Abstract
Subjects were scanned in a single functional MRI (fMRI) experiment that enabled us to localize cortical regions in each subject in the occipital and temporal lobes that responded significantly in a variety of contrasts: faces > objects, body parts > objects, scenes > objects, objects > scrambled objects, and moving > stationary stimuli. The resulting activation maps were coregistered across subjects using spherical surface coordinates [Fischl et al., Hum Brain Mapp 1999;8:272–284] to produce a “percentage overlap map” indicating the percentage of subjects who showed a significant response for each contrast at each point on the surface. Prominent among the overlapping activations in these contrasts were the fusiform face area (FFA), extrastriate body area (EBA), parahippocampal place area (PPA), lateral occipital complex (LOC), and MT+/V5; only a few other areas responded consistently across subjects in these contrasts. Another analysis showed that the spatial profile of the selective response drops off quite sharply outside the standard borders of the FFA and PPA (less so for the EBA and MT+/V5), indicating that these regions are not simply peaks of very broad selectivities spanning centimeters of cortex, but fairly discrete regions of cortex with distinctive functional profiles. The data also yielded a surprise that challenges our understanding of the function of area MT+: a higher response to body parts than to objects. The anatomical consistency of each of our functionally defined regions across subjects and the spatial sharpness of their activation profiles within subjects highlight the fact that these regions constitute replicable and distinctive landmarks in the functional organization of the human brain. Hum Brain Mapp, 2005. © 2005 Wiley‐Liss, Inc.
INTRODUCTION
Functional MRI (fMRI) investigations over the last few years have found strongly selective responses to specific categories of visual stimuli in focal regions of human extrastriate cortex. Thus, the fusiform face area (FFA) responds about twice as strongly to faces as to other stimulus categories [Halgren et al., 1999; Kanwisher et al., 1997a; McCarthy et al., 1997; Puce et al., 1996], the parahippocampal place area (PPA) responds selectively to images depicting places [Aguirre et al., 1998; Epstein et al., 1999; Epstein and Kanwisher, 1998; Ishai et al., 1999], and the extrastriate body area (EBA) responds selectively to images of bodies and body parts [Downing et al., 2001]. The selective responses of these regions are robust enough that each of them can be found, in the same approximate anatomical location, in virtually every normal subject scanned with fMRI. Thus, the FFA, PPA, and EBA are now considered by many to be part of the basic functional architecture of human extrastriate cortex.
But how exactly should we think about these regions of cortex? Are they distinct cortical areas like V1 or MT+/V5? At present we have evidence bearing on only one of the Felleman and Van Essen criteria for cortical areas: function [Felleman and Van Essen, 1991]. We know almost nothing about the connectivity or cytoarchitecture of these regions in the human brain. This means that our main reason for thinking of these regions as distinct entities is their distinctive functional profile and anatomical consistency across subjects. The present study addresses this question directly by scanning 14 normal subjects on the functional localizers for the FFA, PPA, and EBA (as well as other regions), and measuring the consistency across subjects in the location of each of these regions with a new kind of group analysis. We also include a new analysis to determine whether the selectivity of each region drops off abruptly at its functionally defined borders, or whether instead the selectivity profile that defines each region is spatially very diffuse, extending far beyond the borders of that region.
This enterprise is of potential use not only in providing a stronger test of the data on which the existence of each area is based, but also in providing a quantitative measure of the consistency in the location of these regions across subjects in the normal population.
But what counts as the “same place” in two anatomically distinct brains? Comparisons between the anatomical locations of functional responses in different subjects rely on a method for aligning different individuals' brains in the same coordinate system. Most anatomical comparisons of the human brain based on MRI data use the 3‐D Talairach normalization system developed by Talairach and Tournoux [Talairach et al., 1967; Talairach and Tournoux, 1988]. Such normalization, however, underestimates the true distance along the cortical sheet, particularly for points lying on opposite banks of a sulcus. Thus, the metric properties of the Talairach‐based coordinate system do not correctly reflect the metric properties of the cortical sheet. In order to achieve better subject‐to‐subject alignment, surface‐based coordinate systems have recently been developed [Fischl et al., 1999a; Thompson and Toga, 1996; Van Essen and Drury, 1997]. One of them is the spherical coordinate system, where the reconstructed cortical surface of each individual is mapped onto a sphere, using the “maximally isometric transformation” [Fischl et al., 1999a]. This algorithm attempts to preserve as much as possible the distances between cortical locations projected onto the sphere. The normalization then aligns the entire pattern of sulcal/gyral folds to a statistical atlas, and normalization has been shown to produce greater consistency across subjects in the location of structural patterns of the human brain and the functional areas of the retinotopic cortex [Fischl et al., 1999b].
In this study we scanned subjects with fMRI while they viewed either static or moving images from each of five stimulus categories (faces, scenes, body parts, objects, and scrambled objects). We tested the consistency in the anatomical location of several functional areas using the spherical surface‐based coordinate systems. In the portion of the brain we examined we find that only a limited number of regions show a consistently selective activation pattern across subjects for contrasts between these stimuli. The most prominent of these regions are the FFA, PPA, EBA, and MT+/V5.
A second question addressed in this study concerns the sharpness of the spatial selectivity profile around a functionally defined area. The borders of functionally defined areas are typically determined using a t‐test with a relatively stringent statistical P‐level threshold. The choice of threshold is somewhat arbitrary, and the lower the P‐value, the smaller the area. Does weak category selectivity extend far beyond the typically defined borders of each functional region? Here we addressed this question by measuring how response selectivity varies with the distance across cortex from each functionally defined area. We find that category selectivity (preferred minus nonpreferred) drops off rapidly as one moves away from the standard functionally defined borders of each region, becoming zero at a distance of only 4 mm from the borders of the FFA and PPA.
SUBJECTS AND METHODS
Subjects
Fourteen healthy adult volunteers (seven males and seven females) participated in this study. The age of the subjects ranged from 19–44 years.
Stimuli
Stimuli were gray‐scale photographs from five different categories: faces, scenes, body parts, objects, and scrambled objects. In each category 20 different exemplars were used. For the body parts category we used isolated parts of bodies, for instance, a leg, foot, and arm. For the object categories the images represented either man‐made objects or food. These images were also used to generate the “scrambled objects” category. Each “scrambled image” was created by cutting the intact image into a 20 × 20 grid of square subimages, then randomly exchanging the positions of each subimage. The scrambling made the objects unrecognizable. Images were 300 × 300 pixels in size and subtended a visual angle of 9°. They were either static or moved away from the center of the screen at a speed of about 160 pixels/s (causing apparent motion of 1.5°). In a separate set of “meridian mapping” scans run on all but one subject in the same session, we used black and white flickering checkerboard wedges with four different orientations (up, down, right, and left) to map the retinotopic areas. The flickering checkerboard wedges were presented on a 700 × 700 pixel wide screen and subtended a visual angle of 20.8°. The length and width of the wedges were 350 (10.4° of visual angle) and 120 pixels (3.6° of visual angle), respectively. In addition, low‐contrast moving vs. stationary ring stimuli were used in two subjects to localize MT+ according to standard practices [Tootell et al., 1995].
