Abstract
Our previous studies have shown that methyl-2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oate (CDDO-Me), a oleanane synthetic triterpenoid induces apoptosis in prostate cancer cells by inhibiting the Akt/NF-κB/mTOR signaling cascade; however, the mechanism by which CDDO-Me inhibits Akt/NF-κB/mTOR signaling has remained undetermined. Present studies show that Akt plays a critical role in the response of prostate cancer cells to CDDO-Me. Silencing of Akt sensitized PC-3 cells to CDDO-Me, whereas its overexpression rendered them resistant to CDDO-Me. Evaluation of the effect of CDDO-Me on Akt which lies upstream of NF-κB and mTOR showed that CDDO-Me directly inhibits the Akt kinase activity in cell-free kinase activity assay and in vivo without modulating the activity of PDK1, the upstream kinase that phosphorylates and activates Akt. The inhibition of Akt activity resulted in inhibition of phosphorylation/inactivation of proapoptotic procaspase-9, Bad and Foxo3a. Further, inhibition of p-Akt by CDDO-Me was not attributable to an increase in the activity of protein phosphatase 2A (PP2A) or PH domain/leucine-rich repeat protein phosphatase1 (PHLPP1) both of which dephosphorylate p-Akt. These findings show that Akt is a direct target of CDDO-Me in the Akt/NF-κB/mTOR prosurvival signaling axis.
Keywords: CDDO-Me, prostate cancer, apoptosis, Akt/NF-κB/mTOR signaling, PP2A
Introduction
Synthetic oleanane triterpenoid 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid (CDDO) and its C-28 methyl ester (CDDO-Me) and C-28 imidazole (CDDO-Im) derivatives are potent proapoptotic anticancer agents [1–4]. Although the anticancer mechanisms of CDDOs are not fully understood, cancer cell differentiation and activation of caspase-dependent and independent apoptosis contribute to the antitumor activity of CDDOs [5–7]. CDDOs inhibit MAP kinases, STATs, NF-κB, TGF-β/Smad and PPARγ signaling [8–12]. Further, CDDOs exhibit strong chemopreventive activity in mouse models of colon, breast, lung and pancreatic carcinogenesis [13–15].
We have previously shown that CDDO-Me inhibits proliferation and induces apoptosis in hormone-sensitive and hormone-refractory prostate cancer cell lines through the activation of procaspases 3, 8, 9, mitochondrial depolarization and the release of cytochrome c from mitochondria [16]. CDDO and CDDO-Me also slowed the development/progression and metastasis of prostate cancer in the TRAMP mouse model of prostate carcinogenesis [17, 18]. The induction of apoptosis in prostate cancer cell lines and tumor tissue in TRAMP mice was associated with the inhibition of prosurvival Akt, NF-κB and mammalian target of rapamycin (mTOR) signaling proteins [16, 19]. However, the mechanism by which CDDO-Me inhibits prosurvival Akt/ NF-κB/mTOR signaling has remained undetermined. Akt plays a critical role in the survival and resistant of cancer cells to apoptosis and its prosurvival function is amplified through the activation of NF-κB and mTOR, the downstream targets of Akt. The objective of this study was to investigate the mechanism by which CDDO-Me inhibits the activation of Akt in prostate cancer cells. Our data demonstrate that CDDO-Me inhibits the kinase activity of Akt without affecting the activity of PDK1, the upstream kinase that phosphorylates and activates Akt. Further, although CDDO-Me reduced the levels of phosphotases such as phosphatase and tensin homolog (PTEN), protein phosphatase 2A (PP2A) and PH domain/leucine-rich repeat protein phosphatase1 (PHLPP1); it had minimal effect on the activity of PP2A. These studies demonstrate for the first time that in Akt/NF-κB/mTOR signaling cascade CDDO-Me directly inhibits the activity of Akt without affecting the activity of PDK1 or PP2A phosphatase.
Materials and Methods
Reagents
CDDO-Me was obtained from the National Cancer Institute, Bethesda, MD through the Rapid Access to Intervention Development Program. Antibodies against p-Akt (ser473), NF-κB (p65), p-mTOR (Ser2448), p-caspase-9 (p35), p-Bad (ser136), p-Foxo3a (ser2531) and β-actin were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Akt immunoassay kit was from EMD Bioscienes (La Jolla, CA) and recombinant PDK1 was purchsed from Calbiochemicals (La Jolla, CA).
Cell culture
LNCaP and PC-3 human prostate cancer cell lines were obtained from American Type Culture Collection (ATCC, Rockville, MD). LNCaP were grown in RPMI-1640 supplemented with 10% FBS, 1% penicillin/streptomycin, and 25 mM HEPES buffer. PC-3 cells were grown in F-12K nutrient mixture (Invitrogen, Camarillo, CA) supplemented with 10% fetal calf serum, 1% penicillin/streptomycin, and 25 mM HEPES buffer.
Measurement of cell viability
1×104 cells in 100 µl of cell culture medium were seeded into each well of a 96-well plate. After incubation for 24 h, cells were treated with CDDO-Me for 72 h. Cell viability was then determined by the colorimetric MTS assay using CellTiter 96 AQueous One Solution Proliferation Assay System from Promega (Madison, WI). After incubation for 2 h at 37°C absorbance was measured at 490 nm using a microplate reader.
