Abstract
Objective
Inflammatory activity in fat tissue has recently been implicated in mechanisms of insulin resistance and obesity-related metabolic dysfunction. Toll-like receptors (TLRs) play a key role in innate immune responses and recent studies implicate the TLR pathway in mechanisms of inflammation and atherosclerosis. The aim of this study was to examine differential TLR expression and function in human adipose tissue.
Methods and Procedures
We biopsied subcutaneous abdominal fat from 16 obese subjects (age 39 ± 11 years, BMI 49 ± 14 kg/m2) and characterized TLR expression using quantitative real-time PCR and confocal immunofluorescence imaging. In tissue culture, we stimulated isolated human adipocytes with Pam3CSK4 and lipopolysaccharide (LPS) (TLR2 and TLR4 agonists, respectively) and quantified TLR activity, interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) production, and nuclear factor-κB (NF-κB) p65 nuclear activation using real-time PCR, enzyme-linked immunosorbent assay (ELISA), and immunofluorescence.
Results
TLR1, 2, and 4 protein colocalized with adiponectin in human adipocytes with TLR4 exhibiting the highest immunohistochemical expression. Using real-time PCR, we confirmed higher level of gene expression for TLR4 as compared to other members of the TLR family (TLR1, 2, 7, 8) in human adipose depots (P < 0.001). In tissue culture, adipocyte TLR2/TLR4 mRNA expression and protein increased significantly following Pam3CSK4 and LPS (P < 0.001). TLR2/TLR4 stimulation was associated with NF-κB p65 nuclear translocation and proinflammatory cytokine production.
Discussion
The findings demonstrate that TLRs are inducible in adipose tissue and linked with downstream NF-κB activation and cytokine release. Adipose stores may play a dynamic role in the regulation of inflammation and innate immunity in human subjects via modulation of the TLR/NF-κB regulatory pathway.
INTRODUCTION
Obesity represents a disease state associated with chronic sub-clinical inflammation, metabolic dysfunction, and increased cardiovascular risk (1,2). The source and stimulus of persistent inflammatory activation in obesity is largely unknown. In addition to serving as a seemingly passive lipid storage depot, there is a growing recognition of adipose tissue as a highly active metabolic organ, and significant potential source of proatherogenic and proinflammatory adipokines. Circulating inflammatory biomarkers such as C-reactive protein (CRP), interleukin-6 (IL-6), and cellular adhesion molecules are uniformly elevated in obese patients, and frequently correlate with parameters of adiposity, insulin resistance, and type 2 diabetes risk (1,3,4). Fat depots contribute significantly to sustaining a chronic state of inflammation through synthesis and elaboration of cytokines that may be etiologically intertwined with mechanisms of cardiometabolic dysfunction.
The dynamic regulation of inflammatory activity and associated maladaptive adipocytokine production in fat remains incompletely understood. Recent studies suggest that adipocytes may play an important role in the physiological regulation of immune responses in fat depots via toll-like receptor (TLR) signaling (5). TLRs represent a family of innate immune receptors that recognize pathogen-associated molecular patterns (6,7). In addition to host defense against foreign pathogens, recent information directly implicates a role of the TLR pathway in mechanisms of obesity-related insulin resistance and metabolic dysfunction, and clinical studies have linked TLR4 polymorphisms to atherosclerosis and cardiovascular events (8,9). While TLRs are expressed in platelets and vascular cells, specifically atherosclerotic plaques and monocytes, information about TLR expression and function in human fat is limited (10,11). The purpose of this study was to examine ambient expression patterns of various TLR subtypes in subcutaneous fat stores of obese subjects. Additionally, we sought to investigate the inducible functionality of TLRs in relation to nuclear factor-κB (NF-κB) p65 activation and associated cytokine production in human adipocytes.
METHODS AND PROCEDURES
Research subjects
We enrolled consecutive subjects aged 18–60 years who were obese (BMI ≥ 30 kg/m2) and receiving care at the Boston Medical Center, Nutrition and Weight Management Center. Subjects were approached in an outpatient clinic setting and detailed medical history was obtained for each individual. We excluded pregnant women and patients with unstable cardiovascular disease, malignancy, active infection, or treatment with an investigational drug within 30 days of enrollment. All subjects provided written informed consent and the study was approved by the Boston Medical Center Institutional Review Board. Each subject made one baseline visit during which vital signs were recorded, blood sample obtained, and subcutaneous fat pad biopsy performed.