Procedure
Each subject was run on five different functional scans used to localize category‐specific areas (and MT+/V5). A blocked design was used, with each scan consisting of two sets of five epochs (one for each image category). A 16‐s fixation period was present at the beginning of the scan, between the two 5‐epoch sets, and at the end of the scan. Each epoch lasted 16 s, during which 20 images of one category were presented. Each picture was presented for 300 ms, followed by a 500‐ms blank interval. In order to keep subjects alert and attentive to all stimuli, subjects were instructed to press a button whenever they saw two identical images consecutively (a “1‐back” task). This happened twice per epoch. Epochs of each stimulus category were presented in different orders within each scan in a way that balanced the serial position of the category across the scans. During a given epoch we presented either static images located in the center of the screen or images moving away from the center either horizontally, vertically, or along the diagonals. Epochs of static and moving images alternated during each scan. For the additional two meridian mapping scans, subjects viewed in each block a black and white flickering checkerboard wedge with its vertex at fixation (in the middle of the screen) and its base above, below, to the right, or to the left of fixation.
fMRI Data Acquisition
Scanning was done on a Siemens 3T Allegra scanner at the Athinoula A. Martinos Center for Biomedical Imaging in Charlestown, MA. A head coil and a gradient echo, echo‐planar (EPI) pulse sequence with TR, 2 s; TE, 30 ms; flip angle, 90° were used. Twenty contiguous axial slices were acquired, covering the entire temporal and occipital lobes. The voxel size was 3 × 3 × 4 mm, the matrix size was 64 × 64, and the FOV was equal to 192 × 192. We focused on the occipito‐temporal cortex because of its role in object processing.
Brain Reconstruction
Three‐dimensional structural (“MPRAGE”) scans were acquired in order to reconstruct the brain using Freesurfer software [Dale et al., 1999; Fischl et al., 1999a; Fischl et al., 2001]. MPRAGE is an inversion prepared gradient echo sequence, with the following parameters: bandwidth = 190 Hz/pixel, flip angle = 7°, TR/TE/TI = 2.73 s/3.44 ms/1 s, voxel size 1.3 × 1.3 × 1 mm. This software generates an inflated representation of the cortical surface of the brain after several processing steps, including intensity normalization, removal of skull, and segmentation of white matter. Each subject's cortical surface was also registered by matching each individual subject's pattern of cortical folds with a spherical surface‐based atlas [Fischl et al., 1999b].
fMRI Data Analysis
Percentage overlap maps of functional areas
We determined the consistency in the anatomical location of each functional area across subjects as follows: We first motion‐corrected the data from each subject using Fsfast analysis toolbox. Data were then smoothed in the volume using a Gaussian filter with a FWHM equal to 4 mm. For each contrast investigated (faces vs. objects, places vs. objects, body parts vs. objects, objects vs. scrambled objects, moving vs. stationary, and the meridian mapping related contrasts) in each subject we individually calculated P‐values in each voxel to create an activation map of the volume data. For each contrast and each subject, we created a label composed of all the voxels in the brain with a P‐value lower than or equal to 10−4 (uncorrected for multiple comparisons). For each contrast, the labels of all the subjects were mapped onto an statistical template of cortical geometry using the spherical normalization. For each point on the cortical surface, we calculated the percentage of subjects that showed a significant activation for each contrast (with P < 10−4). We call the maps obtained in this manner “percentage overlap maps.” This method is different from the traditional random effect analysis and can be interpreted as the prior probability that a given point in the spherical coordinate system will be within the specified functional area for a novel subject.
Selectivity Profiles
Region of interest (ROI)
Motion correction using the Fsfast analysis toolbox was performed prior to the data analysis [Cox and Jesmanowicz, 1999]. To determine the ROI for each functionally defined region separately for each subject, data from the first two scans were smoothed onto the brain surface in the volume using a Gaussian filter with a FWHM equal to 4 mm and projected onto the inflated brain using the Freesurfer toolbox. A t‐test was then performed on the average surface data from the two scans. The remaining three scans were used for the “ring” analysis (see below). Thus, the selectivity was measured using a dataset independent of that used to define each region. The fusiform face area (FFA) was defined as the area in the mid‐fusiform gyrus that was significantly more active for faces than objects (t‐test, P < 10−5). Similarly, the region in the parahippocampal gyrus that was significantly more active for scenes than objects defines the parahippocampal place area (PPA) (t‐test, P < 10−5). The extrastriate body area (EBA) was defined using the activation contrast for body parts vs. objects (t‐test, P < 10−5). Finally, MT+/V5 was identified by comparing the epochs containing the moving images with the epochs containing the still images (t‐test, P < 10−5). In two subjects we used a more conventional MT+ localizer consisting of low‐contrast moving rings and found an activation at exactly the same place as that found with moving images, validating our choice of the moving‐image localizer.
Ring Analysis
To study how the selectivity of each functionally defined region drops off with the distance from the traditionally defined border of that region, we projected the functional data of the last three scans onto the surface and averaged their values with no smoothing. We determined the relationship between the vertices on the inflated brain as follows. The Freesurfer algorithm identifies a set of vertices, i.e., points on the surface of the inflated brain analogous to voxels in volume data, separated from each other by about 1 mm. Outer and inner rings of vertices were defined as follows: the first outer ring consists of all the surface vertices that are immediately adjacent to the border of the functional area. (see Fig. 5). The second outer ring consists of all the vertices adjacent to the first outer ring. Each new outer ring is similarly defined as the set of vertices immediately adjacent to the previous outer ring that are not part of any previous outer ring, nor part of the functional area (Fig. 5, left). In the case where a functional area is made of more than one continuous region, a given ring could as well be composed of disjoint sets of vertices (Fig. 5, right). The inner rings were defined in a way similar to the outer rings, the first inner ring being identified with the border of the functional area. This procedure is done separately for each subject in their own native space. The percent signal change of a given ring for a given stimulus condition is the average across all subjects of the percent signal increase for that condition above a fixation baseline across all the vertices that constitute that ring in each subject
Figure 5.

