Abstract
The kinetic parameters of the release of Ca2+-dipicolinic acid (CaDPA) during germination of spore populations and multiple individual spores of Bacillus subtilis strains with major alterations in the structure of the spore peptidoglycan (PG) cortex or lacking one or both of the two redundant enzymes involved in cortex hydrolysis (cortex-lytic enzymes [CLEs]) were determined. The lack of the CLE CwlJ greatly slowed CaDPA release with a germinant receptor (GR)-dependent germinant, l-valine, or a non-GR-dependent germinant, dodecylamine. The absence of the cortex-specific PG modification muramic acid–δ-lactam also increased the time needed for full CaDPA release during germination with both types of germinants. In contrast, increased cortex PG cross-linking was associated with faster times for initiation of CaDPA release with both l-valine and dodecylamine but not with faster CaDPA release once this release had been initiated. These data suggest that the precise structure of the spore cortex plays a significant role in determining the timing and the rate of CaDPA release during B. subtilis spore germination and, further, that this effect is independent of effects of GRs.
INTRODUCTION
Spores of Bacillus species that are formed in sporulation are dormant and extremely resistant to a number of stress factors and can remain in this state for years (23, 24). A variety of factors contribute to spores' resistance and dormancy, including the proteinaceous spore coats, the spore core's low water content, and the presence of a large depot (∼25% of core dry weight) of the 1:1 chelate of pyridine-2,6-dicarboxylic acid (dipicolinic acid [DPA]) with divalent cations, predominantly Ca2+ (CaDPA). The low core water content and perhaps the stability of the CaDPA in the spore core are also likely due somehow to the thick layer of spore-specific peptidoglycan (PG), termed the cortex (14, 23, 24). This structure is just outside the spore's nascent or germ cell wall, which in turn is adjacent to the spore's inner membrane. In spores of Bacillus subtilis, the cortex exhibits several differences from growing and germ cell wall PG, including a much lower level of cross-linking between glycan strands, a large number of N-acetylmuramic acid residues with only a single l-alanine residue attached, and a high percentage (∼50%) of muramic acid residues that are present as muramic acid–δ-lactam (MAL) (14, 16). These last two modifications are not present in growing cell or germ cell wall PG.
While the dormant spore is stable for years in the absence of nutrients, various small molecules, including specific nutrients as well as nonnutrient molecules, can rapidly trigger spores' return to life in the process of germination followed by outgrowth (12, 22, 24). Nutrient germinants trigger spore germination by binding to specific nutrient germinant receptors (GRs) present in the spore's inner membrane, while nonnutrient germinants, such as alkylamines (with dodecylamine being the best studied), do not require GRs to trigger germination (19, 22). The most dramatic initial event in spore germination triggered by both types of germinants is the release of the dormant spores' large CaDPA depot. This release generally begins after a lag period of many minutes, the precise timing of which is quite variable for individual spores in populations, although total CaDPA release generally takes only a few minutes once it has begun (4, 6, 7, 13, 25, 29, 32, 33). CaDPA release is followed by degradation of the spore cortex, and with spores of Bacillus species this degradation is carried out by two cortex-lytic enzymes (CLEs), CwlJ and SleB, that are redundant in that either enzyme can catalyze cortex hydrolysis and thus completion of spore germination. These CLEs require MAL for recognition and cleavage of PG and consequently degrade only cortical PG. As expected from the relative timing of CaDPA release and cortex hydrolysis, CaDPA release can take place even if both CLEs are absent (9, 13, 20). However, the absence of CwlJ results in a large increase in the time required for complete CaDPA release during GR-dependent germination (13, 20). The precise mechanism of CaDPA release during spore germination is not known, but it may involve the multiple proteins encoded by the spoVA operon (26, 27), although how any SpoVA-dependent CaDPA channel functions and is gated is not known.
While there have been many detailed studies on the effects of GRs, SpoVA proteins, and CLEs on the kinetics of germination of B. subtilis spores (1, 4, 6, 13, 28, 29, 33), there have been no such detailed studies on the effects of variations in the cortical PG structure, in particular on the germination of individual spores. This is a gap in our knowledge about spore germination, in particular because cortex degradation is such an important part of the germination process. Indeed, variations in cortical PG structure have some significant effects on other spore properties, most notably on their resistance to wet heat and their core water content (15–18). Consequently, in this work we examined the germination of both spore populations and multiple individual spores of a number of B. subtilis strains with large variations in either cortical PG structure or CLEs, using both nutrient germinants and a nonnutrient germinant.
