Abstract
Citrate fermentation by Escherichia coli requires the function of the citrate/succinate antiporter CitT (citT gene) and of citrate lyase (citCDEFXG genes). Earlier experiments suggested that the two-component system CitA/CitB, consisting of the membrane-bound sensor kinase CitA and the response regulator CitB, stimulates the expression of the genes in the presence of citrate, similarly to CitA/CitB of Klebsiella pneumoniae. In this study, the expression of a chromosomal citC-lacZ gene fusion was shown to depend on CitA/CitB and citrate. CitA/CitB is related to the DcuS/DcuR two-component system which induces the expression of genes for fumarate respiration in response to C4-dicarboxylates and citrate. Unlike DcuS, CitA required none of the cognate transporters (CitT, DcuB, or DcuC) for function, and the deletion of the corresponding genes showed no effect on the expression of citC-lacZ. The citAB operon is preceded by a DcuR binding site. Phosphorylated DcuR bound specifically to the promoter region, and the deletion of dcuS or dcuR reduced the expression of citC. The data indicate the presence of a regulatory cascade consisting of DcuS/DcuR modulating citAB expression (and CitA/CitB levels) and CitA/CitB controlling the expression of the citCDEFXGT gene cluster in response to citrate. In vivo fluorescence resonance energy transfer (FRET) and the bacterial two-hybrid system (BACTH) showed interaction between the DcuS and CitA proteins. However, BACTH and expression studies demonstrated the lack of interaction and cross-regulation between CitA and DcuR or DcuS and CitB. Therefore, there is only linear phosphoryl transfer (DcuS→DcuR and CitA→CitB) without cross-regulation between DcuS/DcuR and CitA/CitB.
INTRODUCTION
Escherichia coli can grow on a wide variety of substrates under aerobic or anaerobic conditions. Citrate fermentation by E. coli requires the presence of an oxidizable cosubstrate, like glucose or glycerol, which is used as an electron donor (28). Citrate is taken up by the citrate/succinate antiporter CitT (39) and cleaved to acetate and oxaloacetate (OAA) by citrate lyase (CL). Holo-citrate lyase and the citrate transporter CitT are encoded by the citCDEFXGT gene cluster. Oxaloacetate then is reduced to malate by malate dehydrogenase (Mdh), and malate subsequently is converted to fumarate by fumarase (FumB). Fumarate finally is reduced to succinate by fumarate reductase (FrdABCD). The two-component system CitA/CitB of E. coli is supposed to regulate the expression of the genes for citrate fermentation in response to external citrate under anaerobic conditions (20, 52), similarly to the citrate-responsive two-component system CitA/CitB of Klebsiella pneumoniae (6). CitA/CitB represents a typical extracytoplasmic-sensing two-component system consisting of a membrane-bound sensory histidine kinase, CitA, and the cognate response regulator CitB (30, 50). The perception of the stimulus leads to the autophosphorylation of a conserved histidine residue (His347) in the kinase domain of the sensor CitA. The phosphoryl group subsequently is donated to a conserved aspartate residue (Asp57) of the cognate response regulator CitB, which controls the expression of the target genes. Besides the regulation of citrate fermentation, CitA/CitB (alternative designation, DpiB/DpiA) of E. coli has an effect on the inheritance of iteron-containing plasmids and on the SOS response to β-lactam antibiotics (13, 32, 33). The latter effects were observed under aerobic conditions and the overexpression of the CitB regulator. The periplasmic domain of CitA from E. coli functions as a high-affinity receptor for citrate (Kd [dissociation constant], ∼0.3 μM), isocitrate, and tricarballylate but not for C4-dicarboxylates like fumarate or malate (20). Purified and phosphorylated CitB binds to the promoter regions of the citrate fermentation genes in vitro (52). The overproduction of CitB in vivo leads to an increase of the mRNA level of the citCDEFXGT gene cluster in the presence of citrate (52) and also induces the expression of plasmid-encoded citC-lacZ (13). Thus, the primary physiological role of CitA/CitB of E. coli appears to be the induction of the citrate fermentation genes. Overall, CitA/CitB of E. coli has been shown to represent a two-component system specific for citrate and to autoregulate citAB expression. In contrast to K. pneumoniae, however, CitA/CitB of E. coli has been implied in the regulation of plasmid inheritance, and its role in the regulation of the expression of the citCDEFXGT gene cluster has not been demonstrated under physiological conditions, leaving the question of the role of CitA/CitB in the regulation of anaerobic citrate metabolism unanswered.
The DcuS/DcuR two-component system controls the expression of the genes essential for fumarate respiration (11, 14, 15, 56), including the genes dcuB, fumB, and frdABCD that encode the fumarate/succinate antiporter (DcuB), fumarase (FumB), and fumarate reductase (FrdABCD). The sensor kinase DcuS responds to all types of C4-dicarboxylates and citrate with apparent Kd values in the range of 2 to 13 mM (23). Fumarase and FrdABCD also are required for citrate fermentation, and the respective genes then are induced by the response of DcuS/DcuR to citrate (23, 25). In addition, DcuS requires the fumarate/succinate antiporter (DcuB) as a cosensor and becomes constitutively active when DcuB is missing (22). For cosensing, DcuB contains specific sites that are not essential for transport.
CitA and DcuS constitute the CitA family of sensor histidine kinases and reveal a high topological, functional, and structural similarity (5, 6, 11, 15, 38, 41, 46, 56). In this study, the role of CitA/CitB of E. coli in the regulation of the citrate gene cluster citCDEFXGT was studied using a chromosomal citC-lacZ reporter gene fusion. Additionally, it was tested whether CitA requires, like DcuS, the presence of transporters (CitT and DcuB) of the pathway or of related pathways (the succinate efflux carrier DcuC) to function. Expression studies (25) showed that the induction of dcuB-lacZ expression is diminished slightly in a citA-negative strain but not in a citB-negative strain, suggesting an interaction between the DcuS/DcuR and the CitA/CitB systems in the transcriptional regulation of target genes. The interaction between both systems was studied here in more detail. Such an interference between two regulatory systems can be caused by the interaction of the proteins of the regulatory systems, e.g., DcuS/DcuR and CitA/CitB. Thus, it was studied whether selected proteins of the DcuS/DcuR and the CitA/CitB two-component systems interact. The interaction and transfer of phosphoryl groups between noncognate sensor-response regulator pairs of E. coli has been discussed repeatedly (26, 51), but cross-talk or phosphoryl transfer between the DcuS/DcuR and CitA/CitB systems has not been tested. Experimentally, cross-talk is observed mostly after the overproduction of components of the two-component systems or in vitro. Due to the structural and functional similarity and the interdependence of the regulated metabolic systems, CitA/CitB and DcuS/DcuR might be candidates for physiologically relevant interaction. This was examined by reporter gene expression studies. Furthermore, physical interaction was tested by fluorescence resonance energy transfer (FRET) studies and the bacterial two-hybrid system (BACTH) in vivo. Such an interaction can be a mere physical interaction without physiological relevance (cross-talk) or of physiological significance when the interaction triggers a cross-regulation (26). Alternatively, interference can be the result of the hierarchical control of the two-component systems, where one system controls the expression of the second. Such a hierarchical system is nicely exemplified by the nitrate-responsive NarX/NarL system of E. coli that represses the synthesis of the DcuS/DcuR system (10). Therefore, it was tested by binding studies of DcuR to the citA promoter region whether the DcuS/DcuR system is able to function as a regulator of citAB gene expression and, thus, of the levels of CitA and CitB.
MATERIALS AND METHODS
Bacterial and molecular genetic methods.