Rings of vertices (yellow/white) surrounding a functionally defined area (red/light gray) for two cases in which: (left) the functional area is convex, and (right) the functionally defined area is made out of two discontinuous regions. For visualization purposes, only the third, sixth, and ninth neighboring outer rings and the third neighboring inner rings are drawn. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
RESULTS
Percentage Overlap Maps
We tested the consistency across subjects in the anatomical location of each functionally defined area. In order to avoid any bias in the location of each area, we considered the activation of the entire temporal and occipital cortex for each contrast (“faces vs. objects.” “scenes vs. objects,” “body parts vs. objects,” “objects vs. scrambled objects,” and “moving images vs. static images”; in each case using a threshold of P < 10−4 uncorrected within each subject). For each of these contrasts we created a “percentage overlap map”: a map that indicates at each point in the brain the proportion of subjects that show a significant activation for the contrast in question. We also used the same approach to map V1‐V2 borders (for example, the V1‐V2 superior divisions were derived from the lower field vertical wedges).
Figure 1 shows an example of a percentage overlap map in the right hemisphere for three different contrasts (“faces vs. objects,” “objects vs. scrambled object,” and “scenes vs. objects”) in an average template brain (derived from an average across 40 subjects) [Fischl et al., 1999b]. The maximum of the percentage overlap map varied between 64% and 93%, depending on the contrast (Table I). As expected, the maximum percentage overlap was usually higher with contrasts producing larger activated regions (because, for a given displacement, the overlap will be larger for larger regions). The difference in overlap accuracy might also reflect the fact that some anatomical regions of the brain might have larger location variability across subjects than others. Note that the maximum percentage overlap was not higher for the V1‐V2 borders than for the other contrasts (Table I).
Figure 1.
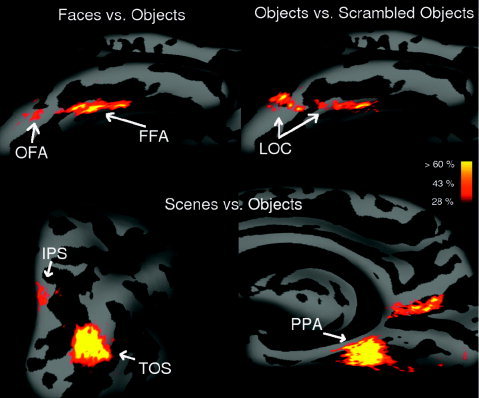
Percentage overlap maps for the “faces vs. objects” (top left),“objects vs. scrambled objects” (top right), and “scenes vs. objects” (bottom) contrasts. The maps indicate the percentage of subjects who showed significant activation at the P < 10−4 level (uncorrected) individually at each point on the surface. Results are represented on an inflated right hemisphere brain of an average template brain. We represent here only overlaps of 4/14 subjects (28%) or higher.
Table I.
Maximum percent overlap for the “best” voxel for each contrast in each hemisphere
| RH (%) | LH (%) | |
|---|---|---|
| Faces vs. objects | 71.5 | 64.3 |
| Scenes vs. objects | 92.9 | 92.9 |
| Body parts vs. objects | 78.6 | 78.6 |
| Objects vs. scrambled objects | 85.7 | 71.4 |
| Moving vs. stationary | 78.6 | 85.7 |
| Upper wedge vs. others | 76.9 | 92.3 |
| Lower wedge vs. others | 76.9 | 69.2 |
Maximum percent overlap = the percentage of subjects showing the contrast in question significantly (P < 10−4, uncorrected).
For the “faces vs. objects” contrast, only two regions of the entire occipital and temporal lobe appear (Fig. 1, top left). One is the well‐known fusiform face area (FFA) in the fusiform gyrus. The second one, located in the inferior occipital gyrus, shows a lesser degree of overlap, and corresponds to the occipital face area (OFA) [Halgren et al., 1999; Haxby et al., 1999; Kanwisher et al., 1997a]. The percentage overlap map for the “object vs. scrambled object” contrast produces a large region starting from the inferior occipital cortex and extending up to the temporal cortex (including the FFA) (Fig. 1, top right). This region is known as the lateral occipital complex (LOC), and has been shown to respond more strongly to stimuli depicting recognizable shapes than to scrambled stimuli that do not depict clear shapes [Kanwisher et al., 1997b; Malach et al., 1995]. No other scanned region shows an activation for this contrast that overlaps across subjects. The “scenes vs. objects” contrast produced four different regions of overlap (Fig. 1, bottom). One, located along the collateral sulcus, is the parahippocampal place area (PPA) [Epstein and Kanwisher, 1998]. We also found three other regions, two of them showing a high degree of overlap. One is in the occipital sulcus, in a region just lateral to the transverse occipital sulcus (TOS) (Fig. 1, bottom left); this scene or building‐selective activation has been reported in several prior studies [Epstein et al., 2005; Grill‐Spector, 2003; Hasson, et al., 2003, Nakamura et al., 2000]. Another region extends from the occipito‐parietal sulcus to the posterior part of the cingulate gyrus (Fig. 1, bottom right). Activation in this area has been found in navigation tasks and place‐encoding [Burgess et al., 2001; Maguire, 2001]. Finally, we found another small region in the lower part of the superior parietal gyrus that is much less consistent across subjects (Fig. 1, bottom left). Grill‐Spector [2003] reported an activation in a similar region (in the intraparietal sulcus, IPS) when subjects view scenes or objects (but not faces) compared to texture patterns.
Figure 2 shows the left and right hemisphere of an averaged template brain. The population activation maps for the different contrasts are represented in different colors. The left and right hemisphere produce very similar results except that the FFA and EBA have a lower amount of overlap in the left than right hemispheres. This finding reflects the fact that not all subjects show an FFA and EBA in the left hemisphere. For clarity of visualization we did not represent the overlap generated by the “objects vs. scrambled objects” contrast, i.e., the LOC. Indeed, the LOC overlaps with both the FFA and EBA. Figure 2 shows the EBA, obtained by contrasting the response to “body parts vs. objects.” This region is located in lateral occipito‐temporal cortex, with most of the overlap in the medial and inferior part of the occipital cortex. The overlap region of the EBA seems to surround the MT+/V5 region obtained with the “moving vs. static images” contrast. The “body parts vs. objects” contrast also shows activation in the FFA, which indicates that the FFA responds fairly strongly to body parts in this study (see next section). The coordinates of the different functional areas obtained with this method are given in Table II.
Figure 2.