MATERIALS AND METHODS
B. subtilis strains used and spore preparation and purification.
The B. subtilis strains used in this work are isogenic derivatives of strain PS832, a prototrophic laboratory 168 strain: (i) PS533 (21), carrying plasmid pUB110, which encodes resistance to kanamycin (10 μg/ml); (ii) PS2066 (15, 17), with a deletion of the dacB gene, which encodes a d,d-carboxypeptidase involved in modifying cortical PG structure; (iii) PS2421 (15), with deletions in the dacB and dacF genes, both of which encode d,d-carboxypeptidases involved in modifying cortex PG structure; (iv) PS2307 (16), with a deletion of the cwlD gene, which is essential for generation of MAL in cortex PG; (v) PS2422 (18), with deletions in both the cwlD and dacB genes; (vi) FB111 (9), with a deletion of the cwlJ gene; (vii) FB112 (9), with a deletion of the sleB gene; and (viii) FB113 (9), with deletions of both the cwlJ and sleB genes. Spores of all these strains were prepared together at 37°C on 2× SG medium agar plates and were harvested, purified, and stored as described previously (8, 10). All spores used in this work were free (>98%) of sporulating cells, germinated spores, and debris, as determined by phase-contrast microscopy.
Spore germination.
Spores were germinated in either (i) 10 mM l-valine in 25 mM K-HEPES buffer (pH 7.4) at 37°C; (ii) a mixture of 10 mM l-asparagine–10 mM d-glucose–10 mM d-fructose–10 mM KCl in 25 mM KPO4 buffer (pH 7.4) (AGFK) at 37°C; or (iii) 0.8 mM dodecylamine with 25 mM K-HEPES buffer (pH 7.4) at 45°C. Spore germination with l-valine and AGFK, but not with dodecylamine, was preceded by a heat shock (70°C; 30 min) of spores in water, followed by cooling on ice for ≥15 min.
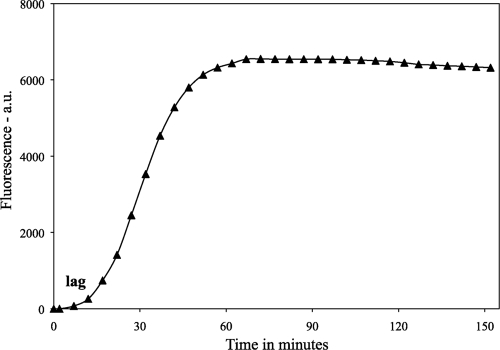
Measurement of the germination of spore populations was carried out in 200 μl of germinant solution also containing 50 μM TbCl3, and germination was monitored by Tb-DPA fluorescence in a multiwell plate reader as described previously (30, 31). Rates of germination of spore populations were obtained from the maximum slopes of plots of Tb-DPA fluorescence versus time, and the initial lag period in germination was determined as the time between the mixing of spores with germinants and the initiation of rapid CaDPA release, as measured by the point at which the extrapolated maximum slope of the Tb-DPA fluorescence curve intersected with the time axis (Fig. 1). Relative maximum rates of DPA release during germination were also corrected for slight differences in different spore preparations' DPA levels. These levels were measured in boiled extracts of spores by measuring Tb-DPA fluorescence as described previously (31). All rates of germination of spore populations were carried out at least in duplicate on two independent spore preparations.
Fig 1.
Kinetics of germination of a spore population. Spores of B. subtilis strain PS533 (wild type) were germinated with l-valine as described in Materials and Methods, and spore germination was monitored by Tb-DPA fluorescence (given in arbitrary units [a.u.]). The lag period prior to attaining the maximum germination rate is indicated. The maximum rate of germination was determined by the maximum linear slope of the Tb-DPA fluorescence increase, and the precise value for the lag time was determined by extrapolating the maximum rate of increase in the Tb-DPA fluorescence to the time axis, allowing the determination of the time between this point and the time of mixing of spores and the germinant.
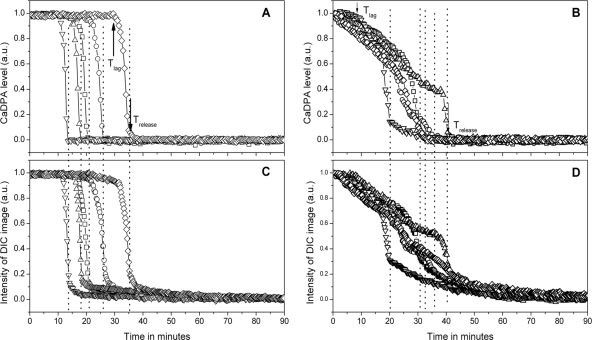
Germination of multiple individual spores was assessed simultaneously by Raman microspectroscopy and differential interference contrast (DIC) microscopy using heat-activated spores adhering to a microscope slide as described previously (5, 33). Previous analysis of the germination of multiple individual Bacillus spores has shown that CaDPA release is minimal for a highly variable lag period and then begins and ends at times defined as Tlag and Trelease, respectively. The exact time period for the release of the great majority of spore CaDPA is defined as ΔTrelease, which is Trelease − Tlag (5, 6). Initial studies of CaDPA release during spore germination used Raman microspectroscopy, generally by measuring the intensity of the most prominent CaDPA-specific Raman peak at 1,017 cm−1 in spores. However, it has been shown that the rapid fall of ∼70% in a spore's DIC image intensity parallels CaDPA release (5, 6, 33), undoubtedly because of the large change in the spore core refractive index upon CaDPA release and the concomitant uptake of water into the core. