and Monod 1961):
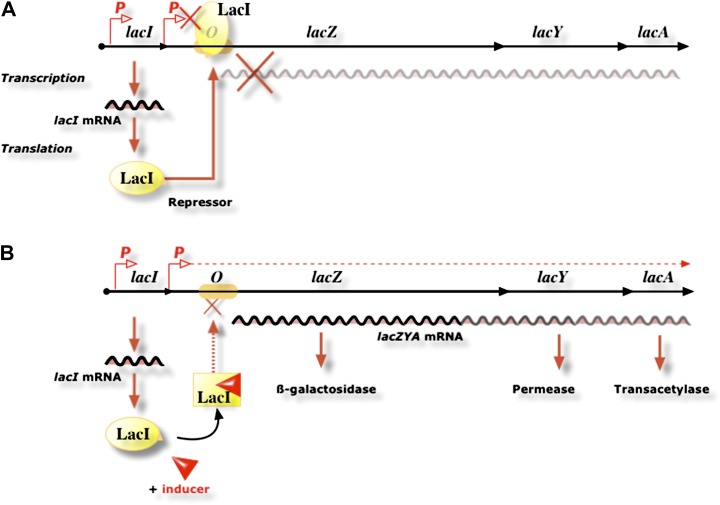
First of all, they defined two categories of genes, structural and regulator genes. Structural genes (lacZ, Y, and A) govern the capacity to synthesize β-galactosidase, permease, and transacetylase; lacI is a regulator gene and codes for a regulator protein, the repressor. The three structural genes are found in a single genetic entity, which Jacob and Monod called the “operon.” According to the model, the repressor acts upon a single receptor on the DNA, named the “operator.” The repressor–operator interaction blocks expression of structural genes. The repressor can be inactivated in the presence of the inducer, a β-galactoside; the proteins of the operon are synthesized in two steps: during transcription, which is the first step, operon genes are copied in a single messenger RNA with a short life span and in the second step, this messenger RNA is translated into proteins via the ribosomes (fig. 6).
FIG. 6.—
The operon model. The lactose operon in the repressed (top) and induced state (bottom). In the absence of an inducer, the LacI repressor binds the operator and prevents lac gene expression, whereas in the presence of the inducer, the LacI repressor is inactivated, and lac genes are expressed (drawing courtesy of Jean-Marc Ghigo).
At the beginning of the 1960s, the molecular mechanisms of repressor-inducer and repressor-operator recognitions were resolved. However the isolation of the repressor met with great difficulties, as its concentration in bacteria was very weak, that is, no more than ten molecules per cell. In the end, however, it was isolated and purified. (Gilbert and Müller-Hill 1966).
One of the major concerns of Monod was how proteins recognize chemical signals, how the repressor recognizes inducer, or how in a biosynthetic pathway, the activity of the first enzyme is inhibited by the product of the last one.
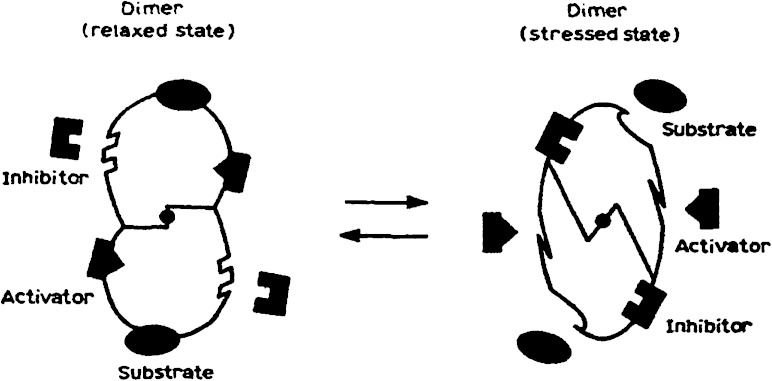
By the end of 1961, one evening quite late Jacques Monod walked into my laboratory looking rather tired and worried. Monod stood silently at my bench and after a few long minutes he said: “I think I have discovered the second secret of life.” I was quite alarmed by this unexpected revelation and asked him if he needed a glass of whisky. After the second or maybe the third glass, he explained the discovery, which he had already given a name: “allostery.” Indeed, he had just understood how effectors of a given protein having different structures, with no steric relationship with one another, could interact with a same protein but at distinct sites. Certain regulator proteins such as the lactose repressor or different metabolic enzymes could exist in two alternative conformational states: in one of them, the protein can associate with a substrate and with an activator ligand; in the other conformation, it can associate with the inhibitory ligand (fig. 7). Allosteric interactions are indirect and are transmitted via a conformational change in the protein (Monod et al. 1965). The concept of allostery was one of the most important ideas to emerge from the study of bacterial regulatory mechanisms; cell signaling for instance, involves allosteric interactions. The prion theory, which implies a transmission of conformational change between identical protein molecules, is inspired from the allosteric concept.
FIG. 7.—
Model of allosteric transition produced in a symmetrical dimer. In one of the two conformations, the protein can attach itself to the substrate as well as to the activating bond. In the other conformation, it can attach itself to the inhibiting bond (from Monod 1965).
As Monod pointed out, the “invention” of indirect allosteric interactions during evolution opened the way of an infinite number of possible regulations. As we now know, such interactions account for a great number of physiological phenomena. Since the explanatory power of the allosteric theory was substantial, virtually nothing was excluded; Boris Magasanik pointed out to Jacques Monod that it was the most decadent theory in biology. And Monod tended to agree with this.
In 1965, the Nobel Prize in Physiology or Medicine was attributed to André Lwoff, Jacques Monod, and François Jacob “for their discoveries concerning genetic control of enzyme and virus synthesis” (fig. 8).
FIG. 8.—
F. Jacob, J. Monod, and A. Lwoff, 1965.
The concepts that Jacques Monod developed are absolutely central to modern biology. The concept of the regulation of gene expression—essentially the Jacob–Monod model, formulated 50 years ago, was the main forerunner of the biotechnical revolution and proved to underlie the systems biology of complex regulatory circuits and to constitute the basis of control mechanisms of gene expression in eukaryotic systems. The regulation of developmental processes in most instances is carried out by trans-activators or repressors binding to specific DNA sites (cis-regulatory sequences), as predicted by the operon model. It seems now established that changes in regulatory systems rather than changes in gene number or protein function are responsible for the evolution of morphological diversity (Carroll 2000). As pointed out by Gann: “Much of the field of EvoDevo employs the language of Jacob and Monod when describing the causes of morphogenetic variation between animals … and much evolutionary variation does indeed come down to changes in the regulation of genes” (Gann 2010). Another new and fertile concept put forward by Jacques Monod dealt with the lactose permease, a membrane-associated protein, believed to allow bacterial cells to pump β-galactosides from the medium (Rickenberg et al 1956). The importance of such membrane-associated pumps in biological phenomena is well recognized today.
In 1967 at the College de France where he was appointed professor, Jacques Monod gave his inaugural lecture “From molecular biology to the ethics of knowledge,” the theme that he would develop later in the “Robbins Lectures,” that took place in Pomona College, Claremont, California in February, 1969. Monod, as a member of the board of the Salk Institute of Biological Science that he helped found, spent every winter a few weeks in La Jolla; there he prepared the Robbins lectures, entitled “Modern Biology and Natural Philosophy.” The four lectures, 1) Living beings as unnatural objects, 2) DNA and emergence, 3) Proteins and teleonomy, and 4) The kingdom of ideas, gave the basic structure of his book: “Chance and necessity,” a philosophical essay on biology and basically a modern view of Darwin’s ideas on evolution and natural selection. It seems evident that the work on regulation led Monod to be more deeply interested in the problem of evolution. He considered the theory of evolution as the most important scientific theory ever formulated because of its philosophical, ideological, and political implications. He gave particular emphasis to the point that there is a profound basic uniformity among living beings, and the basic machinery is the same in all (Monod 1973). At that time, the best example was the universality of the genetic code. Monod would have appreciated the discovery of Hox proteins, highly conserved across vast evolutionary distances and involved in regulation of developmental processes.
As a conclusion of Chance and necessity Monod wrote: “Man at last knows that he is alone in the unfeeling immensity of the universe, out of which he emerged only by chance. Neither his destiny nor his duty have been written down. The kingdom above or the darkness below: it is for him to choose.” The book was published in 1970 and had an unexpected success (Monod 1970). Francis Crick wrote about Chance and necessity in his obituary on Monod: “Written with force and clarity, in an unmistakable personal style, it presented a view of the universe that to many lay readers appeared strange, somber, arid, and austere. This is all the more surprising since the central vision of life that it projected is shared by the great majority of working scientists of any distinction” (Crick 1976).
It was probably not a coincidence that around the same time, Monod, in an unpublished manuscript, revisited his 1948 paper on Lyssenko, analyzed, and demolished the theory of Lyssenko in the light of recent biological knowledge. In 1969, a book of Zhores Medvedev, a Soviet dissident biologist, was published in the United States: “The rise and fall of Lyssenko.” In 1971, prefacing the French translation, Monod analyzed in a powerful style Lyssenko’s career and concluded that “the triumph of Lyssenko was mainly a victory of ideological terrorism.”
