Abstract
Post natal bone repair elicits a regenerative mechanism that restores the injured tissue to its pre-injury cellular composition and structure and is believed to recapitulate the embryological processes of bone formation. Prior studies showed that Nanog, a central epigenetic regulator associated with the maintenance of embryonic stem cells (ESC) was transiently expressed during fracture healing, Bais et al., 2009 [1]. In this study, we show that murine bone marrow stromal cells (MSCs) before they are induced to undergo osteogenic differentiation express ~50× the background levels of Nanog seen in murine embryonic fibroblasts (MEFs) and the W20-17 murine marrow stromal cell line stably expresses Nanog at ~80× the MEF levels. Nanog expression in this cell line was inhibited by BMP7 treatment and Nanog lentivrial shRNA knockdown induced the expression of the terminal osteogenic gene osteocalcin. Lentivrial shRNA knockdown or lentiviral overexpression of Nanog in bone MSCs had inverse effects on proliferation, with knockdown decreasing and overexpression increasing MSC cell proliferation. Surgical marrow ablation of mouse tibia by medullary reaming led to a ~3 fold increase in Nanog that preceded osteogenic differentiation during intramembranous bone formation. Lentiviral shRNA knockdown of Nanog after surgical ablation led to an initial overexpression of osteogenic gene expression with no initial effect on bone formation but during subsequent remodeling of the newly formed bone a ~50% decrease was seen in the expression of terminal osteogenic gene expression and ã50% loss in trabecular bone mass. This loss of bone mass was accompanied by an increased ~2–5 fold adipogenic gene expression and observed increase of fat cells in the marrow space. In summary these data show that Nanog is expressed during surgically induced marrow bone formation and is functionally involved in post natal marrow stromal cell maintenance and differentiation.
Keywords: Nanog, Marrow Stromal Cells, Bone Regeneration
INTRODUCTION
The homeotic gene Nanog is a key mediator of embryonic stem cell (ESC) maintenance [2,3]. BMP in combination with LIF has been shown to maintain ESCs in an undifferentiated state, which is achieved by BMP's up regulation of ID that blocks neural tissue differentiation, while LIF through the up regulation of Nanog blocks Mesodermal/Endodermal differentiation [4]. In subsequent studies Nanog's functions were further elucidated and shown to block BMP induced mesodermal differentiation of ESC, by interacting with Smad1 thereby interfering with its interaction with other activating Smads [5]. Thus Nanog acts like a rheostat to modulate BMP activities and the initial fate decisions of the ESC. While the global deletion of Nanog causes early embryonic lethality, conditional deletion after the blastocyst stage only affects spermatogenesis [4]. While Nanog has not been shown to have any functions in the post natal animals, a handful of studies have demonstrated that Nanog is expressed in various populations of mulitpotential post natal mesenchymal stem cells [6–10].
In previous studies [1], we identified that Nanog was upregulated during fracture healing. This observation led us to hypothesize that Nanog might play a functional role in the maintenance of skeletal stems populations during periods of post natal bone regeneration. Based on this circumstantial evidence, we examined the functional role of Nanog in post natal skeletal stem cell function.
MATERIALS AND METHODS
Materials
W20-17 murine marrow stromal cells were from ATCC (CRL-2623™) and they were cultured as previously described [11]. Expression plasmids for Nanog, and Nanog promoter were from Addgene, Cambridge, MA, USA (plasmid 18920 and 16337). All lentivirus based shRNA DNA clones and packaging cell lines that were used for making the viral transduction particles were from Sigma-Aldrich Inc., St. Louis, MO, USA.
Surgical Models
Research was conducted in conformity with Federal and USDA guidelines, under an IACUC approved protocol. All studies were performed on male 8–10 weeks old C57 BL/6J (B6) mice. Surgical marrow ablation was carried out by reaming of the marrow space as described in Gerstenfeld et al., 2001 [12].
MSC Culture and Osteoinduction
Marrow stromal cell cultures were prepared from C57 BL/6J (B6) male mice of 8–10 weeks of age (Jackson Laboratories, Bar Harbor, ME) and osteoinduction was carried out as previously described [13]
Micro Computer Assisted Tomography (μCT)
Specimens were scanned at 16 μm resolution using a Scanco μCT 40 system (Scanco Medical, Basserdorf, Switzerland) using a region of interest (ROI) as defined in studies by Bais et al., 2009 [13]. Total Bone volume and average mineral density were each compared across groups using a Kruskal-Wallis test (analysis of variance by ranks) [14].
Demineralized Histology
For histological assessment, the tibiae were fixed, decalcified and sectioned as previously described [13]. Serial sections were generated and slides were taken every 100 μM. Slides were stained with either hematoxylin and eosin or Goldener Trichrome.
Lentivirus Preparation and Particle Transduction
All work with lentiviruses was performed under BL2 conditions. All procedures for viral preparation and transduction of either primary MSC or after surgical marrow ablation transduction were as previously described [13].
Murine Embryonic Fibroblast Cultures
MEFs were obtained using standard culture methods [15] for their isolation and expansion, from E13.5 mouse embryos (Charles River; strain #023; CF-1). All results shown are from passage 5 MEFs.
Messenger RNA Analysis
Marrow ablation specimens were prepared by removing the distal cartilage condylar surfaces of the operated tibia and cutting approximately at the center of the mid-diaphyseal region. All bone tissues were collected into liquid nitrogen and stored at −80°C until used for RNA extraction. RNA was prepared from both cell cultures and from bone tissues and qRT-PCR was carried out as previous described [13].
RESULTS
Nanog has a functional role in the maintenance of the undifferentiated state of marrow stromal cells
Nanog levels were assessed in the total population of cells that were initially flushed from the marrow space, at six days after plating when all the non adherent cells were removed but before osteoinductive media had been added to the cultures, and at the end of culture period when the cells have undergone full osteogenic differentiation. Nanog expression was increased ~10 fold during the period when the cells first attached and expanded in culture and this peak level of expression was about 20 fold that seen after the cultures have undergone full osteogenic differentiation. A comparison of Nanog mRNA expression found in primary bone marrow stromal cells was made to the bone marrow stromal cell line (W20-17) that had previously been used to assess BMP2 function in promoting osteogenic differentiation. The W20-17 marrow cell line showed ~1.5 fold higher levels of Nanog expression than seen than in MSCs and ~85 fold higher levels compared to the background levels seen in null expressing MEFs [15]. Using a 2.5 Kb Nanog promoter/luceriferase construct, we verified that the increased steady state mRNA levels of Nanog were reflective of increased transcriptional activity (Figure 1B). Since one of the known mechanisms of action of Nanog in controlling the mesodermal/endodermal differentiation of ESCs is through blocking BMP interaction with its target genes, we next tested whether increasing concentrations of BMP would lead to inhibition of the Nanog promoter activity in the W20-17 cells (Figure 1C). True to its known mechanisms of regulation in ESCs, addition of increasing levels of BMP down regulated Nanog promoter activity by more than 60% in the W20-17 cells. In the final experiment of this series, we tested whether knock down of Nanog expression with lentivirus shNanog particles in W20-17 would mimic the actions of BMP by relieving its repression of osteocalcin, a gene known to be induced by BMP during osteogenic differentiation of MSC. This experiment lead to ~6 fold increase in the level of expression of this terminal osteogenic marker compared to non target shRNA transduced cells (Figure 1D).
Figure 1. Characterization of Nanog Expression in Bone Marrow Stromal Cells.
(A)The relative mRNA expression in adherent mouse marrow stromal cells isolated one day after plating in tissue culture, 6 days after plating but before switching to osteoinductive media and at 21 days in culture (15 days in osteoinductive media) when they are fully differentiated. B) Comparison of undifferentiated marrow stromal cells (MSCs) to W20-17 marrow stromal cell line. Levels are expressed relative to murine embryonic fibroblasts that have been used as a null expressing reference to which ESC are compared. C) Evaluation of relative 2.5 Kb Nanog promoter activities in MEFs and W20-17 cell line. D) BMP-7 down regulation of Nanog promoter activity with exogenous addition of BMP7 protein at 10, 100 and 300 ng concentration compared to control in W20-17 cell line E) Effect of lentiviral Nanog shRNA particle transduction on endogenous Nanog mRNA within W20-17 cells. F) Functional effect of Nanog shRNA mediated knockdown on Osteocalcin mRNA expression. NT= transduced with non target virus. Error bars represent standard deviation from replicate measurements from three experiments.
Assessment of Nanog Function on the Proliferation in Primary Marrow Stromal Cells
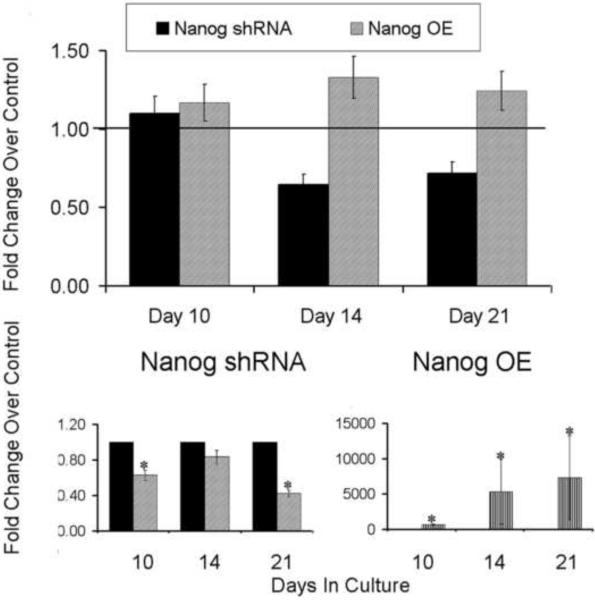
While Nanog is primarily involved in the maintenance of pluripotency in ESCs [16], it also affects the overall proliferative capacity when it is overexpressed in other cell type [3,17]. In order to assess if it has a similar effect in MSCs, we assessed the effect of overexpression and knockdown of Nanog in primary cultures of MSCs (Figure 2). Transduction of lentivirus Nanog shRNA particles inhibited the proliferation of MSCs by 35% and 28.2% on day 14 and 21, respectively. In contrast to the knockdown studies, Nanog overexpression increased the proliferation by 16%, 33%, and 24% on days 10, 14 and 21, respectively. Thus, the loss of Nanog reduced the overall cell number that grew out from the subpopulation of adherent MSCs, whereas ectopic overexpression increased cell number of these primary cells.
Figure 2. Effect of Nanog Knockdown and Overexpression on Bone Marrow Stromal Cell Proliferation.
(Top panel)Effect of nanog shRNA and Nanog OE lentiviral particles transduction on proliferation. The temporal effect of nanog overexpression on cell proliferation was assessed by MTT assay. Horizontal line indicates control level. Mean values are measurements made from three separate preparations of cells. (Bottom Panel) Effect of lentivirus shRNA or Nanog OE particle transduction on expression of Nanog mRNA expression on days 10, 14 and 21. Error bars represent standard deviation from replicate measurements from three experiments. P-value reflects the comparison of different treatment groups calculated by t-test and denoted as * p<0.05. NT= transduced with non target virus. shRNA= lentivirus shRNA to Nanog. OE = overexpression lentivirus for Nanog. NT= non target lentiviral vector. Separate NT viral constructs were used for the shRNA and OE experiments so that the backbone vector that was used for each control was different between experimental groups
In Vivo Assessment of Nanog Function in Marrow Stromal Stem Cells During Induced Bone Formation after Surgical Bone Marrow Ablation
While our previous studies had shown that fracture would induce the expression of Nanog, we wanted to assess if a different model of injury induced bone regeneration (surgical marrow ablation by reaming) would show a similar inductive effect. Unlike fracture healing this model only undergoes a process of intramembraneous bone formation devoid of a cartilage phase. The cells that contribute to bone healing in this model would also presumably be similar to the population of MSCs that are grown out in the primary cultures of adherent marrow cells. The histological events of the regenerative process are seen in Figure 3A. After five days the reamed space fills with an extensive amount of fibrous tissue and by seven days these fibrous cells condense and almost completely fill the medullary space with newly formed trabecular bone. Over the next two weeks this tissue is extensively remodeled and reestablishes its hematopoietic and myolid tissues. Molecular analysis (Figure 3B) showed that Nanog expression was strongly induced at five days post surgery preceding Runx2 and Osteocalcin expression at seven days. Assay of the RankL and TRAP5b expression showed that the expression of RANKL comes up slightly earlier than OC and Runx2 consistent with its known expression during very early osteogenic differentiation. This was followed by a broad period of expression that both preceded and then overlapped with the induction of TRAP5b which peaks at day 14 consistent with the period of coupled remodeling. Thus, in this model Nanog shows an early induction at the time of the first wave of proliferative expansion and regeneration of the stem cell population that is used during the initial round of induced bone formation. While Nanog levels diminishes over the time they continue to be slightly elevated from their baseline level as bone remodeling continues.