Subcutaneous adipose biopsy procedure
We collected human adipose tissue via abdominal subcutaneous fat pad biopsy lateral to the umbilicus using standard sterile technique. In brief, the region was draped, sterilely prepped using alcohol and betadine, and locally anesthetized with 2 ml 2% lidocaine. Through a small superficial 0.5-cm skin incision, fat tissue was collected via punch biopsy and a 3-hole cannula needle. Tissue samples were stored in formalin or temporarily placed on ice and promptly frozen in liquid nitrogen to be stored at −80 °C until all samples were collected from participating subjects. Samples were then used for immunohistochemical and morphologic analyses using established methods described in this article. For functional studies, we used isolated cultured human adipocytes prepared as described previously (12).
Immunofluorescence studies of human adipose tissue biopsies and isolated (cultured) adipocytes
To explore for presence of TLR subtypes and confirm colocalization in human adipose tissue, confocal microscope imaging was performed using double immunofluorescence on harvested fat tissue sections targeted for coexpression of TLR1, TLR2, and TLR4 with adiponectin. Briefly, adipose tissue biopsy specimens were fixed in 10% buffered formalin and embedded in paraffin for sectioning. After dehydration in graded alcohols (100-70%), permeabilization in the presence of 0.2% Triton X-100 and blocking in protein block serum-free solution (DakoCytomation, Carpinteria, CA) adipose sections were incubated with mouse monoclonal antibodies to TLR1 (Cell Science, Canton, MA), TLR2, TLR4 (Imgenex, San Diego, CA) and goat polyclonal antibody to Adiponectin (Abcam, Cambridge, MA) at 4 °C, overnight. After washing in phosphate-buffered saline, slides were incubated with secondary anti-mouse IgG–fluorescein isothiocyanate conjugated and anti-goat IgG-TR conjugated antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) for 1 h at room temperature. Controls included normal mouse and goat IgGs at the same concentrations as the primary antibodies and omitting the primary antibodies. The sections were examined under confocal microscope (Leica TCS SP2 instrument; Leica Microsystems, Exton, PA). The same technique was used to examine TLR2 and TLR4 expression as well as the localization and nuclear translocation of activated NF-κB p65 after Pam3CSK4 and lipopolysaccharide (LPS) stimulation of cultured adipocytes. The p65 subunit of NF-κB was localized by incubating the cells with a mouse monoclonal antibody and visualizing with IgG–fluorescein isothiocyanate conjugated to a goat anti-mouse secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Image analysis and quantification of fluorescence intensity of TLRs, adiponectin, NF-κB p65 were performed using LSM 2.5 software (Carl Zeiss, Microsoft) or US National Institute of Health Image Software (Bethesda, MD, http://rsb.info.nih.gov/nih-image/). Quantification of fluorescence intensity was analyzed from three independent experiments (mean ± s.e.). In each condition, at least 20 cells were quantified.
Stimulation of human isolated (cultured) adipocytes with TLR2 and TLR4 agonists
Ultra pure Escherichia coli K12 LPS as the prototypic TLR4 agonist and synthetic lipoprotein Pam3CSK4 as the TLR2 agonist (InvivoGen, San Diego, CA) were used to stimulate adipocytes at a concentration of 100 ng/ml for 24 h at 37 °C. Adipocytes maintained in untreated media were used as controls. After treatment, conditioned media were removed and stored at −80 °C. Whole-cell, cytoplasmic and nuclear extracts from adipocytes were prepared using a commercial kit (Nuclear Extract Kit; Actif Motif, Carlsbad, CA), and then stored at −80 °C. Total amount of protein was measured using the DC protein assay kit (Bio-Rad, Hercules, CA). A quantity of 10 µg of protein from each whole-cell, cytoplasmic and nuclear extracts were used for NF-κB assay.
Biochemical measurement of activated NF-κB p65 in the nucleus
To detect the localization and nuclear translocation of NF-κB p65 whole-cell, cytoplasmic, and nuclear extracts from adipocytes following treatment with Pam3CSK4 and LPS (TLR2 and TLR4 agonists, respectively) or control were analyzed for total and phosphorylated NF-κB p65 by commercially available enzyme-linked immunosorbent assay (ELISA) kits (Pathscan Total and Phospho-NF-κB p65 Sandwich ELISA Kits; Cell Signaling Technology, Beverely, MA). Jurkat nuclear extract was used as a positive control.
Quantification of cytokines IL-6 and TNF-α secretion from treated adipocytes
Conditioned media from untreated (control) and Pam3CSK4- or LPS-treated adipocytes were assayed for IL-6 and tumor necrosis factor-α (TNF-α) secretion (QuantiGlo ELISA; R&D Systems, Minneapolis, MN) in accordance with manufacturer’s instructions.