Monod had an important role in the creation, in 1973, of the Royaumont Center for a Science of Man, which tried to develop a scientific and synthetic approach of problems concerning modern biology and social sciences. The first meeting, “Unity of Man” discussed problems of fundamental anthropology, animal and human communication, sociology, ethology, among others. The participants were biologists, physicists, sociologists, anthropologists, and psychologists. In October 1975, a conference was held on “Ontogenetic and Phylogenetic Models of Cognitive Development,” with the participation of Noam Chomsky and Jean Piaget. Its scope was to confront and to analyze the foundations and implications of what is innate and what is acquired in the development of language. Jacques Monod participated actively in each conference. It is worth to mention that at the Chomsky–Piaget debate, during a discussion on complexity, Monod argued that knowing the total DNA content in a cell there should be about 1 million genes, most of them involved in regulatory functions, and only 10,000 available for structural functions, numbers that are close to those currently validated (in Piattelli-Palmarini 1979). The Center did not survive the disappearance of Monod.
This scientific and social success did not turn him away from public commitments. For more than a decade, Jacques Monod was continuously fighting for a reform of the French academic and research systems because he realized that France became a scientifically underdeveloped nation and that the rigid structures of research administration and science teaching needed a profound change. Nevertheless, very few reforms took place and those, only after the student revolt in 1968, in which he was deeply involved. His lively teaching courses marked a break between classical university teaching and transmission of modern scientific knowledge. When he was choosing a student, he defined the ideal candidate as being infinitely ignorant and infinitely intelligent.
Monod liked ideas and liked to write; it was always in a clear elegant and incisive style. His logically designed and beautifully written papers remain highlights of the scientific literature. His delight in elegant science can be illustrated by his saying: “A beautiful model or theory may be not right, but an ugly one must be wrong.” He was one of Karl Popper’s admirers and, like Popper, he insisted that scientific advance consisted in the falsification of hypotheses. The foreword of the French translation to Popper’s “The logic of scientific discovery” is revealing of Monod’s intellectual talents. It is of interest to mention that he succeeded to persuade his brother, Philippe Monod to translate Popper’s book, which he did in collaboration with Jacqueline Bernard, a common wartime friend.
In 1971, Monod became Director of the Pasteur Institute. He hesitated for a long time before accepting this task, but he felt he owed the Institute a great deal and wanted to do all he could to maintain its independence and ensure its freedom of research. At the time that Jacques Monod became Director, the Institute was close to bankruptcy. Within the next 5 years (1971–1976), a new scientific and industrial policy was defined and put into practice, and the financial balance was restored. He succeeded in developing public health activities and international relations, especially with the Pasteur Institutes overseas. In other words, he saved the life of an aging institute and propelled it into the modern era. This accomplishment was even more remarkable in light of the fact that, in 1975, he became ill with a disease that would prove fatal only a year later. But his illness did not at any time prevent him from assuming his responsibilities as Director.
Jacques Monod’s commitment to fighting injustice and defending human values was a permanent one. He was continually involved in the struggle against dictatorships and also in the fight against the death penalty and for legalized abortion.
He was an enthusiastic rock climber, in spite of an attack of poliomyelitis in childhood, and an excellent sailor—he was sailing until the last days of his life (figs. 9 and 10)).
FIG. 9.—

Monod on his sailing boat, May 28, 1976 (3 days before he passed away).
FIG. 10.—

Monod in the garden of his house in Cannes, May 29, 1976.
I should like to finish with a quotation of Melvin Cohn who, from 1949 to 1963, with only a few interruptions, spent 10 years in Monod’s laboratory before he joined the Salk Institute in California. Their friendship did not stop because for many years, Jacques Monod used to spend some weeks in winter at the Salk Institute, as a member of the Board of Trusties:
“I believe that Jacques Monod had one of the most creative minds of our time not because he was a leader of righteous causes, not because he was a creator of molecular biology, not because he founded and directed institutes of learning. He had one of the most creative minds simply because he thought deeply, ascetically in a Socratic way about how knowledge is acquired, and it is this process that he insisted should be the only basis for a system of ethical and aesthetic values” (Cohn 1976).