Immunoprecipitation
LNCaP and PC-3 cells were washed after treatment with CDDO-Me with cold PBS and lysed in NP 40 cell lysis buffer (Invitrogen, Camarillo, CA) supplemented with phosphatase inhibitor cocktail (sodium fluoride, sodium orthovanadate, sodium pyrophosphate and beta-glycerophosphate), 5 µg/mL leupeptin, 1 µg/mL aprotinin, 1 µg/mL pepstatinin, and 10 µg/mL 4-2-aminoethyl-benzenesulfinyl fluoride for 30 min on ice. Supernatants were collected after centrifugation at 14000g for 10 min and protein concentration was determined. Each sample (400 µg protein) in 200 µl of antibody binding buffer was incubated with anti-p-Akt or anti-p-PDK1 antibody (2 µg) for 1 h at room temperature followed by incubation with protein A agarose beads for 1 h. Protein A agarose beads were collected and washed first with kinase extraction buffer and then with kinase assay buffer and finally resuspended in 50 µl of kinase assay buffer. GSK-3α or Akt were used as substrates to detect the Akt kinase or PDK1 kinase activity of immune complexes by immunoblotting.
For immunoprecipitation of PP2A, cell lysates were prepared in NP 40 lysis buffer without the phosphatase inhibitors. To the cell lysate (400 µg protein) 50 µl of Dynabeads coated with anti-PP2A antibody (2 µg) was added and incubated for 1 h. Dynabeads were washed three times and resuspended in 20 µl of elution buffer for 10 min. Supernatant was collected by centrifugation and incubated with p-Akt in MOPS buffer (20 mM MOPS, 60 mM 2-Me, 100 mM NaCl, 0.1 mg BSA/ml) for 30 min at 30 °C. The dephophorylation of p-Akt by PP2A IP was analyzed by immunoblotting using anti-p-Akt (ser473) antibody.
Western blotting
Cell lysates were prepared using NP 40 cell lysis buffer. Lysates were clarified by centrifugation at 14,000 × g for 10 min at 4°C and protein concentrations were determined. Samples (50 µg) were boiled in an equal volume of sample buffer (20% glycerol, 4% SDS, 0.2% Bromophenol Blue, 125 mM Tris-HCl (pH 7.5), and 640 mM 2-mercaptoethanol) and separated on pre-casted Tris-glycine polyacrylamide gels using the XCell Surelock™ Mini-Cell, in Tris-Glycine SDS running buffer, all from Novex (Invitrogen, Carlsbad, CA). Proteins resolved on the gels were transferred to PVDF membranes. Membranes were blocked with 5% milk in 10 mM Tris-HCl (pH 8.0), 150 mM NaCl with 0.05% Tween 20 (TPBS) and probed using specific antibodies against proteins of interest or β-actin (loading control) and HRP-conjugated secondary antibody. Immune complexes were visualized with enhanced chemiluminescence. Protein bands were imaged and band densities analyzed using the NIH/Scion image analysis software. The protein band densities were normalized to the corresponding β-actin band densities and percent change in signal strength was calculated.
DNA transfection
Akt overexpression
Semi-confluent cultures of PC-3 cells were transfected with 10 µg of empty or Akt expression vector (pUSEamp) DNA containing Myc-His tagged mouse Akt1 (activated) under the control of CMV promoter (Upstate Cell Signaling, Lake Placid, NY) using LipofectAMINE Plus reagent. After incubation for 24 h, cells were analyzed for the expression of exogenous Akt1 by western blotting using anti-Myc Tag antibody.
Silencing of Akt
PC-3 cells were transfected with double stranded siRNA of Akt using SignalSilence siRNA kit from Cell Signaling Technology (Beverly, MA) according to the instructions provided by the manufacturer. Gene silencing in transfected cells was confirmed by western blotting for Akt.
Statistical analysis
Some data are presented as means ± S.D. The differences between control and treatment groups were analyzed using Dunnett multiple comparison test and differences with p<0.05 were considered statistically significant.
Results
CDDO-Me inhibits prosurvival Akt, NF-κB and mTOR signaling proteins
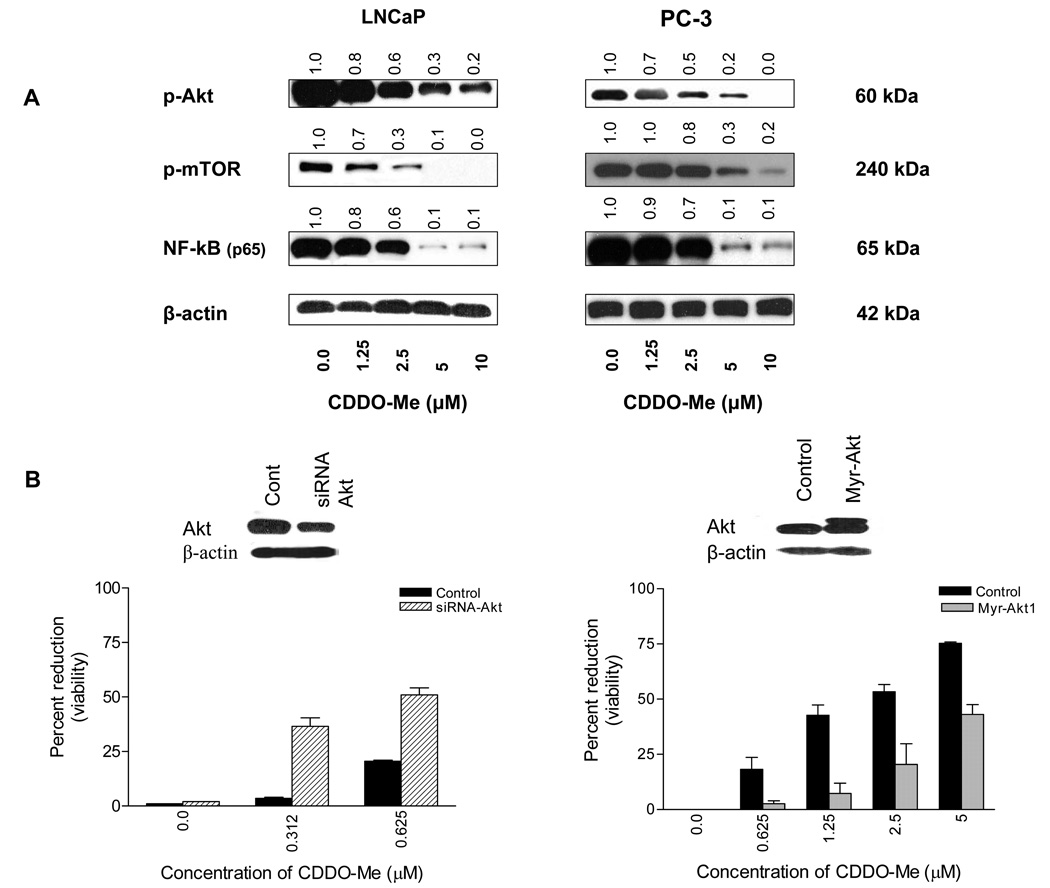
Akt/NF-κB/mTOR is a major antiapoptotic signaling pathway that confers survival advantage to cancer cells. To determine the effect CDDO-Me on Akt, NF-κB and mTOR, LNCaP and PC-3 cells were treated with CDDO-Me (1.25 to 10 µM) for 20 h and cell lysates were analyzed for p-Akt, NF-κB and p-mTOR by western blotting. As shown in Figure 1 A, CDDO-Me significantly to completely reduced the levels of p-Akt, NF-κB and p-mTOR at concentration of 2.5 µM and above in both cell lines.