Figure 3. Time Course of Nanog Expression during Bone Regeneration after Surgical Marrow Ablation.
(A)Histological assessment of endosteal bone formation in response to surgical marrow ablation on days 5, 7, 14 and 21. All images are orientated with the proximal end of the bone at the top (B) Steady state mRNA expression of Nanog, Runx2, Osteocalcin, RankL and TRAP over a 21 day time course of primary bone formation and coupled remodeling following surgical bone marrow ablation. Error bars represent standard deviation from replicates measurements from three different mice.
Loss of Nanog Expression Leads to Diminished Bone Formation during Coupled Remodeling and Increased Adipogenesis
The role of Nanog in osteogenic differentiation in vivo was assessed by introducing lentiviral particles expressing Nanog shRNAs into the marrow space at the time of surgery (Figure 4A–B). Micro-CT analysis of newly formed bone tissue showed robust osteogenesis in non targeted virus group, whereas in the group injected with shNanog lentivirus particles, osteogenesis was impaired. The quantification of bone formation is shown in Fig 4A whereas 3D reconstruction is shown in Fig 4B. On day 7, there was a ~10% decrease in trabecular bone that was formed relative to non-targeted shRNA group; whereas by day 21, there is a ~60 % (p=0.003) reduction in trabecular bone formed in the Nanog lentiviral shRNA treated group. Histological analysis of the tissue at seven and 21 days post surgery confirmed the microCT results (Figure 4C) showing dense sclerotic bone in the shRNA treated samples compared to the controls at day seven but a marked decrease in trabecular bone being observed at day 21. Interestingly the day 21 histological analysis of the shRNA treated tissues showed extensive adipocyte formation replacing the bone compared to the control NT samples (Figure 4C) suggesting that loss of Nanog in MSCs lead to increase in adipogenic differentiation.
Figure 4. Functional Effect of Nanog shRNA Knockdown on Bone Regeneration Following Surgical Bone Marrow Ablation.
(A)Graphical assessment of percent bone tissue volume (BV/TV) as determined from quantitative μCT analysis MicroCT. B) Representative μCT 3D renderings of bone formation from control mice and mice injected with NT and Nanog shRNA lentiviral particles are shown in the bottom panels. All images are orientated with the proximal end of the bone at the top and are from 7 and 21 days after the time of surgery. The Comparisons for all pairs using one way ANOVA showed statistical significant difference in mean in shNanog group. P<.0001 denoted as ***showing significant differences in each group (n=10/ group). C) Histological analysis of bone formation in response to surgical marrow ablation in NT shRNA and Nanog shRNA injected group on day 7 and day 21. Sections were cut in longitudinal orientations and selected sections from the central region of the bone are presented in the space occupied by the regenerating marrow. Sections were stained with Goldners trichome stain. D) Effect of nanog shRNA lentiviral treatment on the expression of various genes involved in regenerative response in skeletal and adipose tissue lineages within marrow tissues formed after surgical ablation. Effect of nanog shRNA treatment relative to NT shRNA on expression of genes involved in skeletal lineage shown in panel on left and major genes involved in adipogenesis are shown in right panel. Levels of expression are relative to unoperated control tibia bones. Error bars represent standard deviation from replicates measurements from pooled three experiments.
The final evaluation examined the underlying effect of inhibition of Nanog on the mRNA expression of a set of genes related to control of MSC differentiation (Fig.4 D). The efficacy of the Nanog shRNA lentiviral knockdown was first evaluated by examining Nanog expression on days 7 and 21. These results showed that there was ~60 to ~70% knock down of Nanog relative to nontargeted shRNA group at both time points. A series of transcription factor regulators of osteogenic MSC differentiation (smad1, Sox9, Osterix, Dlx5 and Runx2) and terminal osteoblast function (OC) were next examined. At day seven all of these factors were upregulated ~ 2 fold while OC levels were minimally reduced. By 21 days post injection, Smad 1, Dlx5, Runx2 and Osteocalcin levels were all reduced from 40 to 60 percent consistent with micro-CT and histology data. Interestingly Sox9 (5 fold) and Osterix (2.5 fold) expression were up regulated relative to non target shRNA group. The key mediators of adipogenesis such as adipsin and PPAR gamma are also increased by 4.5 fold and 2 fold respectively in Nanog shRNA group compared to non-target shRNA also consistent to the observation that down regulation of Nanog leads to increase in the observed adipogenesis.
DISCUSSION
Our results are consistent with prior studies showing that MSCs prepared from various species and multiple tissue including cardiac tissues, adipose tissues, dermis, and the bone marrow all express Nanog [6–10,18–20]. Consistent with the known mechanisms of how Nanog functions in ESCs our data showed that Nanog expression was down regulated as MSCs undergo differentiation and that knockdown of Nanog expression alone could induce osteogenic gene expression in the absence of BMP treatment. In this context, these results are functionally similar to Nanog's regulatory response to BMP signaling during mesodermal cell layer differentiation during embryogenesis [5]. We also show that Nanog regulates the proliferation of MSCs with the knockdown of Nanog decreasing the proliferation of bone MSCs while overexpression induced increased proliferation, similar to results that have shown that Nanog contributes to maintaining embryonic stem cell self renewal [3,16,17,20]. A possible explanation for the modest effects of altering Nanog expression on proliferation in the primary bone MSC cultures, is that the cultures are heterogeneous and contain a number of different cells types that give rise to both hematopoietic and mesenchymal (stromal) cellular lineages.
The most striking results from our study came from the knockdown down of Nanog expression during bone regeneration induced by surgical marrow ablation. While the knockdown of Nanog expression initially appeared to promote more osteogenic differentiation, during the subsequent periods of remodeling there was a loss of bone and an increase in adipose tissues. We interpret these results to suggest that the knockdown of Nanog lead to increased numbers of MSCs cells that are initially committed to terminally differentiate, but as the bone starts to remodel there was a deficiency in the numbers of stem cells that had self renewed and are able to commit to the next round of osteogenesis accompanying remodeling. While adipocytes and osteoblasts are believed to be derived from a common stem cell [21], the increased numbers of adipocytes that were observed in the mice in which Nanog was knocked down suggest that Nanog may have differing effects on the stem maintenance of osteogenic progenitors over those that give rise to apidogenic cells. In this context, several separate studies suggest that Nanog may have a specific function in MSC differentiation associated with the development of skeletogenic cells compared to adipogenic cells. In studies of by Katsara et al., 2011 [22], Nanog was shown to decrease in expression during adipogenesis much more significantly that than during osteogenesis. In other studies, long-term culturing of mouse bone MSCs was shown lead to the loss of their potential to differentiate into osteocytes or chondrocytes while the cells retained their ability to differentiate into adipocytes [23]. In a review to the MSC literature, Sethe et al., 2006 [24], reported that in almost all the studies of MSCs independent of species or origin tended to lose their osteogenic potential with age while their adipogenic potential was found to either remain unaltered or to increase with age. Finally studies of Lui et al., 2004 [20], showed that the overexpression of Nanog in MSCs lead to increased proliferative capacity but decreased the potential of the cells to undergo adipogenic differentiation.
While the conditional deletion of Nanog in post natal mice showed no overt phenotype except for deficient spermatogenesis [4], our studies provide the first demonstration that Nanog might play a role in post-natal skeletal tissue regeneration in response to injury. Although our data appear to be contrary to these prior findings, these previous studies had not examined Nanog function in either an aging context or in animals in which large numbers of stem cells would be mobilized such as happens after repair. In this context, some studies have shown that fracture healing potential decreases with age [25–27] although whether this is consequence of deficient numbers of stem cells [25] or other factors such as deficient vascularization [26] or increased resorption relative to formation [27] has not been fully resolved.
HIGHLIGHTS.
Nanog is related to marrow stromal stem cell maintenance.
Increasing Nanog Expression Is Seen during Post natal Surgical Bone Repai.r
Nanog knockdown decreases post surgical bone regeneration.
Acknowledgments
Supported with grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (PO1AR049920) TAE
Institutional support was provided by the Department of Orthopaedic Surgery Boston University School of Medicine and by Boston University School of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Bais M, McLean J, Sebastiani P, Young M, Wigner N, Smith T, Kotton D, Einhorn T, Gerstenfeld LC. Transcriptional analysis of fracture healing and the induction of embryonic stem cell-related genes. PLoS One. 2009;4:e5393. doi: 10.1371/journal.pone.0005393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jaenisch R, Young R. Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell. 2008;132:567–82. doi: 10.1016/j.cell.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zhang X, Neganova I, Przyborski S, Yang C, Cooke M, Atkinson SP, Anyfantis G, Fenyk S, Keith WN, Hoare SF, Hughes O, Strachan T, Stojkovic M, Hinds PW, Armstrong L, Lako MA. Role for NANOG in G1 to S transition in human embryonic stem cells through direct binding of CDK6 and CDC25A. J Cell Biol. 2009 Jan 12;184(1):67–82. doi: 10.1083/jcb.200801009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chambers I, Silva J, Colby D, Nichols J, Nijmeijer B, Robertson M, Vrana J, Jones K, Grotewold L, Smith A. Nanog safeguards pluripotency and mediates germline development. Nature. 2007;450:1230–4. doi: 10.1038/nature06403. [DOI] [PubMed] [Google Scholar]
- [5].Suzuki A, Raya A, Kawakami Y, Morita M, Matsui T, Nakashima K, Gage FH, Rodriguez-Esteban C, Izpisua Belmonte JC. Nanog binds to Smad1 and blocks bone morphogenetic protein-induced differentiation of embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103:10294–9. doi: 10.1073/pnas.0506945103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Esposito MT, Di Noto R, Mirabelli P, Gorrese M, Parisi S, Montanaro D, Del Vecchio L, Pastore L. Culture conditions allow selection of different mesenchymal progenitors from adult mouse bone marrow. Tissue Eng Part A. 2009;15:2525–36. doi: 10.1089/ten.tea.2008.0509. [DOI] [PubMed] [Google Scholar]
- [7].Kastrinaki MC, Andreakou I, Charbord P, Papadaki H. Isolation of human bone marrow mesenchymal stem cells using different membrane markers: comparison of colony/cloning efficiency, differentiation potential, and molecular profile. Tissue Eng Part C Methods. 2008;14:333–9. doi: 10.1089/ten.tec.2008.0173. [DOI] [PubMed] [Google Scholar]
- [8].Khatri M, O'Brien TD, Sharma JM. Isolation and differentiation of chicken mesenchymal stem cells from bone marrow. Stem Cells Dev. 2009;18:1485–92. doi: 10.1089/scd.2008.0223. [DOI] [PubMed] [Google Scholar]
- [9].Violini S, Ramelli P, Pisani LF, Gorni C, Mariani P. Horse bone marrow mesenchymal stem cells express embryo stem cell markers and show the ability for tenogenic differentiation by in vitro exposure to BMP-12. BMC Cell Biol. 2009;10:29. doi: 10.1186/1471-2121-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cesselli D, Beltrami AP, Rigo S, Bergamin N, D'Aurizio F, Verardo R, Piazza S, Klaric E, Fanin R, Toffoletto B, Marzinotto S, Mariuzzi L, Finato N, Pandolfi M, Leri A, Schneider C, Beltrami CA, Anversa P. Multipotent progenitor cells are present in human peripheral blood. Circ Res. 2009;104:1225–34. doi: 10.1161/CIRCRESAHA.109.195859. [DOI] [PubMed] [Google Scholar]
- [11].Thies RS, Bauduy M, Ashton BA, Kurtzberg L, Wozney JM, Rosen V. Recombinant human bone morphogenetic protein-2 induces osteoblastic differentiation in W-20-17 stromal cells. Endocrinology. 1992;130:1318–24. doi: 10.1210/endo.130.3.1311236. [DOI] [PubMed] [Google Scholar]
- [12].Gerstenfeld LC, Cho TJ, Kon T, Aizawa T, Cruceta J, Graves BD, Einhorn TA. Impaired intramembranous bone formation during bone repair in the absence of tumor necrosis factor-alpha signaling. Cells Tissues Organs. 2001;169:285–94. doi: 10.1159/000047893. [DOI] [PubMed] [Google Scholar]