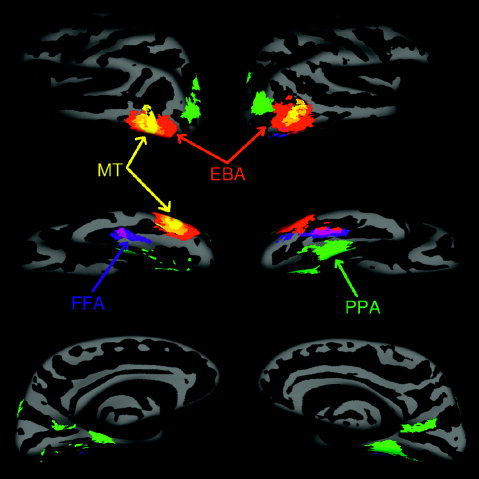
Regions showing at least 28% overlap across subjects in activation from each contrast shown on lateral (top), ventral (middle), and medial (bottom) views of the cortical surface of an averaged template brain; the threshold for each subject individually was P < 10−4 uncorrected. Both left and right hemispheres are represented (in the left and right part of the figure, respectively). Each color represents the population overlap of a given contrast: blue = “faces vs. objects,” green = “scenes vs. objects,” red = “body part vs. objects,” yellow = “moving vs. stationary,” orange = overlap between “body part vs. objects” and “moving vs. stationary,” purple = overlap between “body part vs. objects” and “faces vs. objects,” dark green = overlap between “body part vs. objects” and “scenes vs. objects.”
Table II.
MNI and standard Talairach coordinates of the center of the functional areas obtained in Figure 2
| RH | LH | |
|---|---|---|
| FFA | ||
| MNI | 31.6, −57.2, −10.4 | −50.6, −70.8, −13.0 |
| Tal | 31.3, −55.8, −5.9 | −50.1, −69.2, −7.5 |
| OFA | ||
| MNI | 17.8, −88.9, −11.3 | — |
| Tal | 17.6, −86.6, −5.2 | — |
| EBA (anterior part) | ||
| MNI | 32.0, −67.6, −5.8 | −58.2, −72.3, 4.7 |
| Tal | 31.7, −65.8, −1.5 | −57.6, −69.8, 7.8 |
| EBA (posterior part) | ||
| MNI | 32.6, −77.3, 2.4 | −63.6, −83.3, 21.4 |
| Tal | 32.3, −74.8, 5.9 | −63.0, −79.7, 23.7 |
| MT/V5 | ||
| MNI | 21.3, −67.0, 2.7 | 54.0, −74.3, 13.8 |
| Tal | 21.1, −64.8, 5.7 | −53.5, −71.4, 16.3 |
| LOC (anterior part) | ||
| MNI | 28.8, −49.2, −16.5 | −45.8, −53.7, −11.5 |
| Tal | 28.6, −48.4, −11.5 | −45.4, −52.5, −7.0 |
| LOC (posterior part) | ||
| MNI | 30.6, −81.8, −8.6 | −59.3, −82.5, 5.6 |
| Tal | 30.3, −79.6, −3.2 | −58.7, −79.7, 9.1 |
| PPA | ||
| MNI | 16.5, −45.1, −1.5 | −39.1, −56.9, 1.6 |
| Tal | 16.3, −43.7, 1.0 | −38.7, −55.1, 4.2 |
| Scenes vs. objects (near TOS) | ||
| MNI | 22.3, −88.3, 14.6 | −44.5, −88.7, 32.7 |
| Tal | 22.0, −84.9, 17.7 | −44.0, −84.5, 34.3 |
| Scenes vs. objects (near IPS) | ||
| MNI | 7.6, −76.5, 55.2 | −27.1, −71.5, 62.0 |
| Tal | 7.5, −71.6, 54.4 | −26.7, −66.4, 60.4 |
| Scenes vs. objects (cingulate gyrus) | ||
| MNI | 7.3, −63.5, 24.4 | −37.0, −61.0, 25.4 |
| Tal | 7.2, −60.4, 25.5 | −36.7, −57.9, 26.3 |
MNI, Montreal Neurological Institute space; FFA, fusiform face area; Tal, Talairach; OFA, occipital face area; EBA, extrastriate body area; LOC, lateral occipital complex; PPA, parahippocampal place area.
Figure 3 shows the activations within each of five subjects individually (thresholded at P < 10−4) for each of the contrasts shown in Figure 2. Although the general pattern shown in the group percentage overlap map (Fig. 2) can be seen in each individual subject (Fig. 3), considerable variability is also seen across subjects in the precise location and extent of the activation corresponding to each contrast. Further, considerable patchiness is apparent in the activation for each region in each subject. Some of this patchiness results from sampling at the gray–white‐matter border, which includes a subset of the total activation seen in slice data. Sampling at the gray/white border increases the spatial accuracy of the localization on the cortical surface at the price of reduced statistical power. Further studies in our lab are investigating the patchiness of each functionally defined region by scanning with higher spatial resolution.
Figure 3.
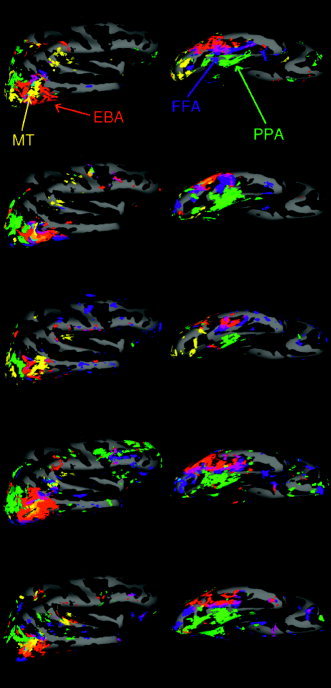
Activation (for P < 10−4) for each contrast in five subjects mapped on an averaged template brain. Only the right hemisphere is represented. The colors identify the contrasts with the same color code as in Figure 2. In addition there is pale blue = overlap between “faces vs. objects” and “moving vs. stationary,” brown = overlap between “scenes vs. objects” and “moving vs. stationary,” and black = overlap between “faces vs. objects” and “scenes vs. objects”.
Percent Signal Change and Selectivity Profile
To test the selectivity of each of the different functional areas, we measured the percent signal increase from fixation baseline for each of the image categories in each functionally defined area (using independent datasets to define the area, and to calculate the percent signal change within that area). A two‐way ANOVA of stimulus categories (“faces,” “scenes,” “body parts,” “objects,” and “scrambled objects”) by regions (“FFA,” “PPA,” “EBA,” and “MT+/V5”) showed a significant interaction (F(12,215) = 10.6, P < 0.001), indicating that the regions differed significantly from each other in their profile of response across stimulus categories (as expected).