Figure 1. CDDO-Me inhibits p-Akt, p-mTOR and NF-κB (p65) and Akt determines the sensitivity of prostate cancer cells to CDDO-Me.
A. LNCaP and PC-3 cells were treated with CDDO-Me at concentrations of 1.25 to 10 µM for 20 h. After treatment, cell lysates were analyzed for p-Akt, p-mTOR, and NF-κB (p65) by western blotting. B. To determine the functional relevance of Akt in response to CDDO-Me, Akt expression was either inhibited or increased by transfecting PC-3 cells with siRNA-Akt or Akt1 expression plasmid (myr-Akt1-pUSE) using LipofectAMINE Plus reagent. The change in Akt expression levels was determined by western blotting (insets) and response of transfected cells to CDDO-Me was measured in MTS assay. Bar graphs represent mean ± SD of two separate experiments. Values above blots represent the change in protein expression level compared to untreated control represented as 1.0.
Akt regulates the sensitivity of prostate cancer cells to CDDO-Me
Both NF-κB and mTOR are targets of Akt and Akt regulates the activation of NF-κB and mTOR through activation of IKKα and small GTPase Rheb, respectively. We investigated the role of Akt in mediating the growth inhibitory effect of CDDO-Me in prostate cancer cells. The effect of altering the expression levels of Akt on the response of tumor cells to CDDO-Me was measured. Thus, PC-3 cells were transfected with either siRNA-Akt or an Akt expression plasmid (myr-Akt1-pUSE) to decrease or increase the levels of Akt. The response of PC-3 cells transfected with siRNA-Akt was then tested at CDDO-Me concentrations that are inactive in MTS assay (e.g. 0.312 or 0.625 µM). As shown in Figure 1 B, control PC-3 cells were unresponsive to CDDO-Me at 0.312 µM with a small reduction in viability occurring at 0.625 µM (13%). On the other hand, PC-3 cells in which Akt has been silenced by transfection with siRNA-Akt (inset) showed increased susceptibility to CDDO-Me both at 0.312 or 0.625 µM (13.6-fold and 4.5-fold increase, respectively). In contrast, overexpression of Akt in cells transfected with Akt1 expression plasmid (Fig. 1B) markedly reduced the susceptibility of PC-3 cells to CDDO-Me at concentrations of 0.625 to 5 µM, (7-fold to 1.8-fold reduction). Transfection with a non-targeting siRNA or empty p-USE vector did not alter the response of PC-3 cells to CDDO-Me (not shown). These data indicate that Akt plays a pivotal role in the response of prostate cancer cells to CDDO-Me.