The Escherichia coli K-12 strains and plasmids used in this study are listed in Table 1. Molecular genetic methods were performed according to standard procedures (43). Plasmids were isolated using a QIAprep spin miniprep kit, and PCR products were purified with a QIAquick PCR purification kit (Qiagen, Hilden, Germany). E. coli strains were transformed by electroporation (8). The dcuS-cfp, dcuS-yfp, citA-yfp, and cfp-yfp fusions for the FRET measurements were constructed as described previously (44, 45). For protein interaction studies by the bacterial two-hybrid system (BACTH) (18, 19), the T25 and T18 domains were separately fused to the N termini of the target proteins (DcuS, CitA, and DcuR). The fusions were produced by cloning the dcuS, citA, and dcuR genes into the pKT25 or pUT18C vector. T25-dcuS was obtained by amplifying dcuS with the primer pair KT25-dcuS-BamHI-for (5′-CACAAGGGATCCGATGAGAC-3′) and KT25-dcuS-EcoRI-rev (5′-CATCGATAATGAATTCATTGATCATC-3′) from pMW181 and cloning into pKT25. For T18-dcuS the primers UT18(C)-dcuS-PstI-for (5′-CAAGGAAGCTCTGCAGACATTC-3′) and UT18(C)-dcuS-EcoRI-rev (5′-CATTGATCATGAATTCGACCTCTCC-3′) were used for amplification from pMW181 and the cloning of dcuS in the pUT18C vector. The dcuR and citA genes for the T25-dcuR and T18-citA gene constructs were amplified from chromosomal DNA of E. coli MG1655 with the primer pairs KT25-dcuR-XbaI-for (5′-GGGAGATCTAGAACAGATGATC-3′) plus KT25-dcuR-EcoRI-rev (5′-GATAACCAGGAATTCGTTATTGGC-3′) and pUT18C-citA-for (5′-CGCAAGGTCTAGACATGTTGCAGC-3′) plus pUT18C-citA-rev (5′-GCCAGCGGCGAATTCGTCCTC-3′) and cloned into pKT25 or pUT18C, respectively. The T25-zip and T18-zip control gene fusions were obtained from the BACTH kit (19).
Table 1.
Strains of Escherichia coli and plasmids used in this study
| Strain, plasmid, or phage | Genotype or characteristic(s) | Reference or source |
|---|---|---|
| E. coli K-12 strains | ||
| MC4100 | F−araD139 Δ(argF-lac)U169 rpsL150 relA1 flbB530 deoC1 ptsF25 rbsR ΔlacZ | 47 |
| MG1655 | CGSC 6300, fnr mutant, λ-F-P1 sensitive | 17 |
| JM109 | recA1 supE44 endA1 hsdR17 gyrA96 relA1 thi e14− F′ traD36 proAB+lacIq (lacZ)M15 (lac-proAB) | 54 |
| BTH101 | F−cya-99 araD139 galE15 galK16 rpsL1 (Strr) hsdR2 mcrA1 mcrB1 | BACTH manual (19) |
| IMW157 | AN387, but dcuC::mini-Tn10(Camr) | 55 |
| IMW205 | MC4100, but dcuR::Kanr | 56 |
| IMW237 | MC4100, but λ[Φ(dcuB-lacZ)Hyb bla+] | 56 |
| IMW238 | MC4100, but λ[Φ(dcuB-lacZ)Hyb bla+] dcuR::Kanr | 56 |
| IMW239 | MC4100, but λ[Φ(dcuB-lacZ)Hyb bla+] citB::Spcr | 56 |
| IMW260 | MC4100, but λ[Φ(dcuB-lacZ)Hyb bla+] dcuS::Camr | 56 |
| IMW262 | MC4100, but dcuS::Camr | 56 |
| IMW280 | MC4100, but λ[Φ(dcuB-lacZ)Hyb bla+] citA::Kanr | 25 |
| IMW370 | MC4100, but λ[Φ(dcuB-lacZ)Hyb bla+] dcuB::Kanr | 22 |
| IMW548 | MC4100, but λ[Φ(citC-lacZ)Hyb bla+] | This study |
| IMW549 | MC4100, but λ[Φ(citC-lacZ)Hyb bla+] citA::Kanr | This study |
| IMW550 | MC4100, but λ[Φ(citC-lacZ)Hyb bla+] citB::Spcr | This study |
| IMW551 | MC4100, but λ[Φ(citC-lacZ)Hyb bla+] ΔdcuB | This study |
| IMW552 | MC4100, but λ[Φ(citC-lacZ)Hyb bla+] citT::Kanr | This study |
| IMW553 | MC4100, but λ[Φ(citC-lacZ)Hyb bla+] dcuS::Camr | P1 (IMW262) × IMW548; this study |
| IMW554 | MC4100, but λ[Φ(citC-lacZ)Hyb bla+] dcuR::Kanr | P1 (IMW205) × IMW548; this study |
| IMW587 | MC4100, but λ[Φ(citC-lacZ)Hyb bla+] dcuC::Camr | P1 (IMW157) × IMW548; this study |
| Plasmids | ||
| pBAD18-Kan | Expression vector; pBR322 ori, pBAD promoter (Kanr) | 12 |
| pBAD30 | Expression vector; pACYC ori, pBAD promoter (Apr) | 12 |
| pDK108 | Tar1-331-YFP expression plasmid; pBR ori, pTrc promoter, pTrc99a derivative (Apr) | 21 |
| pET28a | Expression vector; pBR322 ori, T7 promoter, His tag (Kanr) | Novagen |
| pJL29 | ′lacZ, protein fusion vector (Apr) | 27 |
| pKT25 | C-terminal T25 protein fusion plasmid, pSU40 derivative (Kanr) | BACTH manual (19) |
| pUT18C | C-terminal T18 protein fusion plasmid, pUC19 derivative (Apr) | BACTH manual (19) |
| pKT25-Zip | T25-Zip expression plasmid, pKT25 derivative (Kanr) | BACTH manual (19) |
| pUT18C-Zip | T18-Zip expression plasmid, pUT18C derivative (Apr) | BACTH manual (19) |
| pMW180 | His6-DcuR expression plasmid, pET28a derivative (Kanr) | 14 |
| pMW181 | dcuS with its native promoter in pET28a (Kanr) | 23 |
| pMW407 | DcuS-YFP expression plasmid; pBAD30 derivative (Apr) | 44 |
| pMW408 | DcuS-CFP expression plasmid; pBAD18-Kan derivative (Kanr) | 45 |
| pMW426 | T25-DcuS expression plasmid, pKT25 derivative (Kanr) | This study |
| pMW427 | T25-DcuR expression plasmid, pKT25 derivative (Kanr) | This study |
| pMW429 | T18-DcuS expression plasmid, pUT18C derivative (Apr) | This study |
| pMW442 | CitA-YFP expression plasmid; pBAD30 derivative (Apr) | 44 |
| pMW762 | CFP expression plasmid; pBAD18-Kan derivative (Kanr) | 45 |
| pMW765 | YFP expression plasmid; pBAD30 derivative (Apr) | 45 |
| pMW766 | CFP-YFP expression plasmid; pBAD18-Kan derivative (Kanr) | 45 |
| pMW807 | pJL29, but citC-lacZ | This study |
| pMW1025 | T18-CitA expression plasmid, pUT18C derivative (Apr) | This study |
| Phages | ||
| P1kc | 34 | |
| λRZ5 | λ ′bla ′lacZlacY+ | 37 |
For citC expression studies, a chromosomal citC-lacZ translational fusion was constructed via λRZ5 lysogenization (37). The citC-lacZ fusion was obtained by cloning the complete intergenic region between citA and citC (378 bp) in fusion to the lacZ gene. In the fusion, the citC promoter is located upstream of lacZ. The 693-bp PCR fragment generated with primers citA3-BamHI-for (5′-TTTCGCCGGATCCATTGCCATG-3′) and citC-rev (5′-GCGGACGGATTCACTGATAGC-3′) was cloned into the BamHI and HindIII sites of pJL29 (27), yielding pMW807. The citC-lacZ fusion was transferred into the chromosome of E. coli MC4100 with phage λRZ5 (37). Monolysogens were identified and used for further work (4, 56). The chromosomal citC-lacZ fusion was confirmed by PCR with pJL-Primer (5′-GTGCCACCTGACGTCTAAG-3′) and lacZ-Primer (5′-TGCTGCAAGGCGATTAAGTTGG-3′). The dcuB-lacZ fusion was constructed as described previously (56). The constructs were verified by DNA sequencing. The inactivated genes dcuS and dcuR each were transferred from strain IMW262 or IMW205, respectively, to strain IMW548 by P1 transduction using P1kc phage (34), resulting in IMW553 and IMW554.
β-Galactosidase activity.