Quantification of gene expression using real-time PCR
Quantitative real-time PCR was performed on harvested fat tissue specimens. We examined the relative basal expression of five different TLR subtypes (TLR1, 2, 4, 7, 8) in relation to a housekeeping gene. Briefly, after collection, biopsy specimens were placed in ice-cold saline, cleaned by removing visible blood vessels and clots, immersed in a RNA preserving solution (RNAlater, Sigma, St. Louis, MO), and immediately stored at −20 °C until subsequent isolation of total RNA for gene expression studies. Total RNA was isolated from adipose tissues by Ambion (Austin, TX). High-Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA) was used for cDNA reactions. Several aliquots were stored at −80 °C for PCR experiments. Real-time PCRs were carried out using TaqMan 2× Universal PCR Master Mix (Applied Biosystems, Foster City, CA). TLR1, 2, 4, 7, 8 and glyceraldehyde-3-phosphate dehydrogenase assays were selected and purchased from Applied Biosystems Gene Expression Assay Selection. Real-time PCR experiments were performed on Icycler Real-time PCR instrument (Bio-Rad, Hercules, CA). Total RNA was isolated from adipocyte cell lines using RNeasy Mini Kit (Qiagen, Valencia, CA). High capacity cDNA Archive kit was used for cDNA reaction (Applied Biosystems, Foster City, CA). TaqMan Gene Expression Assays were used to detect relative gene expression levels of TLR2, TLR4 and NF-κB (Applied Biosystems, Foster City, CA). Glyceraldehyde-3-phosphate dehydrogenase gene was used as a housekeeping gene to normalize results. Quantitative real-time PCRs were performed using TaqMan Universal PCR Master Mix in a 7900HT Real-Time PCR Instrument (Applied Biosystems, Foster City, CA).
Biochemical analyses
Serum levels of total cholesterol, triglycerides, high-density lipoprotein cholesterol, and glucose were determined by the Boston Medical Center clinical laboratory using an Advia 2400 Chemistry Analyzer (Bayer Diagnostics, Tarrytown, NY), low-density lipoprotein cholesterol was calculated according to the Friedewald formula. White blood cell count was measured using the LH750 Automated Hematology Analyzer (Beckman Coulter, Miami, FL), and hemoglobin A1C was determined using high-performance liquid chromatography (Bio-Rad, Hercules, CA). High-sensitivity CRP was quantified using the nephelometric methodology (Quest Diagnostic, Cambridge, MA).
Statistical analyses
All data are presented as mean ± s.d., unless otherwise indicated. The relative expression of different TLR subtypes and treatment effects were examined using ANOVA with multiple pairwise Tukey comparison. Correlation between waist circumference, weight, BMI, metabolic and hemodynamic parameters, and TLR expression was examined using linear regression analysis. For all analyses, P value < 0.05 was considered significant. Statistical analysis was performed with Sigmastat for Windows 2.03 software (SPSS, Chicago, IL).
RESULTS
Subject characteristics
A total of 17 consecutive patients participated in the study. One subject was excluded due to an insufficient fat biopsy sample. The clinical characteristics of the remaining 16 subjects are shown in Table 1. All participants were obese women with mean BMI 49 ± 14 kg/m2 (range 35–97 kg/m2) and associated central obesity with mean waist circumference 133 ± 20 cm (range 104–175 cm). High-sensitivity CRP was elevated as described in previous studies of obese subjects (2), while white blood cell count was within the normal clinical range.
Table 1.
Baseline clinical characteristics
| N | 16 |
| Age (years) | 39 ± 11 |
| BMI (kg/m2) | 49 ± 14 |
| Weight (kg) | 127 ± 40 |
| Waist circumference (cm) | 133 ± 20 |
| Total cholesterol (mg/dl) | 186 ± 38 |
| LDL cholesterol (mg/dl) | 116 ± 33 |
| HDL cholesterol (mg/dl) | 48 ± 8 |
| Triglycerides (mg/dl) | 111 ± 42 |
| Glucose (mg/dl) | 112 ± 46 |
| Hemoglobin A1C (%) | 6.5 ± 1.1 |
| Diabetes mellitus (%) | 38 |
| Metabolic syndrome (%) | 50 |
| Systolic BP (mm Hg) | 129 ± 10 |
| WBC (K/UL) | 8.2 ± 1.8 |
| hsCRP (mg/l) | 8.8 ± 8.1 |
BP, blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; hsCRP, high-sensitivity CRP; WBC, white blood cell count.