Figure 4 (left) shows the percent signal change for faces, scenes, body parts, and objects in the FFA, PPA, EBA, and MT+/V5. We did not calculate the percent signal change in LOC, because the localizer we used was not strong enough to reveal the functional signature of LOC (a significantly higher response to objects than scrambled objects) consistently with only two runs. In the PPA and EBA, the response of the preferred category (i.e., scenes and body parts, respectively) is significantly higher than for each of the other categories. On the other hand, the “face” response in the FFA is not significantly different from the “body parts” response (t = 1.35, P = 0.2), although it is significantly higher than for the other image categories. In MT+/V5 we find that the percent signal change for faces, scenes, objects, and scrambled objects has about the same value, but it is substantially higher for body parts. This result is not surprising, given the proximity of MT+/V5 and EBA (see previous section), but note that the higher response for body parts than objects continues inside the border of MT+/V5 (Fig. 6d), suggesting that this body part selectivity is a property of MT+/V5 itself, and does not merely result from partial overlap of MT+/V5 with the EBA. Only the MT+/V5 region showed a significantly higher response for moving than static images, as expected (Fig. 4, right).
Figure 4.
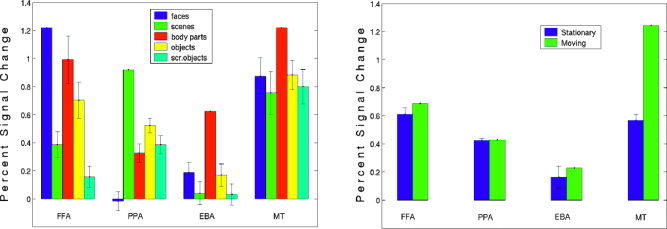
Analysis of the response properties of each region. For this analysis two scans were used to functionally define each region (at the P < 10−5 threshold); the percent signal change in each condition, compared to a fixation baseline, was calculated over the remaining three scans. Because the amount of data used to localize each region was limited, we did not find each area in each subject; the FFA was identified in 12 subjects, the PPA in all 14, the EBA in 12, and MT+ in 9 subjects. a: Mean percent signal change across subjects to each image category in the FFA, PPA, EBA, and MT+ regions in the right hemisphere. The error bars represent the standard error on the difference between the preferred and nonpreferred stimuli. In FFA, PPA, and EBA, paired sample t‐tests on the percent signal change gave values of P < 0.001 for all comparisons between the preferred category and each other category, except for the comparison between “faces” and “body parts” in the FFA, where P = 0.2 (t = 1.35). In MT+ the percent signal change for body parts is significantly higher than for the other categories (body parts vs. faces: t = 2.7 P < 0.02, body parts vs. scenes: t = 3.1 P < 0.01, body parts vs. objects: t = 3.2 P < 0.01, body parts vs. scrambled objects: t = 3.4 P < 0.005). b: Mean percent signal change across subjects to static and moving images in the FFA, PPA, EBA, and MT+ regions in the right hemisphere. The error bars represent the standard error of the difference between the preferred and nonpreferred stimuli. Paired sample t‐tests for responses to moving vs. static images were not significant (all P > 0.05) in any area except MT+, where the responses for moving images are significantly higher than for static images (t = 15.4, P < 0.001).
Figure 6.
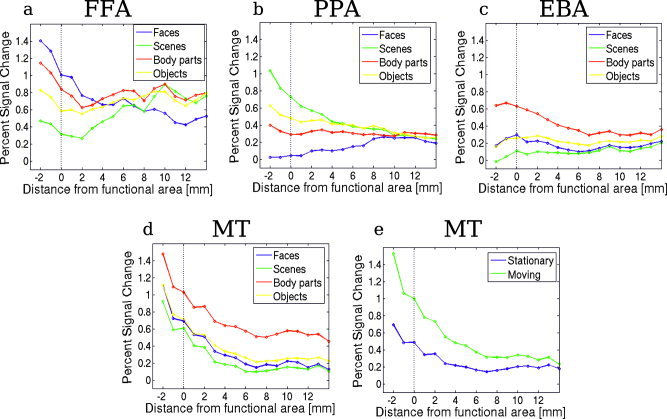
The spatial drop off in selectivity for each functionally defined region (FFA, PPA, EBA, and MT+): Percent signal change for each stimulus category as a function of distance from the borders of the region. Negative distances correspond to the response inside the area, the response at distance 0 being the response at the border of the functionally defined region. The vertical dotted line represents the border of the functional area. [Color figure can be viewed in the online issue, which is available at www.interscience. wiley.com.]
We also studied the selectivity profile around each of the functionally defined areas. To do this, we first defined the borders of each area in each subject using one set of scans and a stringent threshold value (P < 10−5). In the surface data, we then defined a set of outer and inner rings (see Fig. 5), each separated from the next ring by 1 mm, as described in Subjects and Methods. Using a second independent set of scans, we computed the percent signal change of the functional data from each consecutive ring inside and outside each functionally defined area. The value obtained this way was then averaged across all subjects. Figure 6 shows how the selectivity of each area drops off with the distance from the standardly defined border of each area. The response profile for “faces” in the FFA and “scenes” in the PPA is quite sharp; at 3–4 mm from the border of functional area the response is similar for preferred and nonpreferred categories, although interestingly the particularly low response to faces in the PPA extends well beyond the PPA border (Fig. 6a,b, see also Table III). On the other hand, the response profile for body parts in the EBA is much broader (Fig. 6c, Table III). In MT+/V5 the percent signal change for body parts is quite high, possibly because of the proximity of the EBA (Fig. 6d). Figure 6e shows the decrease of the response selectivity for moving images as a function of the distance from the MT+/V5 area. The selectivity for moving images is still present a few mm away from MT+/V5, indicating that the cortex adjacent to MT+/V5 is also responsive to moving stimuli. This motion selectivity was not significant in the EBA in Figure 4 (which surrounds MT+/V5), because it is only the part of the EBA that is close to MT+/V5 that shows motion selectivity, whereas Figure 4 shows an average over the entire area. Table III also shows the gain in surface are for the region between the standard border of the functional area and the perimeter where all selectivity for the preferred category is lost. The expansion of the area is less than 2‐fold, which indicates that the selectivity drops off rapidly as one moves away from the functional area. Note that these values depend on the threshold chosen. The sharp drop‐off can also be seen in Figure 7, which shows the selectivity as a function of the cortical position.
Table III.