- [13].Bais MV, Wigner N, Young M, Toholka R, Graves DT, Morgan EF, Gerstenfeld LC, Einhorn TA. BMP2 is essential for post natal osteogenesis but not for recruitment of osteogenic stem cells. Bone. 2009;45:254–66. doi: 10.1016/j.bone.2009.04.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Theodorsson-Norheim E. Kruskal-Wallis test: BASIC computer program to perform nonparametric one-way analysis of variance and multiple comparisons on ranks of several independent samples. Computer Methods Programs Biomed. 1986;23:57–62. doi: 10.1016/0169-2607(86)90081-7. [DOI] [PubMed] [Google Scholar]
- [15].A EM. Isolation and propagation of mouse embryonic fibroblasts and preparation of mouse embryonic feeder layer cells. Curr Protoc Stem Cell Biol Chapter. 2007;1 doi: 10.1002/9780470151808.sc01c03s3. Unit1C 3. [DOI] [PubMed] [Google Scholar]
- [16].Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, Maruyama M, Maeda M, Yamanaka S. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631–42. doi: 10.1016/s0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- [17].Ma T, Wang Z, Guo Y, Pei D. The C-terminal pentapeptide of Nanog tryptophan repeat domain interacts with Nac1 and regulates stem cell proliferation but not pluripotency. J Biol Chem. 2009;284:16071–81. doi: 10.1074/jbc.M109.005041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Riekstina U, Cakstina I, Parfejevs V, Hoogduijn M, Jankovskis G, Muiznieks I, Muceniece R, Ancans J. Embryonic stem cell marker expression pattern in human mesenchymal stem cells derived from bone marrow, adipose tissue, heart and dermis. Stem Cell Rev. 2009 Dec;5(4):378–8. doi: 10.1007/s12015-009-9094-9. [DOI] [PubMed] [Google Scholar]
- [19].Pierantozzi E, Gava B, Manini I, Roviello F, Marotta G, Chiavarelli M, Sorrentino V. Pluripotency regulators in human mesenchymal stem cells: expression of NANOG but not of OCT-4 and SOX-2. Stem Cells Dev. 2011;20(5):915–23. doi: 10.1089/scd.2010.0353. [DOI] [PubMed] [Google Scholar]
- [20].Liu TM, Wu YN, Guo XM, Hui JH, Lee EH, Lim B. Effects of ectopic Nanog and Oct4 overexpression on mesenchymal stem cells. Stem Cells Dev. 2009;18:1013–22. doi: 10.1089/scd.2008.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Takada I, Kouzmenko AP, Kato S. Wnt and PPARgamma signaling in osteoblastogenesis and adipogenesis. Nat Rev Rheumatol. 2009;5(8):442. doi: 10.1038/nrrheum.2009.137. [DOI] [PubMed] [Google Scholar]
- [22].Katsara O, Mahaira LG, Iliopoulou EG, Moustaki A, Antsaklis A, Loutradis D, Stefanidis K, Baxevanis CN, Papamichail M, Perez SA. Effects of Donor Age, Gender, and In Vitro Cellular Aging on the Phenotypic, Functional, and Molecular Characteristics of Mouse Bone Marrow-Derived Mesenchymal Stem Cells. Stem Cells Dev. 2011 February 15; doi: 10.1089/scd.2010.0280. Ahead of time. [DOI] [PubMed] [Google Scholar]
- [23].Gou S, C Wang, T Liu, H Wu, J Xiong, Zhou F, Zhao G. Spontaneous differentiation of murine bone marrow derived mesenchymal stem cells into adipocytes without malignant transformation after long-term culture. Cells Tissues Organs. 2010;191(3):185–92. doi: 10.1159/000240246. 2010. [DOI] [PubMed] [Google Scholar]
- [24].Sethe S, Scutt A, Stolzing A. Aging of mesenchymal stem cells. Ageing Res Rev. 2006 Feb;5(1):91–1. doi: 10.1016/j.arr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- [25].Strube P, Mehta M, Baerenwaldt A, Trippens J, Wilson CJ, Ode A, Perka C, Duda GN, Kasper G. Sex-specific compromised bone healing in female rats might be associated with a decrease in mesenchymal stem cell quantity. Bone. 2009;45:1065–1072. doi: 10.1016/j.bone.2009.08.005. [DOI] [PubMed] [Google Scholar]
- [26].Lu C, Miclau T, Hu D, Hansen E, Tsui K, Puttlitz C, Marcucio RS. Cellular basis for age-related changes in fracture repair. J Orthop Res. 2005;23(6):1300–7. doi: 10.1016/j.orthres.2005.04.003.1100230610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mehta M, Strube P, Peters A, Perka C, Hutmacher D, Fratzl P, Duda GN. Influences of age and mechanical stability on volume, microstructure, and mineralization of the fracture callus during bone healing: is osteoclast activity the key to age-related impaired healing? Bone. 2010 Aug;47(2):219–28. doi: 10.1016/j.bone.2010.05.029. [DOI] [PubMed] [Google Scholar]