TLR expression in abdominal subcutaneous adipose tissue from obese subjects
To determine the presence of TLRs and confirm colocalization in adipose tissue, we performed high power confocal imaging for TLR1, 2, 4, and adipocyte-specific hormone adiponectin. As shown in Figure 1, there is expression for both TLR2 and 4 (green) and adiponectin (red) in adipocytes with evidence of cellular colocalization in fat tissue. Similar results were obtained for TLR1 expression (immunofluorescence images not shown). As displayed in Figure 2, TLR4 expression exhibited significantly greater fluorescence intensity (in arbitrary units, a.u.) as compared to TLR1 and 2 subtypes (*P < 0.05). Immunofluorescence intensity for TLR4 (29.9 ± 4.2) was threefold greater than TLR2 (9.3 ± 2.6), and TLR1 (8.4 ± 1.9 a.u.). Isotope-matched, irrelevant antibodies were used as negative controls (data not shown). Human blood monocytes (TLR4, n = 3) served as positive control (**P < 0.001).
Figure 1.
High power double immunofluorescence confocal imaging demonstrating presence of Toll-like receptor 2 (TLR2) (left upper panel, green), TLR4 (right upper panel, green), adiponectin (left lower panel, red), and TLR4-adiponectin colocalization (right lower panel, yellow) in human abdominal subcutaneous adipose tissue.
Figure 2.
Immunofluorescence intensity for Toll-like receptor (TLR) protein subtypes 1, 2, and 4 in human adipose tissue. Fluorescence intensity was greatest for TLR4 (*P < 0.05). Human blood monocytes (n = 3) were used as positive control (**P < 0.001). Data are presented as mean ± s.e. a.u., arbitrary unit.
As confirmatory quantitative evidence of TLR expression, we performed real-time PCR on human fat tissue specimens to examine relative RNA expression in these samples. As shown in Figure 3, TLR4 exhibited the highest level of gene expression in adipose tissue (*P < 0.001 as compared to all other TLR subtypes), with lowest expression of TLR8. We observed a significant correlation between fat TLR8 expression and circulating plasma levels of high-sensitivity CRP (r = 0.73, P = 0.002). There was also a similar trend for a correlation between TLR1 and high-sensitivity CRP (r = 0.62, P = 0.1). Otherwise, there were no significant correlation between tissue TLR subtype expression and clinical variables displayed in Table 1, including BMI, weight, or metabolic parameters.
Figure 3.
Toll-like receptor (TLR) expression in human adipose tissue using quantitative real-time PCR. As shown, the highest level of gene expression was for TLR4 (*P < 0.001 compared to other members of the TLR family). TLR7 expression was greater than TLR8 (†P = 0.04). Data are presented as mean ± s.e. a.u., arbitrary unit.
Inducible TLR expression in isolated human adipocytes
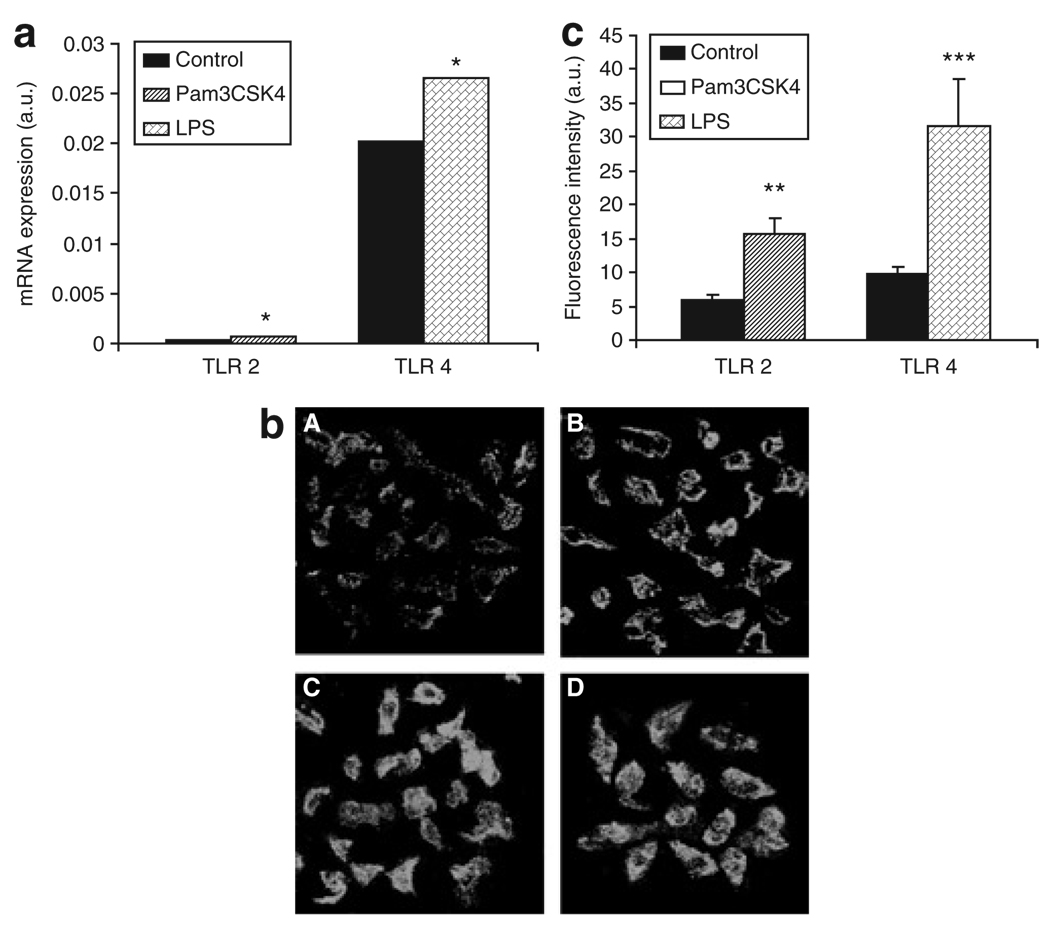