Statistics for the percent signal change in the ring analysis for different areas
| Distance at which t‐test is non‐significant* | P | Distance at which ANOVA test is non‐significant** | P | Increase in size (%)*** |
|---|---|---|---|---|
| FFA | ||||
| Faces‐scenes: 4 mm | 0.13 | 3 mm | 0.09 | 54.6 + −23.7 |
| Faces‐body parts:‐ | — | |||
| Faces‐objects: 2 mm | 0.15 | |||
| PPA | ||||
| Scenes‐faces: 8 mm | 0.24 | 7 mm | 0.07 | 40.1 + −27.5 |
| Scenes‐body parts: 4 mm | 0.12 | |||
| Scenes‐objects: 2 mm | 0.15 | |||
| EBA | ||||
| Body parts‐faces: 7 mm | 0.06 | 7 mm | 0.11 | 70.5 + −34.3 |
| Body parts‐scenes: 7 mm | 0.05 | |||
| Body parts‐objects: 6 mm | 0.06 | |||
| MT+ | ||||
| Moving‐static: 6 mm | 0.1 | 6 mm | 0.1 | 96.1 + −40.3 |
Distance from the border of the functional area at which the percent signal change between the preferred and non‐preferred categories is not significant anymore (t‐test).
Distance at which the one‐way ANOVA test on the percent signal change across categories is not significant anymore.
Gain in surface for the area defined by the drop in selectivity compared to the functional area. The gain in surface is averaged across subjects.
Since the signal for the body parts category was significantly higher than those for the other categories at all the distance studied outside MT+, we did not present the statistics for the object categories signal around MT+.
Figure 7.
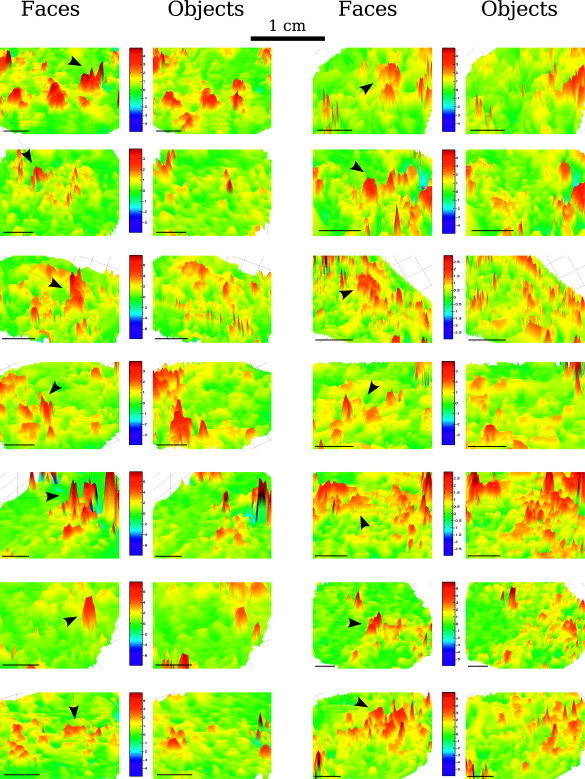
Percent signal change for faces and objects compared to the fixation baseline as a function of the cortical position in all subjects. Only the region around FFA is shown. The color‐coded percent signal change is represented for a flattened portion of the brain. The color bar values represent the percent signal change compared to fixation. The arrows indicate the FFA.
DISCUSSION
This study provides a broad survey of the human occipital and temporal lobes, searching for any cortical regions that respond in a category‐selective fashion consistently across subjects. We did not restrict our analysis to previously identified category‐selective regions of interest such as the FFA, PPA, and EBA [Downing et al., 2001; Epstein and Kanwisher, 1998; Kanwisher et al., 1997], but instead tested the entire scanned area for any category‐selective regions, whether previously identified or not. Using cortical surface representations of our data and a spherical coordinate system [Fischl et al., 1999a] to optimize our registration across subjects, we asked which of the category‐selective responses visible in individual subjects arise in a consistent location across subjects. The result of this analysis is a “percentage overlap map” revealing at each point on the surface the percent of subjects who show a given selectivity at that point. This analysis resembles a random effects group analysis in that it reveals which activations are consistent across subjects. However, because the percentage overlap map reveals the percent of subjects who show a given effect significantly at each point, it provides a particularly useful database for future studies. For example, it can be used to evaluate the probability that a lesion or other anatomical condition in a particular neurological patient has affected the FFA. It also provides a useful database against which activation patterns in subject groups from different ages and/or clinical populations can be compared (for a similar argument about activations in retinotopic cortex, see Dougherty et al. [2003]). In general, the percentage overlap map for an area allows one to compute the probability that a given point in the spherical coordinate system for a novel subject is contained within that area.
The present study also demonstrates several new findings. First, the number of regions of cortex that show a category‐selective functional profile that is consistent across subjects is relatively small. The percentage overlap map reveals the FFA, PPA, EBA, MT+/V5, and LOC, as expected, and the maximal cross‐subject overlap in these regions ranges from between 64–93%. In addition, a small number of other regions were also found in extrastriate cortex that show over 28% overlap across subjects. For example, a scene‐selective region is evident far from the PPA near the transverse occipital sulcus. This region has been noted previously [Grill‐Spector, 2003; Hasson et al., 2003], and its salience in the percentage overlap map (maximal overlap of 86% overlap across subjects) suggests that it is very robust and warrants further detailed study [Epstein et al., 2004]. Note that this method is unable to identify functional entities that are present in all subjects but which show too large anatomical‐position variability across subjects.
These data also reveal two surprises. The first is the significantly higher response in MT+/V5 to body parts than to objects or to any other stimulus class tested (including faces). Although prior studies have demonstrated a higher MT+/V5 response to static stimuli with implied motion [Kourtzi and Kanwisher, 2000; David and Senior, 2000], and to words or pictures related to action [Kable et al., 2002], these effects seem unlikely to explain the higher MT+/V5 response to body parts where any implied or associated motion is likely to be weak (and no higher than it is for faces). The high response to body parts is also unlikely to be due to the partial overlap between the EBA and MT+/V5, because the selective MT+/V5 response to body parts continues 2 mm inside the standard border of MT+/V5 (Fig. 6d), which in many subjects comprises most of MT+/V5, and because the overall response of MT+/V5 is quite strongly selective for body parts (Fig. 4). This selectivity for body parts is not easily explainable within current views about the function of MT+/V5. One could argue that these results could be due to the use of nonstandard MT+/V5 localizer (i.e., moving objects instead of low‐contrast moving rings). However, we also used low‐contrast moving‐ring stimuli [Tootell et al., 1995] to localize MT+/V5 in two subjects and found an activation in a place entirely consistent with those found using moving objects (see Subjects and Methods), making the existence of a bias in our localization of MT+/V5 little probable. Also, Beauchamp et al. [2002] found a larger response for humans and tools than moving rings in MT+/V5. One possibility is that the effect arises not in MT/V5, but in the nearby MST/FST, which may be included in our MT+/V5 localizer [Huk et al., 2002].