The expression of the dcuB-lacZ and the citC-lacZ reporter gene fusions was determined by measuring the β-galactosidase activity (34) of exponentially growing cultures (optical density at 578 nm [OD578] of 0.5 to 0.8) at 37°C under anaerobic conditions in enriched mineral (eM9) medium supplemented with acid-hydrolyzed Casamino Acids (0.1%) and tryptophan (0.005%). Glycerol (50 mM) and dimethyl sulfoxide (20 mM) plus sodium fumarate (20 mM) or sodium citrate (20 mM) were used as the substrates as indicated. For anaerobic growth, cultures were incubated in degassed medium in rubber-stoppered infusion bottles under N2. Growth experiments were performed in three or more replicates, and the β-galactosidase activities were measured at least in triplicate for each experiment.
Gel retardation assays with His6-DcuR.
For gel retardation assays, His6-DcuR, encoded by pMW180, was overproduced and purified (16). Prior to use in gel retardation, His6-DcuR was phosphorylated by incubation with 50 mM acetyl phosphate for 60 min at 37°C (16). The DNA fragments were obtained by PCR from E. coli MG1655 DNA. The dcuB promoter fragment (603 bp) was amplified with oligonucleotide primers dcuB_for (5′-TACTCACTACTGAAACAATA-3′) and dcuB_rev (5′-TGGATAGTAAATAACATGTG-3′). The DNA fragment (652 bp) with the citA promoter was amplified with primers citAB_for (5′-AGGCGAGGTTTATCAATTCAG-3′) and citAB_rev (5′-TCTCGTTAAGCTGCAACATTG-3′). The dcuS promoter region (220 bp) was obtained with primers dcuS_for (5′-AGTAGCGCCTGATCCATGAC-3′) and dcuS_rev (5′-CGGTAGGGCAATGAATGTCTC-3′). Gel retardation assays were performed as described previously (16). The reaction mixture was applied to a nondenaturing polyacrylamide gel (5%) buffered with Tris-borate-EDTA (TBE) buffer (43). The gel subsequently was stained with SYBR green I according to the instructions of the supplier (Sigma-Aldrich) and photographed.
In vivo FRET spectroscopy.
FRET measurements were performed as described previously (45). DcuS and CitA were genetically fused to the enhanced variants of the cyan (CFP) and yellow fluorescent proteins (YFP), respectively. The sensor proteins (DcuS and CitA) in the fusions are functional in sensing and signal transduction in vivo, and the fluorescent proteins (CFP and YFP) show their normal fluorescence (44, 45). DcuS-CFP, DcuS-YFP, CitA-YFP, and CFP-YFP were expressed in E. coli JM109 by induction with l-arabinose (133 μM) to mid-exponential growth. Tar1-331-YFP (21) was expressed by induction with 50 μM isopropyl-β-d-thiogalactopyranoside (IPTG). The validation of the FRET efficiency in living cells, including flexible background subtraction, was performed as described earlier (45).
BACTH measurements.
All cultures for the BACTH tests (18, 19) were grown at 30°C and supplemented with ampicillin (100 μg/ml), kanamycin (50 μg/ml), and 500 μM IPTG. E. coli BTH101 was cotransformed with the plasmids encoding the T25- and T18-protein fusions. The bacteria were grown for 40 h on LB agar plates. For the ß-galactosidase assays, fresh cultures were inoculated from liquid overnight cultures (4%, vol/vol) and grown aerobically in LB to the mid-exponential phase (OD578 of 0.5 to 0.7).The ß-galactosidase activity was measured as described previously (34). Each cotransformation was performed twice independently, and two cultures of each transformation were assayed in quadruplicate.
RESULTS
Regulation of citC-lacZ expression by CitA/CitB in vivo.
The transcriptional regulation of the citCDEFXGT gene cluster in Escherichia coli by CitA/CitB and environmental factors was studied in vivo under physiological conditions using a chromosomal citC-lacZ reporter gene fusion (Table 2). Under anaerobic conditions, the expression of citC-lacZ was very low in the wild-type strain (IMW548) and the mutant strains citA (IMW549) and citB (IMW550) in citrate-deficient medium. The addition of citrate strongly induced the expression in the wild-type strain but not in the citA or citB deletion strains. The addition of fumarate had no positive effect on the expression of citC-lacZ. The presence of oxygen or nitrate repressed citrate induction (Table 2). Thus, the expression of the citCDEFXGT gene cluster of E. coli depends on CitA/CitB, and the system confers induction by citrate in the absence of oxygen and nitrate. The anaerobic induction by citrate was independent of the growth phase (lag, exponential, and stationary phases; data not shown).
Table 2.
Influence of CitA, CitB, effectors, and electron acceptors on the expression of a chromosomal citC-lacZ reporter gene fusion in Escherichia colia
| Effector |
citC-lacZ expression (MU) |
||
|---|---|---|---|
| IMW548 (wild type) |
IMW549 (CitA−) | IMW550 (CitB−) | |
| H2O | 5 ± 4 | 3 ± 2 | 3 ± 2 |
| Citrate | 320 ± 20 | 5 ± 2 | 8 ± 1 |
| Fumarate | 3 ± 1 | 12 ± 2 | 11 ± 3 |
| Citrate and O2 | 3 ± 1 | 3 ± 2 | 3 ± 2 |
| Citrate and NO3− | 3 ± 2 | 1 ± 0 | 1 ± 1 |
The expression of citC-lacZ was tested in strains deleted of citA or citB genes in the absence and presence of an effector (citrate or fumarate) and an electron acceptor (O2 or NO3−). The E. coli strains were grown anaerobically or aerobically (O2), as indicated, in eM9 medium containing glycerol (50 mM) and dimethyl sulfoxide (20 mM) as growth substrates, with and without sodium fumarate or sodium citrate (20 mM) as an effector and with or without sodium nitrate (20 mM) as an alternative electron acceptor. Activities (in Miller units [MU]) are shown as the averages from at least four independent measurements. The standard deviations are shown.
The citC-lacZ translational fusion contains the complete intergenic region upstream of citC (378 bp) that separates the divergently organized citCDEFXGT and citAB operons. In a citC154-lacZ fusion (where the upstream region extends only to position 154 bp upstream of the translational start codon of citC), citrate regulation and CitB-dependent expression was lost (data not shown). This is consistent with the position of the CitB-binding sites, which are located between 190 and 280 bp upstream of the translational start codon (13, 53).
A citT-lacZ fusion did not show any expression under all conditions tested (data not shown), indicating that citT encoding the citrate/succinate antiporter CitT has no promoter of its own. The cotranscription of citT with the cit operon was previously proposed for E. coli (13) and demonstrated for the citCDEFG operon of Klebsiella pneumoniae (31).
Influence of cognate transporters on the expression of citC-lacZ.
In E. coli the fumarate/succinate antiporter DcuB exerts a regulatory effect on genes regulated by DcuS/DcuR (22). For normal regulatory function, the DcuS/DcuR system requires the presence of DcuB. The deletion of the dcuB gene results in the permanent derepression of dcuB-lacZ regardless of the presence of fumarate. Similarly, the aerobic C4-dicarboxylate transporter DctA exerts a regulatory effect on DcuS function and the expression of DcuS-regulated genes under aerobic conditions (7). Here, it was tested if the related CitA/CitB two-component system requires a transporter for its normal function (Table 3). Obvious candidates for cosensing are the citrate/succinate antiporter CitT and the fumarate/succinate antiporters DcuB and DcuC. DcuC functions as a succinate efflux transporter during anaerobic growth on glucose (55), and it might export succinate during citrate fermentation when glucose is used as the cosubstrate. The dcuC gene is divergently located next to the citAB operon.
Table 3.
Influence of cognate transporters on the expression of citC-lacZ and dcuB-lacZa
| Effector | Activity (MU) |
|||||
|---|---|---|---|---|---|---|
|
citC-lacZ |
dcuB-lacZ |
|||||
| IMW548 (wild type) | IMW552 (ΔCitT−) | IMW587 (ΔDcuC−) | IMW551 (ΔDcuB−) | IMW237 (wild type) | IMW370 (ΔDcuB−) | |
| H2O | 5 ± 4 | 2 ± 1 | 0 ± 0 | 10 ± 1 | 22 ± 4 | 812 ± 32 |
| Citrate | 320 ± 20 | 364 ± 15 | 390 ± 37 | 339 ± 8 | ND | ND |
| Fumarate | 3 ± 1 | 3 ± 1 | ND | 3 ± 0 | 1,096 ± 67 | 802 ± 1 |
The expression of citC-lacZ or dcuB-lacZ was tested in strains with the citT, dcuC, or dcuB gene deleted in the absence and presence of an effector (citrate or fumarate). E. coli strains were grown anaerobically in eM9 medium containing glycerol (50 mM) and dimethyl sulfoxide (20 mM) with and without sodium fumarate (20 mM) or sodium citrate (20 mM), as indicated. Activities in Miller units (MU) are shown as the averages from at least four independent measurements. Standard deviations for the activities are shown. ND, not determined.
The effect of the transporters on citC-lacZ expression was tested in strains deleted of citT, dcuB, or dcuC. In the strains deficient in CitT, DcuC, or DcuB (Table 3), the induction of citC-LacZ depended on citrate comparably to the citrate-dependent induction of citC-lacZ in the wild-type strain. This is in contrast to the expression of dcuB-lacZ, which requires fumarate for induction in the wild-type strain, whereas the induction is fumarate independent in the dcuB mutant strain (IMW370), in agreement with earlier observations (22). Thus, lacking the requirement for a transporter as a cosensor for CitA is a clear difference to the otherwise structurally and functionally similar DcuS.
Mutual effects of CitA/CitB and DcuS/DcuR on the induction of target gene expression.
DcuS and CitA belong to the CitA family of sensor histidine kinases (24, 46). The DcuS-regulated pathway of fumarate respiration, and in particular fumarase (FumB) and fumarate reductase (FrdABCD), are also a part of the otherwise CitA-regulated citrate fermentation pathway. The expression of the frdABCD, fumB, and dcuB genes is induced under anaerobic conditions by DcuS/DcuR in the presence of C4-dicarboxylates and citrate (23, 56). For a more detailed understanding of the joint regulation and coordination of both DcuS/DcuR and CitA/CitB systems, mutual effects of DcuS and CitA on the expression of both pathways were tested. The individual effects of both regulatory systems on the expression of the DcuS/DcuR-dependent dcuB gene and of the CitA/CitB-dependent citC gene were studied using the corresponding reporter gene fusions (dcuB-lacZ and citC-lacZ) in strains lacking the dcuS, dcuR, citA, and citB genes individually. The single-gene mutants were generated by the insertion of an antibiotic resistance cassette into the intact genes by homologous recombination (56). To avoid polar effects by terminating sequences, such as that downstream of the Camr resistance cassette in the dcuS mutant strain IMW260, the sequences were removed after creating the deletion to enable the independent transcription of dcuR without polar effects. For complementation, plasmid-carried dcuS, which is known to substitute for a chromosomal dcuS deficiency, was used (45).
The contribution of CitA/CitB to the citrate regulation of the DcuS/DcuR-dependent dcuB-lacZ expression is shown in Table 4 for growth under anaerobic conditions. In strain IMW237, which is wild type for dcuS and citA, dcuB-lacZ expression was strongly induced by fumarate and by citrate, yielding about half the activity with citrate of that obtained with fumarate. In the dcuS or dcuR mutant (IMW260 or IMW238, respectively), the stimulation of dcuB-lacZ expression by either effector was completely lost. Therefore, the induction of dcuB depends on functional DcuS/DcuR. Nevertheless, the deletion of citA led to a decrease of dcuB-lacZ induction by citrate by a factor of about 1.4. A similar result was obtained when the bacteria were grown anaerobically in eM9 medium with gluconate as the carbon source and citrate as the effector, where the citrate induction of dcuB-lacZ decreased by a factor of 1.7 in the CitA deletion strain (data not shown). An even stronger decrease (factor of 3.3) was observed earlier for the citA mutant after the anaerobic growth of the bacteria in eM9 medium with glucose and citrate (25). The decrease in dcuB-lacZ expression in the CitA mutant was not observed with fumarate and is citrate specific (Table 4). In contrast, the inactivation of CitB (IMW239) resulted in a slight increase of dcuB-lacZ expression. Overall, it appears that CitA affects the maximal expression of dcuB-lacZ in an indirect manner (possibly through CitA-DcuS interaction) during anaerobic growth on citrate.
Table 4.
Influence of DcuS, DcuR, CitA, and CitB on the expression of citC-lacZ and dcuB-lacZa
| Strain | Activity (MU) |
|||
|---|---|---|---|---|
|
citC-lacZ |
dcuB-lacZ |
|||
| Fumarate | Citrate | Fumarate | Citrate | |
| IMW548/IMW237 (wild type) | 3 ± 1 | 320 ± 20 | 1,040 ± 125 | 586 ± 60 |
| IMW553/IMW260 (ΔDcuS−) | 2 ± 1 | 215 ± 24 | 23 ± 11 | 29 ± 1 |
| IMW554/IMW238 (ΔDcuR−) | 2 ± 2 | 95 ± 24 | 7 ± 1 | 5 ± 1 |
| IMW549/IMW280 (ΔCitA−) | 12 ± 2 | 5 ± 2 | 1,035 ± 75 | 434 ± 64 |
| IMW550/IMW239 (ΔCitB−) | 11 ± 3 | 8 ± 1 | 1,209 ± 181 | 628 ± 77 |
The expression of citC-lacZ or dcuB-lacZ was tested in strains deleted of dcuS, dcuR, citA, or citB in the absence and presence of an effector (fumarate or citrate). E. coli strains were grown under anaerobic conditions in eM9 medium with glycerol (50 mM) and dimethylsulfoxide (DMSO) (20 mM) plus sodium fumarate (20 mM) or sodium citrate (20 mM), as indicated. Activities in Miller units (MU) are the results from at least four independent measurements, and the standard deviations are given.
A potential contribution of DcuS/DcuR to the citrate regulation of the CitA/CitB-dependent genes was tested using the citC-lacZ fusion. Again, E. coli wild type for dcuS and citaA and a series of mutants lacking either one of the kinases (DcuS or CitA) or one of the response regulators (DcuR or CitB) were tested for the expression of a chromosomal citC-lacZ reporter gene fusion during anaerobic growth (Table 4). The citC-lacZ reporter fusion was induced by citrate but not by fumarate in the wild-type strain (IMW548). The expression was completely lost in the absence of CitA or CitB (IMW549 or IMW550, respectively). Thus, the induction of citC-lacZ depended on functional CitA/CitB. However, the inactivation of DcuS (IMW553) led to a decrease of citC-lacZ expression by a factor of 1.5, which could be due to interaction between CitA and DcuS. A 3.4-fold decrease of citC-lacZ induction was observed when DcuR was inactivated (IMW554). The impact of DcuR on citC expression might be due to a transcriptional activation of citAB by DcuR, as discussed below.
Binding of His6-DcuR to the citA promoter region.
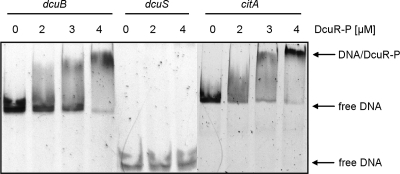
The binding of His6-DcuR to the citA promoter region was tested by gel retardation assays. Phosphorylated His6-DcuR (DcuR-P) was incubated with DNA fragments comprising the promoter regions of citA, dcuB, or dcuS, respectively. The DNA fragment of the citA promoter covered the complete citA promoter region shown in Fig. 5. In native gel electrophoresis, the fragments showed distinct bands (Fig. 1). Upon the incubation of the fragments with increasing concentrations of DcuR-P, the band of the citA promoter DNA blurred and was shifted to a position with lower mobility. The half-maximal concentration of DcuR-P required for the shift was 2 to 3 μM. The promoter region of dcuB that is known to bind DcuR-P (1, 16) was shifted in the presence of DcuR-P with a similar half-maximal concentration of 2 to 3 μM under given experimental conditions. Therefore, the response of the citA and dcuB promoter regions is similar in the mobility shift with DcuR-P. The promoter region of dcuS, on the other hand, was not shifted by DcuR-P, which is in accordance with previous suggestions that dcuSR is not autoregulated (1, 10).
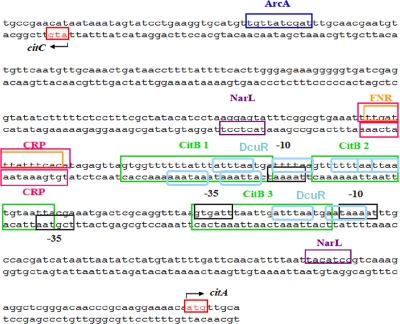
Fig 5.
Intergenic region between citC and citA. Putative binding sites of the regulators FNR, NarL, ArcA, and CRP as well as citC and citA promoter regions are based on the Prodoric Virtual Footprint software (35). The positions of the CitB binding sites are taken from Ingmer et al. (13). Conserved putative DcuR binding motifs (light blue rounded boxes), consisting of a tandem repeat of (T/A)(A/T)(T/C)(A/T)AA (1), are located upstream of citC and citA, respectively. Note that DcuR and CitB binding sites overlap. The 652-bp DNA fragment for the mobility shift assays shown in Fig. 1 covers the complete intergenic region shown in the figure and extends to the sequence at the 5′ and the 3′ ends shown here.
Fig 1.
Gel retardation of dcuB, dcuS, and citA promoter DNA by phosphorylated DcuR (DcuR-P). The promoter fragments of citA (652 bp), dcuB (603 bp), and dcuS (220 bp) were incubated in the presence of a 300-fold excess of competitor DNA with increasing concentrations of His6-DcuR-P as indicated. The protein-DNA mixture was subjected to nondenaturing DNA PAGE. The locations of the free promoter DNA and of the retarded DNA/DcuR-P complexes are indicated by arrows.
Physical interaction between CitA and DcuS: FRET and BACTH studies.
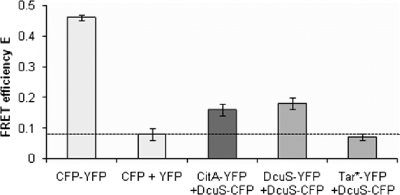
Table 4 shows the mutual effects of the DcuS/DcuR and the CitA/CitB systems on the expression of the alternate target genes, which was obviously not due to the direct regulation of the genes by the noncognate regulatory system. To further analyze these observations, various forms of interaction between DcuS/DcuR and CitA/CitB were tested. Protein-protein interaction between the related sensor histidine kinases DcuS and CitA was analyzed by in vivo fluorescence resonance energy transfer (FRET) measurements with cells coexpressing DcuS and CitA fused to CFP or YFP derivatives of the green fluorescent protein (GFP). The DcuS-CFP (or DcuS-YFP) and CitA-YFP fusion proteins are functional in sensing and signal transduction in vivo, and the fused CFP and YFP proteins show their normal fluorescence (44, 45). In vivo FRET measurements (Fig. 2) were performed as described previously (45). The FRET efficiency was calibrated by comparing the efficiency of a fusion protein with maximal FRET efficiency (CFP-YFP fusion protein) to a pair of proteins with no interaction (background FRET) in the Tar1-331-YFP/DcuS-CFP pair and cytosolic CFP/YFP. In the cytosolic CFP-YFP fusion protein encoded by pMW766 that exhibited maximal FRET efficiency, CFP and YFP are fused by a short linker of 9 amino acid residues (45). Cells containing CFP-YFP revealed an average donor fraction (fD) of 0.49 ± 0.01 with a FRET efficiency of 0.46 ± 0.01. The donor fraction represents the amount of donor in a sample containing donor and acceptor and should be approximately 0.5 to allow an accurate determination of the FRET efficiency (45). The FRET efficiency of 0.46 (Fig. 2) thus represents the maximal efficiency that can be obtained under the given experimental conditions. As a negative control, cytosolic CFP (pMW762) and cytosolic YFP (pMW765) were coexpressed. A donor fraction of 0.47 ± 0.02 and a FRET efficiency of 0.08 ± 0.02 were observed, corresponding to 17% of the FRET efficiency of the optimal FRET pair (CFP-YFP fusion). Furthermore, DcuS-CFP (pMW408) was coexpressed with the membrane protein Tar1-331-YFP (pDK108), a C-terminal truncated variant of the chemotaxis receptor Tar which is homogeneously distributed in the cell membrane (21) and does not interact with DcuS (45). A donor fraction of 0.41 ± 0.01 with a FRET efficiency of 0.07 ± 0.01 was obtained, corresponding to 15% of the activity of the optimal FRET pair. The FRET efficiencies of about 0.07 obtained in both control measurements define a background level for the FRET measurements and a threshold value for noninteracting proteins under given experimental conditions (Fig. 2, dashed line). Cells coexpressing DcuS-CFP (pMW408) and DcuS-YFP (pMW407) yielded a donor fraction of 0.41 ± 0.01 and a FRET efficiency of 0.18 ± 0.02 when grown in the absence of an effector (fumarate or citrate). This is equivalent to a relative FRET efficiency of 39%, indicating specific FRET. The FRET of this pair can be attributed to specific DcuS/DcuS interaction due to an oligomeric state of DcuS in living cells (45). When the interaction of DcuS with CitA was tested by coexpressing CitA-YFP (pMW442) and DcuS-CFP (pMW408), a FRET efficiency of 0.16 ± 0.01 at a donor fraction of 0.37 ± 0.01 was observed (n = 46), which is close to that of the DcuS-YFP/DcuS-CFP pair, clearly indicating interaction between DcuS-CFP and CitA-YFP.
Fig 2.
In vivo FRET between CitA-YFP and DcuS-CFP. Shown are the FRET efficiencies (means ± standard deviations of the means) of E. coli JM109 cells expressing CFP-YFP fusion protein (pMW766) and cells coexpressing protein pairs of either CFP (pMW762) and YFP (pMW765), CitA-YFP (pMW442) and DcuS-CFP (pMW408), DcuS-YFP (pMW407) and DcuS-CFP (pMW408), or Tar1-331-YFP (Tar*-YFP; pDK108) and DcuS-CFP (pMW408). The average transfer efficiencies of the CFP plus YFP and Tar1-331-YFP plus DcuS-CFP pairs define a background level (dashed horizontal line) for the FRET measurements to identity false-positive results due to CFP-YFP interaction. Results are from at least 6 independent test series (at least 26 data points for each test series). All samples were measured in phosphate-buffered saline (PBS) buffer (pH 7.5). The FRET efficiencies were calculated from emission spectra, which were recorded after excitation at 433 and 488 nm, respectively, and subsequently analyzed as described previously (45).
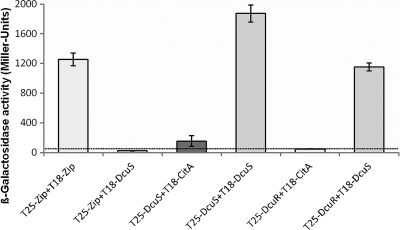
In an alternative approach, the interaction between DcuS and CitA as well as the interaction between DcuR and DcuS or CitA were tested by BACTH (19). Protein-protein interaction was determined by the reconstitution of the Bordetella pertussis adenylate cyclase subdomains T25 and T18 to the functional holoenzyme. Each subdomain is inactive, but activity is regained when each subdomain is fused to one protein of an interacting protein-protein pair. This results in cyclic AMP (cAMP) production which can be measured in an adenylate cyclase-negative E. coli strain (BTH101) with lacZ as the reporter gene.
Here, the domains T25 and T18 of the adenylate cyclase were separately fused to the N-terminal ends of the respective interaction partners (DcuS or CitA) (Fig. 3). Fusing the T25 and T18 domains to the C terminus of DcuS for homo-oligomerization assays resulted in the very high expression of the reporter activity and the inhibition of bacterial growth (not shown). Upon the coexpression of the fusion proteins T25-DcuS (pMW426) and T18-CitA (pMW1025) in BTH101, the strain showed significant and stable activity for β-galactosidase. For this reason, the N-terminal fusions were used throughout for further studies. The activity indicates that the adenylate cyclase domains reconstitute through CitA and DcuS interaction. The activities were 7-fold higher than those for the noninteracting control proteins T25-zip coexpressed with T25-DcuS. On the other hand, when the domains were fused to separate entities of DcuS, promoting the formation of T25-DcuS (pMW426) and T18-DcuS (pMW429) dimers, the β-galactosidase activities for the T25-DcuS/T18-DcuS dimers exceeded those of the T25-DcuS/T18-CitA heterodimers by a factor of 12.2. DcuS is a dimer, tetramer, or higher oligomer when functionally incorporated in the membrane (45), supplying maximal interaction for T25 and T18 in this system. The data demonstrate the interaction and hetero-oligomer formation between DcuS and CitA, but the degree of interaction between DcuS and CitA appears to be clearly lower than that between the DcuS homo-dimer or homo-oligomer. When T25 and T18 were fused to DcuR (pMW427) and DcuS (pMW429), respectively, a high degree of interaction was detected, reflecting the interaction between the sensor kinase and the cognate response regulator. In the corresponding experiment for T18-CitA (pMW1025) and T25-DcuR (pMW427), the activity (and interaction) was the same as the background level. Overall, the BACTH measurements indicate an interaction between the DcuS and CitA sensor kinases but no interaction between CitA and DcuR, which is consistent with the expression studies.
Fig 3.
Interaction of the CitA sensor kinase with the components of the DcuS/DcuR system according to studies using the bacterial two-hybrid system (BACTH). E. coli BTH101 cotransformed with plasmids in pairs encoding T18-DcuS (pMW429), T25-DcuS (pMW426), T18-CitA (pMW1025), and T25-DcuR (pMW427) were grown to an OD578 of 0.5 to 0.7 and tested for ß-galactosidase activity. The strain expressing the pair of leucine zipper proteins, T25-Zip (pKT25) plus T18-Zip (pUT18C), was used as a positive control for two cytosolically located interacting proteins. The level of 55 Miller units is the background activity obtained for noninteracting proteins like the T25-Zip and T18-DcuS pair.
DISCUSSION
Citrate fermentation genes of E. coli are regulated in a citrate-specific manner by the CitA/CitB two-component system.
CitA/CitB of E. coli (20, 52) functions in vivo, similarly to CitA/CitB of K. pneumoniae, as the transcriptional regulator of the citCDEFXGT gene cluster exerting citrate-specific transcriptional regulation. The physiological role of CitA/CitB in both bacteria thus is the regulation of citrate fermentation. Purified and phosphorylated CitB of E. coli binds to the promoters of target genes citC, citA, mdh, and exuT (52), supporting its role in the regulation of citrate fermentation and galacturonate degradation. CitA/CitB of E. coli additionally regulates iteron-containing plasmid stability and the SOS response to β-lactam antibiotics (13, 32, 33). The latter effects were observed during aerobic growth and the overexpression of CitB. To our knowledge, it is not known whether this type of regulation also occurs under anaerobic conditions. The role of CitA in the SOS response to ß-lactam antibiotics in E. coli could not be reproduced here (data not shown) or by others (29), and it might occur under specific conditions, e.g., when CitB is overproduced.
CitA functions, unlike DcuS, without using Dcu or Cit carriers as cosensors.
DcuS and CitA belong to the same family of sensor histidine kinases and have similar sequences, structures, and functions (24, 46). Both sensors respond to citrate, but the Kd of CitA is very low (0.3 μM) (20) compared to the apparent Kd of 7 mM DcuS for citrate (23). However, CitA is independent of the function of related transporters in cosensing. Thus, the deletion of the transporter CitT, DcuB, or DcuC, which are functionally (CitT, DcuB) or genetically (DcuC) linked to CitA/CitB or citrate fermentation, have no effect on CitA/CitB function. The structural basis for this difference is not clear, and there are no obvious sequence differences related to this. Using secondary transporters as cosensors appears to be a common feature of C4-dicarboxylate sensor kinases, including DcuS of E. coli (7, 22), DctB of Rhizobium meliloti (40), and DctS of Bacillus subtilis (2).
Interaction between DcuS and CitA without cross-regulation.
Figure 4 summarizes the physical and functional interaction between DcuS/DcuR and CitA/CitB systems. First, the expression studies demonstrate linear signal transfer, CitA→CitB→citC and DcuS→DcuR→dcuB, and exclude branched signal transfer between both systems in accordance with earlier suggestions (25). The linear signal transfer is confirmed by the interaction studies by BACTH that show interaction between DcuS and DcuR but the lack of interaction between CitA and DcuR. DcuS/CitA interaction was clearer in the FRET than the BACTH assay. It is well known, however, that both FRET and BACTH provide a relative measure for the interaction, which depends on various parameters, including geometric parameters of the reporters and their linking to the interacting partners.
Fig 4.
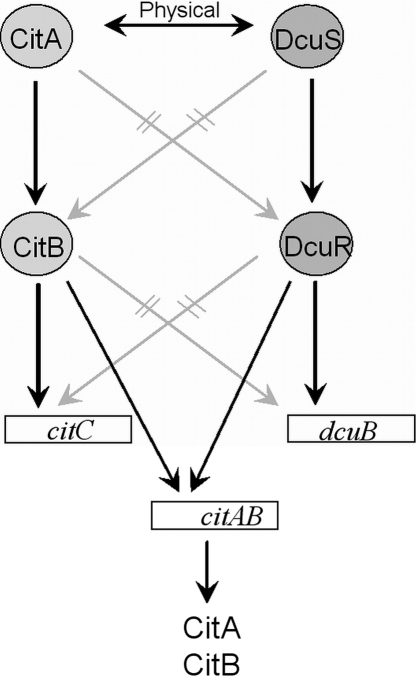
Scheme showing the interaction between components of the CitA/CitB and the DcuS/DcuR systems. Interaction (physical or functional) between individual components is indicated by arrows. Physical interaction is derived from FRET and BACTH studies between the proteins (this work) and between the response regulators and target genes (1, 16, 52). Functional interaction was derived from the requirement of the respective component for the expression of citC or dcuB (this work and Krämer et al. [25]). Interrupted lines (gray slashes) indicate that the lack of interaction has been demonstrated either by FRET and BACTH or by expression studies (this work). The physical interaction between CitA and DcuS was derived from BACTH and FRET studies (see Discussion for details).
Cross-talk has been discussed for various two-component systems of E. coli (51). However, cross-talk by phosphoryl transfer occurred mainly under artificial or in vitro conditions and was absent under physiological conditions or in vivo (9, 26). The expression and interaction studies shown here suggest that this applies even to the closely related DcuS/DcuR and CitA/CitB systems. The highly specific interaction of the sensor kinases with the respective response regulators relies on specific sites and amino acid residues (48).
There was, however, significant interaction between the DcuS and CitA sensors, as demonstrated by the FRET and BACTH studies. In addition, both DcuS and CitA are accumulated at the cell poles, which may be related to the interaction between DcuS and CitA (44). In contrast, DcuR is localized also at the cell poles (P. D. Scheu and G. Unden, unpublished data) but interacts specifically only with DcuS and not with CitA. Part of the sensor population might be present as permanent or transient DcuS/CitA heterodimers or as DcuS2/CitA2 hetero-oligomers. However, the interaction seems to have no direct functional relevance and is not involved in signal transfer between both systems (Fig. 4). This becomes evident from the finding that possible DcuS/CitA heteroforms obviously do not phosphorylate CitB and do not stimulate citC expression by fumarate in vivo. Recently, a detailed study showed that the related histidine kinases EnvZ and RstB of E. coli form only homodimers (3). This suggests that DcuS and CitA preferentially form hetero-oligomers rather than heterodimers, which would be in agreement with the lack of signal transfer between CitA and DcuS.
Nevertheless, CitA is required for the maximal induction of dcuB-lacZ by citrate (but not by fumarate) as shown here and earlier (25), and DcuS/DcuR is required for the maximal expression of citC-lacZ. The modulation of citC-lacZ expression by DcuS/DcuR can be explained by a regulation of citAB expression by DcuS/DcuR. The promoter region of citAB contains at least two DcuR consensus binding sites (Fig. 5), and the DcuR binding in the gel retardation studies (Fig. 1) suggests that the sites are functional. These findings indicate that DcuS/DcuR regulates citAB expression and thus CitA/CitB levels, resulting in the indirect regulation of CitA/CitB-dependent genes by the DcuS/DcuR system (Fig. 4). Above that, the nitrate-responsive NarX/NarL regulatory system represses the synthesis of DcuS/DcuR (10). Thus, nitrate and NarX/NarL have negative effects on the expression of the fumarate respiratory and the citrate fermentation genes by decreasing the levels of DcuS/DcuR and CitA/CitB. Nitrate and NarX/NarL might repress citC-lacZ further and directly (Table 2), similarly to the fumarate reductase genes (for a review, see reference 49). In addition, CitA/CitB positively autoregulates the expression of the citAB operon by binding to CitB sites (52) that precede the citAB promoter region (Fig. 5). A complex regulation of citC-lacZ expression thus is achieved using direct transcriptional regulation and the control of the levels of the regulators. Unfortunately, the citAB operon is not available for expression studies so far. A transcriptome analysis of all two-component regulatory system mutants of E. coli that was performed only under aerobic conditions gave, not surprisingly, no indication of changes in citAB expression in a ΔdcuSR mutant (36). Direct studies on the transcriptional regulation of citAB by DcuS/DcuR using dcuR or dcuS deletions have been complicated by the very low level of citA expression, and a chromosomal citA-lacZ fusion showed no detectable expression (data not shown). The expression of citAB also is repressed by the small regulatory RNA RybC (29), which could contribute to the low expression level. On the other hand, the dcuSR operon is not autoregulated (Fig. 1) (1, 10), and no DcuR or CitB binding sites are present in the dcuS promoter region. DcuS is constitutively expressed but induced 2-fold under anaerobic conditions by FNR (10, 42).
Cooperation of DcuS/DcuR and CitA/CitB in the regulation of fumarate respiration and citrate fermentation.
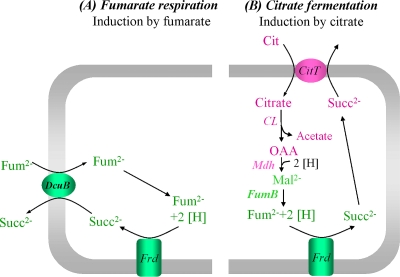
Fumarate respiration and citrate fermentation in E. coli are interconnected at various levels. Under anaerobic conditions, fumarate respiration is induced by DcuS/DcuR and fumarate or l-malate and aspartate (Fig. 6). In the presence of citrate, on the other hand, the citrate fermentation genes (citCDEFXGT and mdh), encoding citrate lyase, the citrate/succinate antiporter, and malate dehydrogenase are induced by CitA/CitB and citrate, and the additionally required fumarate respiration genes (frdABCD and fumB) are induced by DcuS/DcuR and citrate. The specific dependence of the citrate fermentation genes on CitA/CitB, and that of the fumarate respiration genes on DcuS/DcuR as well as the dual specificity of DcuS for fumarate and citrate, allows the expression of the systems according to need. In addition, DcuS/DcuR binds to the citAB promoter region and appears to regulate citAB expression. Therefore, DcuS/DcuR serves as an overriding regulatory system for establishing a hierarchical control in the anaerobic C4-dicarboxylate and citrate metabolism that helps to coordinate the regulation of both metabolic systems, since fumarate respiration is part of citrate fermentation. On the other hand, the DcuS/DcuR system appears to be independent of regulation by citrate and CitA/CitB.
Fig 6.
Induction of fumarate respiration (A) and citrate fermentation (B) enzymes and transporters by fumarate and citrate. (A) Fumarate reductase FrdABCD (Frd) and fumarate/succinate antiporter (DcuB) are induced by fumarate (or other C4-dicarboxylates or citrate) via DcuS/DcuR. (B) The citrate-induced transporter (CitT; citrate/succinate antiporter) and enzymes (CL, citrate lyase; Mdh, malate dehydrogenase) are shown in pink (induction by CitA/CitB) and green (induction by DcuS/DcuR). The enzymes and the carrier shown in pink are unique for citrate fermentation; the enzymes shown in green are used both in citrate fermentation and fumarate respiration. Abbreviations: Cit, citrate; Fum, fumarate; Mal, malate; OAA, oxaloacetate; Succ, succinate.
ACKNOWLEDGMENTS
We thank M. Wagner and C. Marutschke for help with the CitA/DcuS FRET measurements.
The support of G.U. and T.B. by a grant of the Deutsche Forschungsgemeinschaft (DFG) is gratefully acknowledged.
The supply of strains and plasmids for BACTH by G. Karimova and D. Ladant (Paris, France) is gratefully acknowledged.
Footnotes
Published ahead of print 18 November 2011
REFERENCES
- 1. Abo-Amer AE, et al. 2004. DNA interaction and phosphotransfer of the C4-dicarboxylate-responsive DcuS-DcuR two-component regulatory system from Escherichia coli. J. Bacteriol. 186:1879–1889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Asai K, Baik SH, Kasahara Y, Moriya S, Ogasawara N. 2000. Regulation of the transport system for C4-dicarboxylic acids in Bacillus subtilis. Microbiology 146:263–271 [DOI] [PubMed] [Google Scholar]
- 3. Ashenberg O, Rozen-Gagnon K, Laub MT, Keating AE. 2011. Determinants of homodimerization specificity in histidine kinases. J. Mol. Biol. 413:222–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bongaerts J, Zoske S, Weidner U, Unden G. 1995. Transcriptional regulation of the proton translocating NADH dehydrogenase genes (nuoA-N) of Escherichia coli by electron acceptors, electron donors and gene regulators. Mol. Microbiol. 16:521–534 [DOI] [PubMed] [Google Scholar]
- 5. Bott M. 1997. Anaerobic citrate metabolism and its regulation in enterobacteria. Arch. Microbiol. 167:78–88 [PubMed] [Google Scholar]
- 6. Bott M, Meyer M, Dimroth P. 1995. Regulation of anaerobic citrate metabolism in Klebsiella pneumoniae. Mol. Microbiol. 18:533–546 [DOI] [PubMed] [Google Scholar]
- 7. Davies SJ, et al. 1999. Inactivation and regulation of the aerobic C4-dicarboxylate transport (dctA) gene of Escherichia coli. J. Bacteriol. 181:5624–5635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dower WJ, Miller JF, Ragsdale CW. 1988. High efficiency transformation of Escherichia coli by high voltage electroporation. Nucleic Acids Res. 16:6127–6145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gao R, Stock AM. 2009. Biological insights from structures of two-component proteins. Annu. Rev. Microbiol. 63:133–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goh EB, et al. 2005. Hierarchical control of anaerobic gene expression in Escherichia coli K-12: the nitrate responsive NarX-NarL regulatory system represses synthesis of the fumarate-responsive DcuS-DcuR regulatory system. J. Bacteriol. 187:4890–4899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Golby P, Davies S, Kelly DJ, Guest JR, Andrews SC. 1999. Identification and characterization of a two-component sensor-kinase and response regulator system (DcuS-DcuR) controlling gene expression in response to C4-dicarboxylates in Escherichia coli. J. Bacteriol. 181:1238–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guzman LM, Belin D, Carson MJ, Beckwith J. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121–4130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ingmer H, Miller CA, Cohen SN. 1998. Destabilized inheritance of pSC101 and other Escherichia coli plasmids by DpiA, a novel two-component system regulator. Mol. Microbiol. 29:49–59 [DOI] [PubMed] [Google Scholar]
- 14. Janausch IG, Garcia-Moreno I, Unden G. 2002. Function of DcuS from Escherichia coli as a fumarate-stimulated histidine protein kinase in vitro. J. Biol. Chem. 277:39809–39814 [DOI] [PubMed] [Google Scholar]
- 15. Janausch IG, Zientz E, Tran QH, Kröger A, Unden G. 2002. C4-dicarboxylate carriers and sensors in bacteria. Biochim. Biophys. Acta 1553:39–56 [DOI] [PubMed] [Google Scholar]
- 16. Janausch IG, Garcia-Moreno I, Lehnen D, Zeuner Y, Unden G. 2004. Phosphorylation and DNA-binding of the response regulator DcuR of the fumarate two-component system DcuSR. Microbiology 150:877–883 [DOI] [PubMed] [Google Scholar]
- 17. Jensen KF. 1993. The Escherichia coli K-12 “wild-types” W3110 and MG1655 have an rph frameshift mutation that leads to pyrimidine starvation due to low pyre expression levels. J. Bacteriol. 175:3401–3407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Karimova G, Pidoux J, Ullmann A, Ladant D. 1998. A bacterial two-hybrid system based on a reconstituted signal transducing pathway. Proc. Natl. Acad. Sci. U. S. A. 95:5752–5756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Karimova G, Dautin N, Ladant D. 2005. Interaction network among Escherichia coli membrane proteins involved in cell division as revealed by bacterial two-hybrid analysis. J. Bacteriol. 187:2233–2243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaspar S, Bott M. 2002. The sensor kinase CitA (DpiB) of Escherichia coli functions as a high-affinity citrate receptor. Arch. Microbiol. 177:313–321 [DOI] [PubMed] [Google Scholar]
- 21. Kentner D, Thiem S, Hildenbeutel M, Sourjik V. 2006. Determinants of chemoreceptor cluster formation in Escherichia coli. Mol. Microbiol. 61:407–417 [DOI] [PubMed] [Google Scholar]
- 22. Kleefeld A, Ackermann B, Bauer J, Krämer J, Unden G. 2009. The fumarate/succinate antiporter DcuB of Escherichia coli is a bifunctional protein with sites for regulation of DcuS dependent gene expression. J. Biol. Chem. 284:265–275 [DOI] [PubMed] [Google Scholar]
- 23. Kneuper H, et al. 2005. The nature of the stimulus and of the fumarate binding site of the fumarate sensor DcuS of Escherichia coli. J. Biol. Chem. 280:20596–20603 [DOI] [PubMed] [Google Scholar]
- 24. Kneuper H, et al. 2010. Sensing ligands by periplasmic sensing histidine kinases with sensory PAS domains, p. 41–61 In Spiro S, Dixon R. (ed.), Sensory mechanisms in bacteria. Horizon Press, Norwich, United Kingdom [Google Scholar]
- 25. Krämer J, et al. 2007. Citrate sensing by the C4-dicarboxylate/citrate sensor kinase DcuS of Escherichia coli: binding site and conversion of DcuS to a C4-dicarboxylate- or citrate-specific sensor. J. Bacteriol. 189:4290–4298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Laub MT, Goulian M. 2007. Specificity in two-component signal transducing pathways. Annu. Rev. Genet. 41:121–145 [DOI] [PubMed] [Google Scholar]
- 27. Lucht JM, Dersch P, Kempf B, Bremer E. 1994. Interactions of the nucleoid-associated DNA-binding protein H-NS with the regulatory region of the osmotically controlled proU operon of Escherichia coli. J. Biol. Chem. 269:6578–6586 [PubMed] [Google Scholar]
- 28. Lütgens M, Gottschalk G. 1980. Why a co-substrate is required for anaerobic growth of Escherichia coli on citrate. J. Gen. Microbiol. 119:63–70 [DOI] [PubMed] [Google Scholar]
- 29. Mandin P, Gottesman S. 2009. A genetic approach for finding small RNAs regulators of genes of interest RybC as regulating the DpiA/DpiB two-component system. Mol. Microbiol. 72:551–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mascher T, Helman JD, Unden G. 2006. Stimulus perception in bacterial signal-transducing histidine kinases. Microbiol. Mol. Biol. Rev. 70:910–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Meyer M, Dimroth P, Bott M. 1997. In vitro binding of the response regulator CitB and of its carboxy-terminal domain to A+T-rich DNA target sequences in the control region of the divergent citC and citS operons of Klebsiella pneumoniae. J. Mol. Biol. 269:719–731 [DOI] [PubMed] [Google Scholar]
- 32. Miller C, Ingmer H, Thomsen LE, Skarstad K, Cohen SN. 2003. DpiA binding to the replication origin of Escherichia coli plasmids and chromosomes destabilizes plasmid inheritance and induces the bacterial SOS response. J. Bacteriol. 185:6025–6031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Miller C, et al. 2004. SOS response induction by β-lactams and bacterial defense against antibiotic lethality. Science 305:1629–1631 [DOI] [PubMed] [Google Scholar]
- 34. Miller JH. 1992. A short course in bacterial genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 35. Münch R, et al. 2005. Virtual footprint and PRODORIC: an integrative framework for regulon prediction in prokaryotes. Bioinformatics 21:4187–4189 [DOI] [PubMed] [Google Scholar]
- 36. Oshima T, Aiba HY, Masuda Y, et al. 2002. Transcriptome analysis of all two-component regulatory system mutants of Escherichia coli K-12. Mol. Microbiol. 46:281–291 [DOI] [PubMed] [Google Scholar]
- 37. Ostrow KS, Silhavy TJ, Garrett S. 1986. cis-acting sites required for osmoregulation of ompF expression in Escherichia coli K-12. J. Bacteriol. 168:1165–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pappalardo L, et al. 2003. The NMR structure of the sensory domain of the membraneous two-component fumarate sensor (histidine protein kinase) DcuS of Escherichia coli. J. Biol. Chem. 278:39185–39188 [DOI] [PubMed] [Google Scholar]
- 39. Pos KM, Dimroth P, Bott M. 1998. The Escherichia coli citrate carrier CitT: a member of a novel eubacterial transporter family related to the 2-oxoglutarate/malate translocator from spinach chloroplasts. J. Bacteriol. 180:4160–4165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Reid CJ, Poole PS. 1998. Roles of DctA and DctB in signal detection by the dicarboxylic acid transport system of Rhizobium leguminosarum. J. Bacteriol. 180:2660–2669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Reinelt S, Hoffmann E, Gerharz T, Bott M, Madden DR. 2003. The structure of the periplasmic ligand-binding domain of the sensor kinase CitA reveals the first extracellular PAS domain. J. Biol. Chem. 278:39189–39196 [DOI] [PubMed] [Google Scholar]
- 42. Salmon K, et al. 2003. Global gene expression profiling in Escherichia coli K12. The effects of oxygen availability and FNR. J. Biol. Chem. 278:29837–29855 [DOI] [PubMed] [Google Scholar]
- 43. Sambrook J, Russel DW. 2001. Molecular cloning: a laboratory manual, 3rd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 44. Scheu P, et al. 2008. Polar accumulation of the metabolic sensory histidine kinases DcuS and CitA in Escherichia coli. Microbiology 154:2463–2472 [DOI] [PubMed] [Google Scholar]
- 45. Scheu PD, et al. 2010. Oligomeric sensor kinase DcuS in the membrane of Escherichia coli and in proteoliposomes: chemical cross-linking and FRET spectroscopy. J. Bacteriol. 192:3474–3483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Scheu PD, Kim OB, Griesinger C, Unden G. 2010. Sensing by the membrane-bound sensor kinase DcuS: exogenous versus endogenous sensing of C4-dicarboxylates in bacteria. Fut. Microbiol. 5:1383–1402 [DOI] [PubMed] [Google Scholar]
- 47. Silhavy TJ, Berman ML, Enquist LW. 1984. Experiments with gene fusions. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 48. Skerker JM, et al. 2008. Rewiring the specificity of two-component signal transduction systems. Cell 133:1043–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Stewart V. 1993. Nitrate regulation of anaerobic respiratory gene expression in Escherichia coli. Mol. Microbiol. 9:425–434 [DOI] [PubMed] [Google Scholar]
- 50. West AH, Stock AM. 2001. Histidine kinases and response regulator proteins in two-component signaling systems. Trends Biochem. Sci. 26:369–376 [DOI] [PubMed] [Google Scholar]
- 51. Yamamoto K, et al. 2005. Functional characterization in vitro of all two-component signal transducing systems from Escherichia coli. J. Biol. Chem. 280:1448–1456 [DOI] [PubMed] [Google Scholar]
- 52. Yamamoto K, et al. 2008. Anaerobic regulation of citrate fermentation by CitAB in Escherichia coli. Biosci. Biotechnol. Biochem. 72:3011–3014 [DOI] [PubMed] [Google Scholar]
- 53. Yamamoto K, et al. 2009. Characterization of CitA-CitB signal transduction activating genes involved in anaerobic citrate metabolism in Escherichia coli. Biosci. Biotechnol. Biochem. 73:346–350 [DOI] [PubMed] [Google Scholar]
- 54. Yanisch-Perron C, Vieira J, Messing J. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103–119 [DOI] [PubMed] [Google Scholar]
- 55. Zientz E, Six S, Unden G. 1996. Identification of a third secondary carrier (DcuC) for anaerobic C4-dicarboxylate transport in Escherichia coli: role of the three Dcu carriers in uptake and exchange. J. Bacteriol. 178:7241–7247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zientz E, Bongaerts J, Unden G. 1998. Fumarate regulation of gene expression in Escherichia coli by the DcuSR (dcuSR genes) two-component regulatory system. J. Bacteriol. 180:5421–5425 [DOI] [PMC free article] [PubMed] [Google Scholar]