CDDO-Me inhibits Akt activity
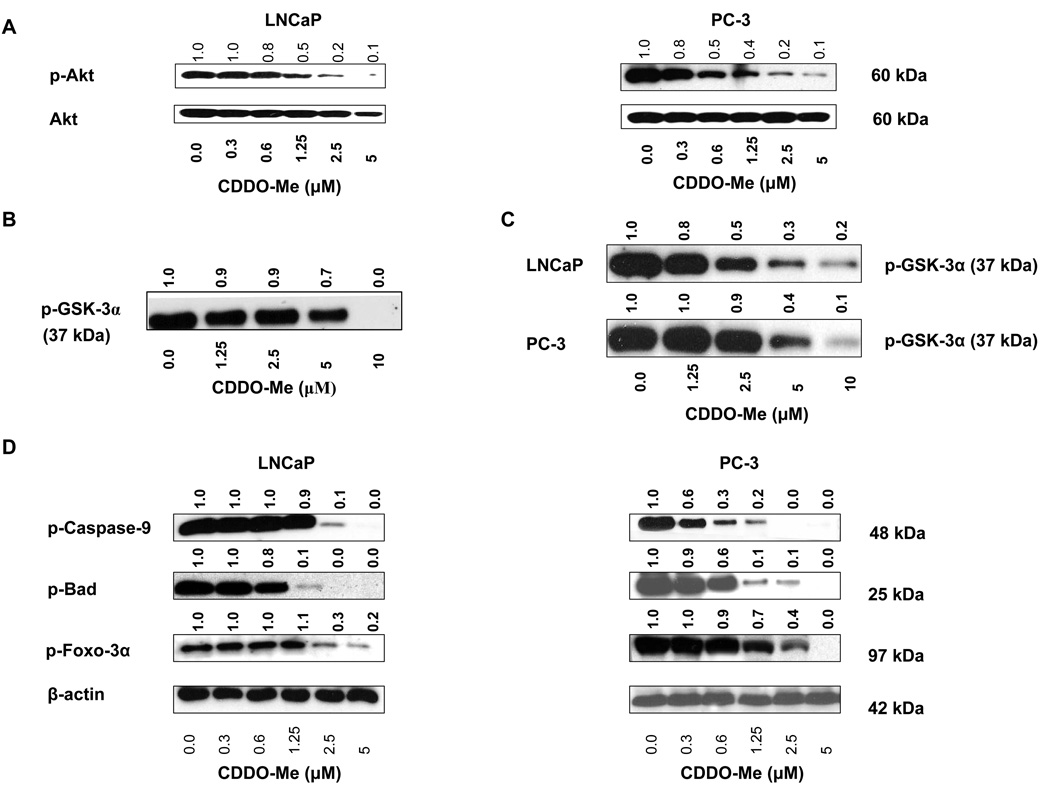
Since Akt promotes oncogenesis by inhibiting apoptosis and promoting proliferation of cancer cells directly as well as indirectly by regulating the activation of NF-κB and p-mTOR, we investigated the mechanism by CDDO-Me inhibits Akt. Treatment with CDDO-Me inhibited the phosphorylation of Akt in LNCaP and PC-3 cells in a dose-dependent manner without significantly affecting basal Akt (Fig. 2 A). GSK-3α is a well known substrate of Akt kinase; therefore, we tested the effect of CDDO-Me on phosphorylation of GSK-3α on ser21 by activated Akt. As shown in Figure 2 A, CDDO-Me measurably reduced the phosphorylation of GSK-3α at 5 µM but completely inhibited it at 10 µM, indicating that CDDO-Me can inhibit the kinase activity of Akt. Data in Figure 1 A showed that treatment with CDDO-Me inhibited the level of p-Akt (active) in cells; therefore we next determined whether it also inhibits Akt kinase activity in vivo. For this, LNCaP and PC-3 cells were treated with CDDO-Me (0–10 µM) for 20 h and p-Akt from treated and untreated cells was immunoprecipitated (IP) under identical conditions and tested for its ability to phosphorylate GSK-3α. p-Akt IP from untreated cells (both cell lines) heavily phosphorylated GSK-3α. On the other hand, treatment with 2.5 to 10 µM CDDO-Me inhibited the p-Akt kinase activity in a dose-related manner in LNCaP cells, whereas 5 to 10 µM CDDO-Me was markedly effectively in inhibiting it in PC-3 cells (Fig. 2 B). Thus data in Figure 2 A and B show that CDDO-Me can inhibit the Akt kinase activity in vitro and in vivo.
Figure 2. CDDO-Me inhibits Akt activity in vitro and in vivo.
A. Effect of CDDO-Me on phosphorylation of Akt in cells. B. Effect on Akt activity in cell-free kinase activity assay. C. Effect on p-Akt activity in vivo. Akt was immunoprecipitated from LNCaP and PC-3 cells treated with CDDO-Me (1.25–10 µM) for 20 h and then analyzed for the ability of p-Akt IP to phosphorylate GSK-3α by western blotting. D. Effect of CDDO-Me on constitutive phosphorylation of caspase-9, Bad and Foxo-3α. LNCaP and PC-3 cells were treated with CDDO-Me (0.3 to 5 µM) for 20 h and cell lysates were analyzed for p-caspase-9, p-Bad and p-Foxo-3α by western blotting. Each experiment was repeated at least two times.
Akt protects tumor cells from apoptosis through phosphorylation and inactivation of proapoptotic caspase-9, Bad and Foxo-3α transcription factor. Whether inhibition of activated Akt (p-Akt) by CDDO-Me also reduces the levels of p-caspase-9, p-Bad and p-Foxo-3α was determined. Indeed, treatment with CDDO-Me, especially at 2.5 and 5 µM, significantly to completely inhibited phosphorylation of caspase-9, Bad and Foxo-3α. (Fig. 2 C).