The second surprise revealed in our ROI analysis is the very strong response of the FFA to body parts. This finding is revealed both in the overlap of the body part‐selective response and the face‐selective response (the purple region on the ventral view in Fig. 2), and even more strikingly in the percent signal change for each stimulus condition in each region (Fig. 4). Prior reports of high FFA responses to whole or headless bodies of humans or animals [Chao et al., 1999] could be accounted for in terms of mental imagery [O'Craven and Kanwisher, 2000] or completion of faces [Cox et al., 2004; Peelen and Downing, 2005]. However, it seems unlikely that faces are being inferred [Kourtzi and Kanwisher, 2000], imagined [O'Craven and Kanwisher, 2000], or contextually completed [Cox et al., 2004] from the presentation of individual body parts. This raises the possibility that the FFA is not solely selective for faces, but also quite selective for bodies and body parts. On the other hand, this account leaves unexplained our earlier findings that the FFA responds only weakly to human hands [Kanwisher et al., 1997] and to the backs of human heads [Tong et al., 2000]. Ongoing work in our lab using event‐related designs and higher spatial resolution suggests that under these conditions the standardly defined FFA itself shows no selectivity to body parts per se [Schwarzlose, Yovel, and Kanwisher, unpublished observations]. Although further research will be necessary to resolve this question, our current hypothesis is that the FFA itself is not body part‐selective, but can respond to the inference of a face from a headless body [Cox et al., 2004; Peelen and Downing, 2005], and that cortical regions adjacent to the FFA are selective for body parts [McCarthy et al., 1999; Peelen and Downing, 2005; Puce et al., 1999], and the response of these regions often gets averaged in with the FFA when scanning at standard resolution.
The present work also addressed the question of whether functionally defined extrastriate regions such as the FFA, EBA, and PPA represent simply the peaks of very broad swaths of selectivity spanning centimeters of cortex, or whether instead these regions have fairly sharp edges. Using a new analysis method in which we measured the selectivity in concentric rings drawn on the cortical surface around each functionally defined region in individual subjects, we showed that the selectivity in some of these areas drops off quite sharply around their typically defined borders. For example, selectivity for faces is gone at a distance of about 4 mm outside the standardly defined border of the FFA, and all selectivity for places is gone at roughly the same distance outside the standard borders of the PPA. Thus, these regions are not simply the tips of very large icebergs of selectivity that extend far beyond the borders of each area, but fairly discrete regions of cortex with distinctive functional profiles that drop away quite sharply outside their cortical borders. This emerging picture of sharp changes in the functional profile across the cortex is reinforced by ongoing work in our lab using higher resolution [Baker et al., 2004].
Note that these results do not address the function of each of these regions and the question of whether the representation of each object spans a distributed swath of occipito‐temporal cortex including many of these regions [Haxby et al., 2001]. Although evidence from one study suggested that information about nonpreferred stimuli is carried within selectively responsive regions of occipitotemporal cortex, Spiridon and Kanwisher [2002] found no evidence that the pattern of response across voxels in the FFA and PPA contain information useful for discriminating between nonpreferred stimuli [see also Tsao et al., 2003]. This issue is the topic of much current research [Carlson et al., 2003; Cox and Savoy, 2003; Hanson et al., 2004].
The well‐defined functional borders of the regions investigated here help support their status as distinct cortical regions. However, it should be noted that work in humans can so far only straightforwardly test one criterion (function) of the multiple criteria for cortical areas that have been proposed and tested in the macaque [Felleman and Van Essen, 1991]. Evidence that these cortical regions constitute true cortical areas will be greatly strengthened if future work finds that they also differ from each other (and their cortical neighbors) in cytoarchitecture and connectivity.
Acknowledgements
We thank Chris Baker, Galit Yovel, and Becca Schwarzlose for comments on the manuscript.
REFERENCES
- Aguirre GK, Zarahn E, D'Esposito M (1998): An area within human ventral cortex sensitive to “building” stimuli: evidence and implications. Neuron 21: 373–383. [DOI] [PubMed] [Google Scholar]
- Baker CI, Knouf N, Wald L, Kwong K, Benner T, Fischl B, Kanwisher N (2004): Functional selectivity of human extrastriate visual cortex at high resolution. J Vis 4: 88a. [Google Scholar]
- Beauchamp MS, Lee KE, Haxby JV, Martin A (2002): Parallel visual motion processing streams for manipulable objects and human movements. Neuron 34: 149–159. [DOI] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, Spiers HJ, O'Keefe J (2001): A temporoparietal and prefrontal network for retrieving the spatial context of lifelike events. Neuroimage 14: 439–453. [DOI] [PubMed] [Google Scholar]
- Carlson TA, Schrater P, He S (2003): Patterns of activity in the categorical representations of objects. J Cogn Neurosci 15: 704–717. [DOI] [PubMed] [Google Scholar]
- Chao LL, Martin A, Haxby JV (1999): Are face‐responsive regions selective only for faces? Neuroreport 10: 2945–2950. [DOI] [PubMed] [Google Scholar]
- Cox RW, Jesmanowicz A (1999): Real‐time 3D registration for functional MRI. Magn Reson Med 42: 1014–1018. [DOI] [PubMed] [Google Scholar]
- Cox DD, Savoy RL (2003): Functional magnetic resonance imaging (fMRI) “brain reading”: detecting and classifying distributed patterns of fMRI activity in human visual cortex. Neuroimage 19: 261–270. [DOI] [PubMed] [Google Scholar]
- Cox DD, Meyers E, Sinha P (2004): Contextually evoked object‐specific responses in human visual cortex. Science 304: 115–117. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI (1999): Cortical surface‐based analysis. I. Segmentation and surface reconstruction. Neuroimage 9: 179–194. [DOI] [PubMed] [Google Scholar]
- David AS, Senior C (2000): Implicit motion and the brain. Trends Cogn Sci 4: 293–295. [DOI] [PubMed] [Google Scholar]
- Dougherty RF, Koch VM, Brewer AA, Fischer B, Modersitzki J, Wandell BA (2003): Visual field representations and locations of visual areas V1/2/3 in human visual cortex. J Vis 3: 586–598. [DOI] [PubMed] [Google Scholar]
- Downing PE, Jiang Y, Shuman M, Kanwisher N (2001): A cortical area selective for visual processing of the human body. Science 293: 2470–2473. [DOI] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N (1998): A cortical representation of the local visual environment. Nature 392: 598–601. [DOI] [PubMed] [Google Scholar]