To determine functional TLR activity, we stimulated isolated (cultured) adipocytes with either Pam3CSK4 (TLR2 agonist) or LPS (TLR4 agonist). As shown in Figure 4a, TLR2 and TLR4 mRNA expression increased significantly following agonist stimulation compared to controls (*P < 0.01). We also demonstrated inducible TLR protein expression quantified by confocal immunofluorescence. As shown in Figure 4b and c, TLR2 and TLR4 protein expression increased significantly following stimulation with either Pam3CSK4 (15.3 ± 2.5) vs. untreated control (5.7 ± 0.8), **P < 0.005 or LPS (32.4 ± 7.4) vs. untreated control (9.8 ± 1.2 units), ***P < 0.001, respectively.
Figure 4.
Toll-like receptor 2 (TLR2)/TLR4 mRNA and protein expression in response to Pam3CSK4 and lipopolysaccharide (LPS) stimulation of adipocytes. (a) TLR2 /TLR4 gene expression increased significantly in treated adipocytes as compared to untreated control (*P < 0.01, n = 3). (b,c) TLR2/TLR4 protein expression quantified by immunofluorescence increased significantly in stimulated adipocytes compared to control. (b) Upper panels show basal expression of (A) TLR2 and (B) TLR4 in untreated cells; lower panels demonstrate (C) expression of TLR2 in Pam3CSK4-treated cells; (D) expression of TLR4 in LPS-treated cells (**P < 0.005; ***P < 0.001, respectively). Data are presented as mean ± s.e. a.u., arbitrary unit.
Inducible NF-κB p65 activation and nuclear translocation in adipocytes
NF-κB is an inducible transcription factor that plays a central role in regulation of inflammation. To address the question whether the stimulatory effect of Pam3CSK4/LPS on TLR2/TLR4 expression is associated with stimulation of the NF-κB pathway, we measured mRNA and protein levels of NF-κB p65 in the untreated (control) and treated adipocytes using real-time PCR, ELISA, and immunofluorescence techniques. As shown in Figure 5a, NF-κB p65 mRNA expression increased significantly following Pam3CSK4 (0.233 ± 0.0002) and LPS (0.300 ± 0.0002) treated adipocytes as compared to control (0.173 ± 0.0003 units), *P < 0.005; **P < 0.001, respectively. Additionally, NF-κB p65 activation in treated adipocytes was confirmed at the protein level (ELISA). As shown in Figure 5b, extent of phosphorylated NF-κB p65 in whole-cell extract was markedly increased after Pam3CSK4 (0.319 ± 0.029) and LPS (0.471 ± 0.060) as compared with unstimulated cells (0.069 ± 0.015 optical density units), *P < 0.005. Also, as shown in Figure 5c, we demonstrated NF-κB activation and its nuclear translocation following Pam3CSK4 (0.350 ± 0.042) and LPS (0.469 ± 0.125) compared to untreated cells (0.068 ± 0.017 optical density units), *P < 0.005. Finally, we confirmed nuclear translocation of activated NF-κB p65 by immunofluorescence as clearly demonstrated in Figure 5d.
Figure 5.