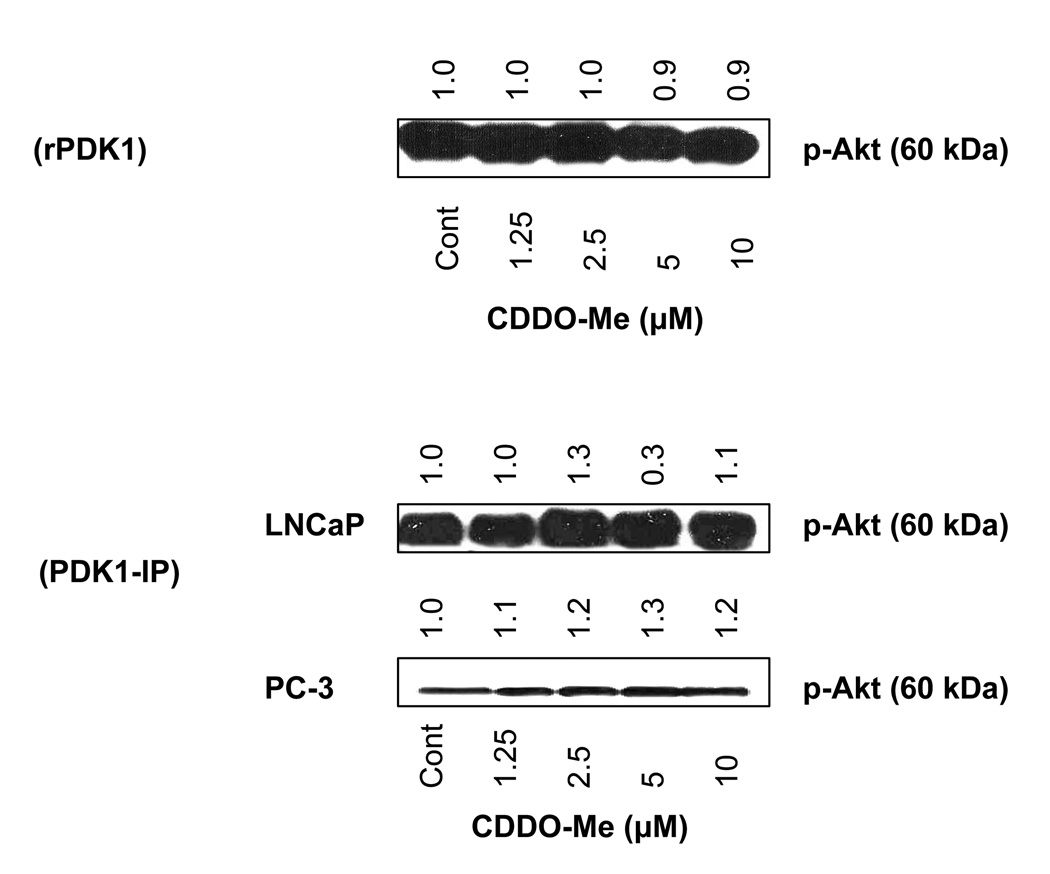
CDDO-Me does not inhibit PDK1 activity
We considered the possibility that inhibition of phosphorylation of Akt by CDDO-Me might also be due to the inhibition of PDK1 activity, an immediate upstream kinase that phosphorylates Akt on Thr308. First we tested the effect of CDDO-Me (1.25 to 10 µM) on phosphorylation of Akt by recombinant PDK1 in vitro. Figure 3 A shows that CDDO-Me does not inhibit the phosphorylation of Akt by PDK1 at any concentration tested. To test whether CDDO-Me affects PDK1 activity in vivo, LNCaP and PC-3 cells were treated with CDDO-Me for 20 h and p-PDK1 was immunoprecipitated from control (untreated) and treated cells under identical conditions and the ability p-PDK1 IP to phosphorylate Akt was determined. Data in Figure 3 B show that unlike the inhibition of Akt kinase activity, CDDO-Me does not inhibit PDK1 kinase activity in these cell lines. This result demonstrates that inhibition of Akt phosphorylation by CDDO-Me is not due to the inhibition of PDK1 activity.
Figure 3. CDDO-Me does not inhibit PDK1 activity.
A. Effect on PDK1 activity in cell-free kinase activity assay. B. Effect on PDK1 activity in vivo. PDK1 was immunoprecipitated from lysates of LNCaP and PC-3 cells treated with CDDO-Me (1.25–10 µM) for 20 h and then analyzed for the ability of PDK1 IP to phosphorylate Akt by western blotting. Each experiment was repeated two times.
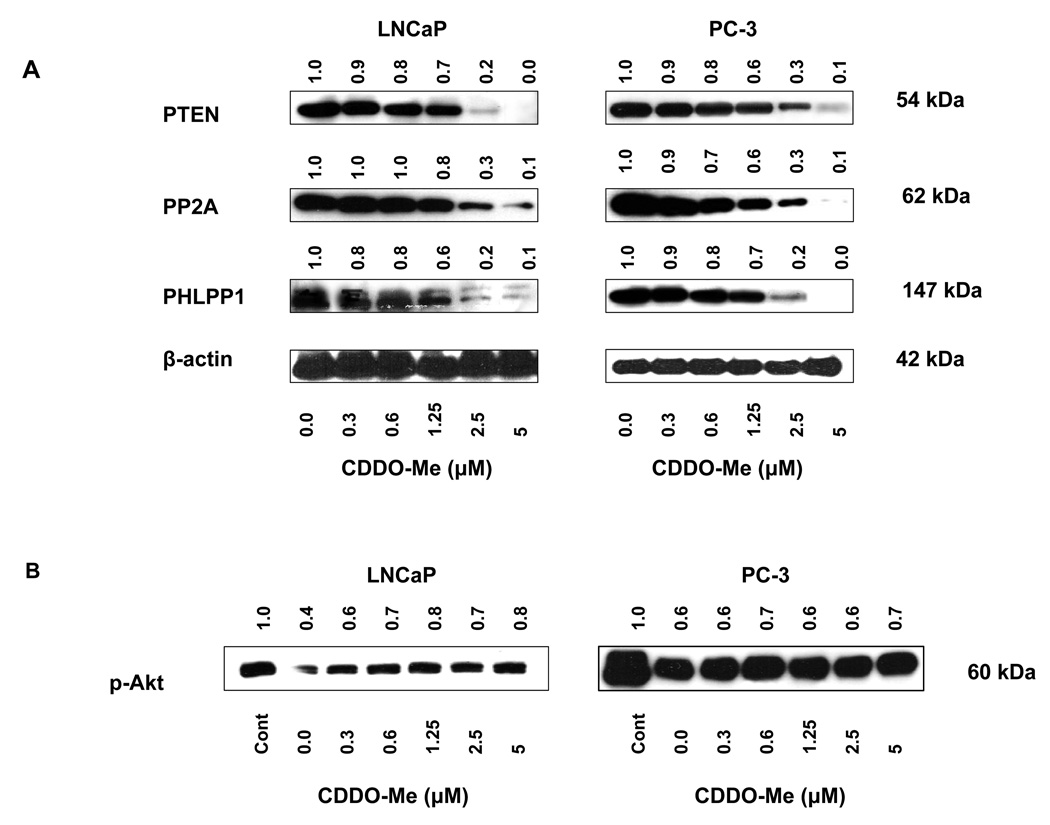
CDDO-Me inhibits phosphatases
The possibility that CDDO-Me could reduce levels of p-Akt by enhansing the activity of phosphatases was tested next. We first analyzed the effect of CDDO-Me on levels of lipid phosphatase PTEN and phosphatases PP2A and PHLPP1 that dephosphorylate p-Akt. LNCaP and PC-3 cells were treated with CDDO-Me (0.0312 to 5 µM) for 20 h and levels of PTEN, PP2A and PHLPP1 were analyzed by western blotting. Treatment with CDDO-Me at 1.25 to 5 µM partially to completely inhibited these phosphatases in both cell lines (Fig. 4 A). For the effect of CDDO-Me on PP2A phosphatase activity, PP2A was immunoprecipitated from control (untreated) cells and cells treated with CDDO-Me and the ability of PP2A IP to dephosphorylate p-Akt was determined. As shown in Figure 4 B, PP2A IP from untreated LNCaP cells significantly reduced the level of p-Akt (~60%). Compared to untreated LNCaP cells, phosphatase activity of PP2A IP from LNCaP cells treated with CDDO-Me was partially reduced. This however was not the result obtained in PC-3 cells. PP2A IP from control PC-3 cells also reduced the level of p-Akt (~60%); however, phosphatase activity of PP2A IP from PC-3 cells treated with CDDO-Me (0.3 to 5 µM) was not different from PP2A IP from untreated PC-3 cells, indicating that CDDO-Me does not affect PP2A phosphatase activity in PC-3 cells.
Figure 4. Effect of CDDO-Me on cellular phosphatases and protein phosphatase PP2A activity.