References
- Avery OT, Macleod CM, McCarthy M. Studies of the chemical nature of the substance inducing transformation of pneumococcal types. Induction of transformation by a deoxyribonucleic acid fraction isolated from pneumococcus type III. J Exp Med. 1944;89:137–158. doi: 10.1084/jem.79.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll SB. Endless forms: the evolution of gene regulation and morphological diversity. Cell. 2000;101:577–580. doi: 10.1016/s0092-8674(00)80868-5. [DOI] [PubMed] [Google Scholar]
- Cohn M. In Memoriam. In: Ullmann A, editor. Origins of molecular biology: a tribute to Jacques Monod. Washington, (DC): ASM Press; 1976. pp. 93–104. [Google Scholar]
- Cohn M, Monod J, Pollock S, Spiegelman S, Stanier RY. Nature. 1953;172:1906. doi: 10.1038/1721096a0. [DOI] [PubMed] [Google Scholar]
- Crick FHC. Jacques Monod. Nature (London) 1976;262:429–430. [Google Scholar]
- Gann A. Jacob and Monod: from Operons to EvoDevo. Curr Biol. 2010;20:R718–R723. doi: 10.1016/j.cub.2010.06.027. [DOI] [PubMed] [Google Scholar]
- Gilbert W, Müller-Hill B. Isolation of the Lac repressor. Proc Natl Acad Sci U S A. 1966;56:1891–1898. doi: 10.1073/pnas.56.6.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogness DS, Cohn M, Monod J. Studies on the induced synthesis of β-galactosidase in Escherichia coli: the kinetics and mechanism of sulfur incorporation. Biochim Biophys Acta. 1955;16:99–116. doi: 10.1016/0006-3002(55)90188-8. [DOI] [PubMed] [Google Scholar]
- Jacob F, Monod J. Gènes de structure et gènes de régulation dans la biosynthèse des proteins. C R Acad Sci. 1959;249:778–780. [PubMed] [Google Scholar]
- Jaob F, Monod J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- Jacob F, Wollman E. Sur les processus de conjugaison et de recombinaison chez Escherichia coli: l’induction par conjugaison ou induction zygotique. Ann Inst Pasteur. 1956;91:486–510. [PubMed] [Google Scholar]
- Luria SE, Delbrück M. Mutations of bacteria from virus sensitivity to virus resistance. Genetics. 1943;28:491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monod J. Sur un phénomène nouveau de croissance complexe dans les cultures bactériennes. C R Acad Sci. 1941;212:934–936. [Google Scholar]
- Monod J. Recherche sur la croissance des cultures bactériennes. In: Hermann, editor. Paris (France): 1942. [Google Scholar]
- Monod J. The growth of bacterial cultures. Ann Rev Microbiol. 1949;3:371–394. [Google Scholar]
- Monod J. La technique de culture continue. Théorie et applications. Ann Inst Pasteur. 1950;79:390–410. [Google Scholar]
- Monod J. From enzymatic adaptation to allosteric transitions, Nobel Lecture. Science. 1966;154:1475–1483. doi: 10.1126/science.154.3748.475. [DOI] [PubMed] [Google Scholar]
- Monod J. Le hasard et la necessité: essai sur la philosophie naturelle de la biologie moderne. Editions du Seuil, Paris. English translation 1972 Chance and Necessity. An Essay on Natural Philosophu of Modern Biology. London: Collins; 1970. [Google Scholar]
- Monod J. Problems Of Scientific Revolution: Progress and obstacles to progress in the sciences (The Herbert Spencer Lectures 1973) Oxford, (United Kingdom): Oxford University Press; 1973. On the molecular theory of evolution; pp. 11–24. [Google Scholar]
- Monod J, Wyman J, Changeux J-P. On the nature of allosteric transitions: a plausible model. J Mol Biol. 1965;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Pardee AB, Jacob F, Monod J. The genetic control and cytoplasmic expression of “inducibility” in the synthesis of β-galactosidase in Escherichia coli. J Mol Biol. 1959;1:165–178. [Google Scholar]
- Piattelli-Palmarini M, editor. Théories du languamge, théories de l’apprentissage [language and learning] London: Routledge and Kegan Paul PLC; 1979. p. 291. [Google Scholar]
- Rickenberg H, Cohen GN, Buttin G, Monod J. La galactoside–perméase d’ Escherichia coli. Ann Inst Pasteur. 1956;91:829–857. [PubMed] [Google Scholar]
- Stanier RY. Jacques Monod 1910–1976. J Gen Microbiol. 1977;101:1–12. doi: 10.1099/00221287-101-1-1. [DOI] [PubMed] [Google Scholar]
- Zabin I, Kepes A, Monod J. Thiogalactoside transacetylase. J Biol Chem. 1962;237:253–257. [PubMed] [Google Scholar]