Activation and nuclear translocation of nuclear factor-κB (NF-κB) p65 following Pam3CSK4 and lipopolysaccharide (LPS) stimulation of adipocytes. (a) NF-κB p65 mRNA expression increased significantly in treated adipocytes (*P < 0.005; **P < 0.001, respectively, n = 3) as compared to untreated control. (b, ELISA) Phosphorylated NF-κB p65 in whole-cell extract increased significantly in treated adipocytes as compared to control (*P < 0.005, n = 3). (c, ELISA; d, confocal microscope) Panels depict expression of NF-κB p65 in (A) untreated cells followed by nuclear translocation of NF-κB p65 in (B) Pam3CSK4-treated cells and in (C) LPS-treated cells. Jurkat nuclear extract was used as a positive control (n = 3). Data are presented as mean ± s.e. ELISA, enzyme-linked immunosorbent assay.
Proinflammatory cytokine secretion in human adipocytes
Stimulation of NF-κB may lead to enhanced expression of inflammatory mediators including TNF-α and IL-6. Therefore, we examined TNF-α and IL-6 protein secretion in human cultured adipocytes using ELISA. As shown in Figure 6a and b, TNF-α production increased significantly in adipocytes treated with Pam3CSK4 (162.1 ± 17.1) and LPS (298.2 ± 27.7) vs. untreated controls (74.1 ± 10.9 pg/ml), *P < 0.005. Similarly, IL-6 secretion rose significantly with Pam3CSK4 (1,670 ± 246.7) and LPS (2,544 ± 237.5) vs. untreated cells (760.3 ± 135.8 pg/ml), **P < 0.001.
Figure 6.
(a) Tumor necrosis factor-α (TNF-α) and (b) interleukin-6 (IL-6) secretion in response to Pam3CSK4 and lipopolysaccharide (LPS) treatment of adipocytes. Cytokine release from treated adipocytes were significantly increased compared to control (*P < 0.005; **P < 0.001, n = 3). Data are presented as mean ± s.e.
DISCUSSION
In this study of obese subjects, we demonstrated both qualitative and quantitative evidence of differential TLR activity in human adipose tissue, extending knowledge from previous animal studies. Under basal conditions, we demonstrated greatest expression of the TLR4 subtype using two separate complementary methods that included enhanced immunofluorescence and gene expression, and confirmed TLR colocalization with adiponectin in adipocytes. Additionally, we demonstrated that TLRs are functionally inducible and associated with downstream NF-κB activation and proinflammatory cytokine production. These current findings provide evidence that adipose stores play a dynamic role in mechanisms of innate or adaptive immune responses in human subjects, and provide additional data to our growing understanding of the active inflammatory pathways in human adipose tissue.
TLRs represent a family of receptors that are critical to the innate immune response against foreign pathogens and microorganisms (6,7,13). The TLR family has significant homology in its cytoplasmic domain to the IL-1 receptor, and recognizes both endogenous and exogenous ligands. Currently, up to 10 mammalian TLRs have been identified, primarily in vascular and immune cells, myocytes, and platelets, with receptors functioning both within the plasma membrane and intracellular space. Adipose tissue has also been shown to express TLR in experimental studies (5). TLR2 has been cloned and characterized in mouse adipocytes, and their inflammatory stimulation induces changes in TLR2 expression and secretory cytokines, such as IL-6. In agreement with our findings, LPS has been shown to induce proinflammatory chemokine gene expression in differentiated human adipocytes through TLR and NF-κB action (14,15). In this study, we additionally demonstrated that human adipocytes respond robustly to other agonists such as Pam3CSK4 as manifest by significant upregulation of TLR2. We also provide novel data by demonstrating NF-κB activation and nuclear translocation in human adipocytes using confocal immunofluorescence imaging techniques which had been previously reported in vascular endothelial cells (16). These findings lend support to the presence of a functionally intact and diverse pathway of innate immunity in fat that is subject to activation and immunomodulation.
The functional significance of differential TLR expression patterns in human adipose tissue remains unknown. Although TLR expression in immune cells appears teleologically adaptive about host defense, its functional presence in fat cells raises the question of whether adipocytes are not only intricately involved in homeostatic immune regulation but may also play a functional role in disease states pathophysiologically linked to low-grade systemic inflammation. Stimulation of TLRs initiates intracellular signaling cascades resulting in downstream NF-κB and mitogen-activated protein kinase activation and production of proinflammatory chemokines associated with mechanisms of metabolic dysfunction and cardiovascular disease progression. Chronic inflammation owing to excessively efficient or hyperactive innate immunity, while protective against pathogens, may contribute to mechanisms of systemic disease. This observation is best clinically supported by a study demonstrating that the TLR4 polymorphism is associated with attenuated receptor signaling, reduced levels of circulating proinflammatory mediators, and decreased carotid atherosclerosis, while conferring increased susceptibility to bacterial infection (8).