- Epstein R, Harris A, Stanley D, Kanwisher N (1999): The parahippocampal place area: recognition, navigation, or encoding? Neuron 23: 115–125. [DOI] [PubMed] [Google Scholar]
- Epstein RA, Higgins S, Thompson‐Schill SL (2005): Learning places from views: variation in scene processing as a function of experience and navigational ability. J Cogn Neurosci 17: 73–83. [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC (1991): Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex 1: 1–47. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM (1999a): Cortical surface‐based analysis. II. Inflation, flattening, and a surface‐based coordinate system. Neuroimage 9: 195–207. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM (1999b): High‐resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp 8: 272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM (2001): Automated‐manifold surgery constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging 20: 70–80. [DOI] [PubMed] [Google Scholar]
- Grill‐Spector K (2003): The neural basis of object perception. Curr Opin Neurobiol 13: 159–166. [DOI] [PubMed] [Google Scholar]
- Grill‐Spector K, Kourtzi Z, Kanwisher N (2001): The lateral occipital complex and its role in object recognition. Vis Res 41: 1409–1422. [DOI] [PubMed] [Google Scholar]
- Halgren E, Dale AM, Sereno MI, Tootell RB, Marinkovic K, Rosen BR (1999): Location of human face‐selective cortex with respect to retinotopic areas. Hum Brain Mapp 7: 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson SJ, Matsuka T, Haxby JV (2004): Combinatorial codes in ventral temporal lobe for object recognition: Haxby (2001) revisited: is there a “face” area? Neuroimage 23: 56–166. [DOI] [PubMed] [Google Scholar]
- Hasson U, Harel M, Levy I, Malach R (2003): Large‐scale mirror‐symmetry organization of human occipito‐temporal object areas. Neuron 37: 1027–1041. [DOI] [PubMed] [Google Scholar]
- Haxby J, Ungerleider LG, Clark VP, Schouten JL, Hoffman EA, Martin A (1999): The effect of face inversion on activity in human neural systems for face and object perception. Neuron 22: 189–199. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P (2001): Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science 293: 2425–2430. [DOI] [PubMed] [Google Scholar]
- Huk AC, Dougherty RF, Heeger DJ (2002): Retinotopy and functional subdivision of human areas MT and MST. J Neurosci 15: 7195–7205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishai A, Ungerleider LG, Martin A, Schouten JL, Haxby JV (1999): Distributed representation of objects in the human ventral visual pathway. Proc Natl Acad Sci U S A 96: 9379–9384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Lease‐Spellmeyer J, Chatterjee A (2002): Neural substrates of action event knowledge. J Neurosci 14: 795–805. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, Chun MM, McDermott J, Ledden PJ (1996): Functional imagining of human visual recognition. Brain Res Cogn Brain Res 5: 55–67. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM (1997a): The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci 17: 4302–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, Woods RP, Iacoboni M, Mazziotta JC (1997b): A locus in human extrastriate cortex for visual shape analysis. J Cogn Neurosci 9: 133–142. [DOI] [PubMed] [Google Scholar]
- Kourtzi Z, Kanwisher N (2000): Activation in human MT/MST for static images with implied motion. J Cogn Neurosci 12: 48–55. [DOI] [PubMed] [Google Scholar]
- Maguire EA (2001): The retrosplenial contribution to human navigation: a review of lesion and neuroimaging findings. Scand J Psychol 42: 225–238. [DOI] [PubMed] [Google Scholar]
- Malach R, Reppas JB, Benson RR, Kwong KK, Jiang H, Kennedy WA, Ledden PJ, Brady TJ, Rosen BR, Tootell RB (1995): Object‐related activity revealed by functional magnetic resonance imaging in human occipital cortex. Proc Natl Acad Sci U S A 92: 8135–8139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy G, Puce A, Gore JC, Allison T (1997): Face‐specific processing in the human fusiform gyrus. J Cogn Neurosci 9: 604–609. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Puce A, Berger A, Allison T (1999): Electrophysiological studies of human face perception. II. Response properties of face‐specific potentials generated in occipitotemporal cortex. Cereb Cortex 9: 431–444. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kawashima R, Sato N, Nakamura A, Sugiura M, Kato T, Hatano K, Ito K, Fukuda H, Schormann T, Zilles K (2000): Functional delineation of the human occipito‐temporal areas related to face and scene processing: a PET study. Brain 123: 1903–1912. [DOI] [PubMed] [Google Scholar]
- O'Craven KM, Kanwisher N (2000): Mental imagery of faces and places activates corresponding stimulus‐specific brain regions. J Cogn Neurosci 12: 1013–1023. [DOI] [PubMed] [Google Scholar]
- Peelen M, Downing P (2005): Selectivity for the human body in the fusiform gyrus. J Neurophysiol 93: 603–608. [DOI] [PubMed] [Google Scholar]
- Puce A, Allison T, Asgari M, Gore JC, McCarthy G (1996): Differential sensitivity of human visual cortex to faces, letter strings, and textures: a functional magnetic resonance imaging study. J Neurosci 16: 5205–5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puce A, Allison T, McCarthy G (1999): Electrophysiological studies of human face perception. III. Effects of top‐down processing on face‐specific potentials. Cereb Cortex 9: 445–458. [DOI] [PubMed] [Google Scholar]
- Spiridon M, Kanwisher N (2002): How distributed is visual category information in human occipito‐temporal cortex? An fMRI study. Neuron 35: 1157–1165. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988): Co‐planar stereotaxic atlas of the human brain. New York: Thieme Medical. [Google Scholar]
- Talairach J, Szikla G, Tournoux P (1967): Atlas d'anatomie stereotaxique du telencephale. Paris: Masson. [Google Scholar]
- Thompson PM, Toga W (1996): A surface‐based technique for warping 3‐dimensional images of the brain. IEEE Trans Med Imaging 15: 1–16. [DOI] [PubMed] [Google Scholar]
- Tong F, Nakayama K, Moscovitch M, Weinrib O, Kanwisher N (2000): Response properties of the human fusiform face area. Cogn Neuropsychol 17: 257–279. [DOI] [PubMed] [Google Scholar]
- Tootell RB, Reppas JB, Kwong KK, Malach R, Born RT, Brady TJ, Rosen BR, Belliveau JW (1995): Functional analysis of human MT and related visual cortical areas using magnetic resonance imaging. J Neurosci 15: 3215–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao DY, Freiwald WA, Knutsen TA, Mandeville JB, Tootell RB (2003): Faces and objects in macaque cerebral cortex. Nat Neurosci 6: 989–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Drury HA (1997): Structural and functional analyses of human cerebral cortex using a surface‐based atlas. J Neurosci 17: 7079–7102. [DOI] [PMC free article] [PubMed] [Google Scholar]