A. LNCaP and PC-3 cells were treated with CDDO-Me (0.3–5 µM) for 20 h and cell lysates were analyzed for PTEN, PP2A and PHLPP1 by immunoblotting. B. Effect on PP2A activity in vivo. PP2A was immunoprecipitated from LNCaP and PC-3 cells after treatment with CDDO-Me (0.3–5 µM) for 20 h. PP2A IP was incubated with p-Akt and dephosphorylation of p-Akt was analyzed by western blotting. Each experiment was repeated two times.
Discussion
Our previous studies have demonstrated that among the three synthetic CDDOs (eg., CDDO, CDDO-Im and CDDO-Me), CDDO-Me was most active against prostate cancer cells regardless of their responsiveness to androgen or androgen receptor (AR) expression [16]. Treatment with CDDO-Me inhibited the constitutively active prosurvival Akt, mTOR and NF-κB in prostate cancer cells and suppression of these signaling proteins was accompanied by inhibition of cell proliferation and induction of apoptosis [16, 19]. Akt/NF-κB/mTOR signaling axis is a major prosurvival/antiapoptotic pathway which is frequently hyperactivated in most cancers [20–23]. Akt promotes cell proliferation and survival by inactivating proapoptotic substrates such as procaspase-9, Bad and Foxo-3α and activating NF-κB and mTOR, which control proliferation, oncogenesis and ribogenesis. Data showed that Akt controls the response of prostate cancer cells to CDDO-Me, since inhibition of Akt increased and its overexpression decreased the sensitivity of cancer cells to CDDO-Me. CDDO-Me also inhibited p-Akt, NF-κB (p65) and p-mTOR in prostate cancer cells.
To obtain an insight into the mechanism by which CDDO-Me inhibits Akt we investigated the effect CDDO-Me on the activity of Akt in vitro in cell-free kinase activity assay and in vivo as well as on the activity PDK1, the immediate upstream kinase that phosphorylates Akt and on phosphatases that dephosphorylate and inactivate Akt. CDDO-Me partially to completely inhibited the phosphorylation of GSK-3α by activated Akt at concentrations of 5 to 10 µM in cell-free kinase assay. Further, p-Akt immunoprecipitated from tumor cells treated with CDDO-Me (2.5 to 10 µM) also showed significant to almost complete inhibition of Akt activity. These results demonstrate that CDDO-Me not only inhibits the levels of p-Akt (activated) but also its kinase activity. Others have argued that formation of complexes between CDDOs and reducing agents (DTT, 2-ME etc.) present in kinase assay buffers makes it difficult to measure the effect of synthetic triterpenoids on the activity of kinases in cell-free kinase assays [1, 9]. However, our data suggest that perhaps only at nanomolar concentrations these reducing agents are able to completely chelate CDDO-Me. It is obvious that at micromolar concentrations, such as those used in our experiments, not all of CDDO-Me is chelated, leaving enough free CDDO-Me to block Akt activity. Our studies however do not address the actual mechanism by which CDDO-Me inhibits the catalytic activity of Akt. The inhibition of Akt activity would be expected to impact phosphorylation of its downtstream substrates including those which participate in apoptosis. Indeed, the phosphorylation of proapoptotic caspase-9, Bad and Foxo-3α by Akt which renders these molecules inactive was inhibited in cells treated with CDDO-Me in a concentration dependent manner.