A salient finding in our study was evidence for greatest expression of TLR4 in fat tissue among the TLR family, raising the possibility that its activity may play a prominent role in obesity-associated inflammation and cardiometabolic risk. In support of this, both animal and human studies have supported a mechanistic role for TLR4 in systemic disease. TLR4-deficient mice crossed with ApoE−/− mice develop smaller and fewer atherosclerotic lesions than ApoE−/− mice alone (17,18). TLR4 has been shown to recognize endogenous ligands such as oxidized-low-density lipoprotein and fibrinogen that may have relevance to atherothrombotic events. TLR4 expression is enhanced in both murine atherosclerotic lesions and lipid-rich, macrophage-infiltrated human coronary plaques, particularly in shoulder regions prone to rupture (10,19). Elevated fatty acids levels associated with obesity activate TLR4 signaling in fat cells and macrophages, and induce insulin resistance in murine models (20,21). Genotypic variations in TLR4 have been associated with carotid intimal thickening and increased risk of myocardial infarction (8,9). In addition, cardiac myocytes constitutively express TLR4 that is upregulated in failing hearts (22). Other members of the TLR family have also been implicated in atherogenesis. TLR1–6 are differentially expressed in human atherosclerotic plaques, although TLR2 and TLR4 appear to be expressed more widely (23). TLR2 in mice is linked to increased atherosclerotic lesion formation, and TLR2 genetic variants have been associated with diabetes mellitus (24). Meanwhile, adiponectin suppresses NF-κB activation in vascular cells, macrophages, and adipocytes (25). Collectively, the findings lend support to a functional link between TLR signaling and obesity-related inflammation and cardiometabolic dysfunction.
This study has several limitations. First, the study was largely exploratory with the primary objective of examining different TLR subtype expression patterns in human adipose tissue under basal conditions. Our functional studies however were limited to TLR2 and TLR4 immunomodulation owing to clinical relevance based on previous studies. Second, we examined TLRs exclusively in obese patients as securing fat tissue was technically safer and more feasible in these subjects. Additionally, a recent report had previously examined this issue in adipose tissue from lean subjects (26). Finally, we did not find significant correlation between TLR4 expression and cardiovascular risk factors, which is likely a limitation of the small sample size. The finding of a relationship between tissue TLR8 expression and circulating CRP levels supports a parallel link in local and systemic inflammatory activation in obesity and warrants further investigation.
In summary, this study demonstrated that human adipose tissue expresses TLRs with greatest expression of TLR4. TLR expression is inducible in adipose tissue and linked with downstream NF-κB activation and upregulated cytokine release. Enhanced TLR-dependent inflammatory activation owing to excess fat mass may represent a mechanistic link between obesity and cardiometabolic risk.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants R01 HL 74097 and HL 084213 (to N.G.), and P01 HL83801 (to J.F.).
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1.Klein S, Burke LE, Bray GA, et al. Clinical implications of obesity with specific focus on cardiovascular disease—a statement for professionals from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism—Endorsed by the American College of Cardiology Foundation. Circulation. 2004;110:2952–2967. doi: 10.1161/01.CIR.0000145546.97738.1E. [DOI] [PubMed] [Google Scholar]
- 2.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131–2135. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- 3.Engstrom G, Hedblad B, Stavenow L, et al. Incidence of obesity-associated cardiovascular disease is related to inflammation-sensitive plasma proteins—a population-based cohort study. Arterioscler Thromb Vasc Biol. 2004;24:1498–1502. doi: 10.1161/01.ATV.0000134293.31512.be. [DOI] [PubMed] [Google Scholar]
- 4.Ziccardi P, Nappo F, Giugliano G, et al. Reduction of inflammatory cytokine concentrations and improvement of endothelial functions in obese women after weight loss over one year. Circulation. 2002;105:804–809. doi: 10.1161/hc0702.104279. [DOI] [PubMed] [Google Scholar]
- 5.Lin Y, Lee H, Berg AH, et al. The lipopolysaccharide-activated toll-like receptor (TLR)-4 induces synthesis of the closely related receptor TLR-2 in adipocytes. J Biol Chem. 2000;275:24255–24263. doi: 10.1074/jbc.M002137200. [DOI] [PubMed] [Google Scholar]
- 6.Akira S, Takeda K. Toll-like receptor signaling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto M, Takeda K, Akira S. TIR domain-containing adaptors define the specificity of TLR signaling. Mol Immunol. 2004;40:861–868. doi: 10.1016/j.molimm.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Kiechl S, Lorenz E, Reindl M, et al. Toll-like receptor 4 polymorphisms and atherogenesis. N Engl J Med. 2002;347:185–192. doi: 10.1056/NEJMoa012673. [DOI] [PubMed] [Google Scholar]
- 9.Edfeldt K, Bennet AM, Eriksson P, et al. Association of hypo-responsive toll-like receptor 4 variants with risk of myocardial infarction. Eur Heart J. 2004;25:1447–1453. doi: 10.1016/j.ehj.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Xu XH, Shah PK, Faure E, et al. Toll-like receptor-4 is expressed by macrophages in murine and human lipid-rich atherosclerotic plaques and upregulated by oxidized LDL. Circulation. 2001;104:3103–3108. doi: 10.1161/hc5001.100631. [DOI] [PubMed] [Google Scholar]
- 11.Shiraki R, Inoue N, Kawasaki S, et al. Expression of toll-like receptors on human platelets. Thromb Res. 2004;113:379–385. doi: 10.1016/j.thromres.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 12.Tchkonia T, Tchoukalova YD, Giorgadze N, et al. Abundance of two human preadipocyte subtypes with distinct capacities for replication, adipogenesis, and apoptosis varies among fat depots. Am J Physiol Endocrinol Metab. 2005;288:E267–E277. doi: 10.1152/ajpendo.00265.2004. [DOI] [PubMed] [Google Scholar]
- 13.Medzhitov R, PrestonHurlburt P, Janeway CA. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 14.Chung S, LaPoint K, Martinez K, et al. Preadipocytes mediate lipopolysaccharide-induced inflammation and insulin resistance in primary cultures of newly differentiated human adipocytes. Endocrinology. 2006;147:5340–5351. doi: 10.1210/en.2006-0536. [DOI] [PubMed] [Google Scholar]
- 15.Bes-Houtmann S, Roche R, Hoareau L, et al. Presence of functional TLR2 and TLR4 on human adipocytes. Histochem Cell Biol. 2007;127:131–137. doi: 10.1007/s00418-006-0230-1. [DOI] [PubMed] [Google Scholar]
- 16.Rogers JA, Fuseler JW. Regulation of NF-kappa B activation and nuclear translocation by exogenous nitric oxide (NO) donors in TNF-alpha activated vascular endothelial cells. Nitric Oxide Biol Chem. 2007;16:379–391. doi: 10.1016/j.niox.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Bjorkbacka H, Kunjathoor VV, Moore KJ, et al. Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat Med. 2004;10:416–421. doi: 10.1038/nm1008. [DOI] [PubMed] [Google Scholar]
- 18.Michelsen KS, Wong MH, Shah PK, et al. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci USA. 2004;101:10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edfeldt K, Swedenborg J, Hansson GK, Yan ZQ. Expression of toll-like receptors in human atherosclerotic lesions—a possible pathway for plaque activation. Circulation. 2002;105:1158–1161. [PubMed] [Google Scholar]
- 20.Suganami T, Tanimoto-Koyama K, Nishida J, et al. Role of the Toll-like receptor 4/NF-kappa B pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler Thromb Vasc Biol. 2007;27:84–91. doi: 10.1161/01.ATV.0000251608.09329.9a. [DOI] [PubMed] [Google Scholar]
- 21.Shi H, Kokoeva MV, Inouye K, et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frantz S, Kobzik L, Kim YD, et al. Toll4 (TLR4) expression in cardiac myocytes in normal and failing myocardium. J Clin Invest. 1999;104:271–280. doi: 10.1172/JCI6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansson GK, Edfeldt K. Toll to be paid at the gateway to the vessel wall. Arterioscler Thromb Vasc Biol. 2005;25:1085–1087. doi: 10.1161/01.ATV.0000168894.43759.47. [DOI] [PubMed] [Google Scholar]
- 24.Park Y, Park S, Yoo E, Kim D, Shin H. Association of the polymorphism for toll-like receptor 2 with type 1 diabetes susceptibility. Ann NY Acad Sci. 2004;1037:170–174. doi: 10.1196/annals.1337.028. [DOI] [PubMed] [Google Scholar]
- 25.Ajuwon KM, Spurlock ME. Adiponectin inhibits LPS-induced NF-kappa B activation and IL-6 production and increases PPAR gamma 2 expression in adipocytes. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1220–R1225. doi: 10.1152/ajpregu.00397.2004. [DOI] [PubMed] [Google Scholar]
- 26.Creely SJ, McTernan PG, Kusminski CM, et al. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am J Physiol Endocrinol Metab. 2007;292:E740–E747. doi: 10.1152/ajpendo.00302.2006. [DOI] [PubMed] [Google Scholar]