We also considered the possibility that inhibition of Akt by CDDO-Me could also result from inactivation of activating kinases such as PI3K or PDK1 upstream of Akt. However, our results showed that CDDO-Me does not inhibit PDK1 activity either in cell-free kinase activity assay or in vivo in cells treated with CDDO-Me up to a concentration of 10 µM. Similarly, there was no decrease in the activity of PDK1 immunoprecipitated from tumor cells treated with CDDO-Me (1.25–10 µM). Once again it can be argued that lack of effect on PDK1 activity in cell-free kinase assay is because of chelation of CDDO-Me, but such an interpretation is contradicted by the fact that there was no effect on the activity of PDK1 in vivo either. The lack of effect on the activity of PDK1 would also imply that CDDO-Me does not affect the activity of PI3K either, which regulates activation of PDK1 through the generation of second messenger PIP3. We also investigated the possibility that CDDO-Me might reduce p-Akt by enhancing the activity of phosphatases. Treatment with CDDO-Me reduced PTEN which negatively regulates PI3K/Akt signaling through dephorylation of PIP3. In addition, levels PP2A and PHLPP1 that dephosphorylate p-Akt were also reduced by CDDO-Me. Examination of the phosphatase activity of PP2A immunoprecipitated from cells treated with CDDO-Me showed that in contrast to marked reduction in levels of PP2A at higher concentrations of CDDO-Me it only minimally inhibited PP2A activity in LNCaP cells without affecting it in PC-3 cells. It appears from these data that cellular context and the phosphatase being examined determine the effect of CDDOs on phosphatase activity. Contrary to our finding that CDDO-Me does not significantly modulate the phosphatase activity of PP2A, CDDO-Im was shown to inhibit PTEN activity in retinal epithelial cells and of SHP-1, which dephosphorylates STATs in myeloma cells [11, 24].
In summary, our data show that CDDO-Me inhibits the activity of Akt without affecting the activity of PDK1 with minimal effect on PP2A phosphatase activity. Although these studies provide an insight into the level of PI3K/Akt signaling cascade at which CDDO-Me is inhibitory, further studies are warranted to determine whether CDDO-Me blocks the association of Akt with the substrate or directly interferes with its catalytic activity. Further, whether inactivation of NF-κB and mTOR takes place subsequent to the inactivation of Akt or whether they are direct targets of CDDO-Me remains to be determined.
Highlights.
-
➢
CDDO-Me inhibits prosurvival Akt, NF-kB and mTOR signaling proteins
-
➢
CDDO-Me inhibits Akt kinase activity but not its upstream kinase PDK1
-
➢
CDDO-Me does not inhibit PP2A phosphatase activity
Acknowledgements
This work was supported by NIH grant 1R01 CA130948-01.
Abbreviations
- CDDO-Me
methyl-2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oate
- Akt (protein kinase B)
a serine/threonine protein kinase
- PDK1
phosphoinositide-dependent kinase-1
- MAP kinase
mitogen activated protein kinase
- NF-κB
nuclear factor kappa B
- mTOR
mammalian target of rapamycin
- STAT
signal transducer and activator of transcription
- Foxo3a, forkhead box O3
a transcription factor encoded by FOXO3 gene
- PTEN
phosphatase and tensin homolog
- PP2A
protein phosphatase 2A
- PHLPP1
PH domain/leucine-rich repeat protein phosphatase1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liby KT, Yore MM, Sporn MB. Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat Rev Cancer. 2007;7:357–369. doi: 10.1038/nrc2129. [DOI] [PubMed] [Google Scholar]
- 2.Gao X, Deeb D, Jiang H, Liu Y, Dulchavsky SA, Gautam SC. Synthetic triterpenoids inhibit growth and induce apoptosis in human glioblastoma and neuroblastoma cells through inhibition of prosurvival Akt, NF-kappaB and Notch1 signaling. J Neurooncol. 2007;84:147–157. doi: 10.1007/s11060-007-9364-9. [DOI] [PubMed] [Google Scholar]
- 3.Ito Y, Pandey P, Sporn MB, Datta R, Kharbanda S, Kufe D. The novel triterpenoid CDDO induces apoptosis and differentiation of human osteosarcoma cells by a caspase-8 dependent mechanism. Mol Pharmacol. 2001;59:1094–1099. doi: 10.1124/mol.59.5.1094. [DOI] [PubMed] [Google Scholar]
- 4.Konopleva M, Tsao T, Ruvolo P, Stiouf I, Estrov Z, Leysath CE, Zhao S, Harris D, Chang S, Jackson CE, Munsell M, Suh N, Gribble G, Honda T, May WS, Sporn MB, Andreeff M. Novel triterpenoid CDDO-Me is a potent inducer of apoptosis and differentiation in acute myelogenous leukemia. Blood. 2002;99:326–335. doi: 10.1182/blood.v99.1.326. [DOI] [PubMed] [Google Scholar]
- 5.Ikeda T, Sporn M, Honda T, Gribble GW, Kufe D. The novel triterpenoid CDDO and its derivatives induce apoptosis by disruption of intracellular redox balance. Cancer Res. 2003;63:5551–5558. [PubMed] [Google Scholar]
- 6.Konopleva M, Tsao T, Estrov Z, Lee RM, Wang RY, Jackson CE, McQueen T, Monaco G, Munsell M, Belmont J, Kantarjian H, Sporn MB, Andreeff M. The synthetic triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid induces caspase-dependent and -independent apoptosis in acute myelogenous leukemia. Cancer Res. 2004;64:7927–7935. doi: 10.1158/0008-5472.CAN-03-2402. [DOI] [PubMed] [Google Scholar]
- 7.Ito Y, Pandey P, Sporn MB, Datta R, Kharbanda S, Kufe D. The novel triterpenoid CDDO induces apoptosis and differentiation of human osteosarcoma cells by a caspase-8 dependent mechanism. Mol Pharmacol. 2001;59:1094–1099. doi: 10.1124/mol.59.5.1094. [DOI] [PubMed] [Google Scholar]
- 8.Konopleva M, Contractor R, Kurinna SM, Chen W, Andreeff M, Ruvolo PP. The novel triterpenoid CDDO-Me suppresses MAPK pathways and promotes p38 activation in acute myeloid leukemia cells. Leukemia. 2005;19:1350–1354. doi: 10.1038/sj.leu.2403828. [DOI] [PubMed] [Google Scholar]
- 9.Liby K, Voong N, Williams CR, Risingsong R, Royce DB, Honda T, Gribble GW, Sporn MB, Letterio JJ. The synthetic triterpenoid CDDO-Imidazolide suppresses STAT phosphorylation and induces apoptosis in myeloma and lung cancer cells. Clin Cancer Res. 2006;12:4288–4293. doi: 10.1158/1078-0432.CCR-06-0215. [DOI] [PubMed] [Google Scholar]
- 10.Ahmad R, Raina D, Meyer C, Kharbanda S, Kufe D. Triterpenoid CDDO-Me blocks the NF-kappaB pathway by direct inhibition of IKKbeta on Cys-179. J Biol Chem. 2006;281:35764–35769. doi: 10.1074/jbc.M607160200. [DOI] [PubMed] [Google Scholar]
- 11.Suh N, Roberts AB, Birkey Reffey S, Miyazono K, Itoh S, ten Dijke P, Heiss EH, Place AE, Risingsong R, Williams CR, Honda T, Gribble GW, Sporn MB. Synthetic triterpenoids enhance transforming growth factor beta/Smad signaling. Cancer Res. 2003;63:1371–1376. [PubMed] [Google Scholar]
- 12.Chintharlapalli S, Papineni S, Konopleva M, Andreef M, Samudio I, Safe S. 2-Cyano-3,12-dioxoolean-1,9-dien-28-oic acid and related compounds inhibit growth of colon cancer cells through peroxisome proliferator-activated receptor gamma-dependent and -independent pathways. Mol Pharmacol. 2005;68:119–128. doi: 10.1124/mol.105.011437. [DOI] [PubMed] [Google Scholar]
- 13.Ling X, Konopleva M, Zeng Z, Ruvolo V, Stephens LC, Schober W, McQueen T, Dietrich M, Madden TL, Andreeff M. The novel triterpenoid C-28 methyl ester of 2-cyano-3, 12-dioxoolen-1, 9-dien-28-oic acid inhibits metastatic murine breast tumor growth through inactivation of STAT3 signaling. Cancer Res. 2007;67:4210–4218. doi: 10.1158/0008-5472.CAN-06-3629. [DOI] [PubMed] [Google Scholar]
- 14.Liby K, Royce DB, Williams CR, Risingsong R, Yore MM, Honda T, Gribble GW, Dmitrovsky E, Sporn TA, Sporn MB. The synthetic triterpenoids CDDO-methyl ester and CDDO-ethyl amide prevent lung cancer induced by vinyl carbamate in A/J mice. Cancer Res. 2007;67:2414–2419. doi: 10.1158/0008-5472.CAN-06-4534. [DOI] [PubMed] [Google Scholar]
- 15.Liby KT, Royce DB, Risingsong R, Williams CR, Maitra A, Hruban RH, Sporn MB. Synthetic triterpenoids prolong survival in a transgenic mouse model of pancreatic cancer. Cancer Prev Res (Phila) 2010;3:1427–1434. doi: 10.1158/1940-6207.CAPR-10-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deeb D, Gao X, Dulchavsky SA, Gautam SC. CDDO-me induces apoptosis and inhibits Akt, mTOR and NF-kappaB signaling proteins in prostate cancer cells. Anticancer Res. 2007;27:3035–3044. [PubMed] [Google Scholar]
- 17.Deeb D, Gao X, Liu Y, Jiang D, Divine GW, Arbab AS, Dulchavsky SA, Gautam SC. Synthetic triterpenoid CDDO prevents the progression and metastasis of prostate cancer in TRAMP mice by inhibiting survival signaling. Carcinogenesis. 2011;32:757–764. doi: 10.1093/carcin/bgr030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao X, Deeb D, Liu Y, Arbab AS, Divine GW, Dulchavsky SA, Gautam SC. Prevention of Prostate Cancer with Oleanane Synthetic Triterpenoid CDDO-Me in the TRAMP Mouse Model of Prostate Cancer. Cancers (Basel) 2011;3:3353–3369. doi: 10.3390/cancers3033353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deeb D, Gao X, Jiang H, Dulchavsky SA, Gautam SC. Oleanane triterpenoid CDDO-Me inhibits growth and induces apoptosis in prostate cancer cells by independently targeting pro-survival Akt and mTOR. Prostate. 2009;69:851–860. doi: 10.1002/pros.20937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vivanco, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 21.Mayo MW, Baldwin AS. The transcription factor NF-kappaB: control of oncogenesis and cancer therapy resistance. Biochim Biophys Acta. 2000;1470:M55–M62. doi: 10.1016/s0304-419x(00)00002-0. [DOI] [PubMed] [Google Scholar]
- 22.Altomare DA, Testa JR. Perturbations of the AKT signaling pathway in human cancer. Oncogene. 2005;24:7455–7464. doi: 10.1038/sj.onc.1209085. [DOI] [PubMed] [Google Scholar]
- 23.Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4:335–348. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- 24.Pitha-Rowe I, Liby K, Royce D, Sporn M. Synthetic triterpenoids attenuate cytotoxic retinal injury: cross-talk between Nrf2 and PI3K/AKT signaling through inhibition of the lipid phosphatase PTEN. Invest Ophthalmol Vis Sci. 2009;50:5339–5347. doi: 10.1167/iovs.09-3648. [DOI] [PubMed] [Google Scholar]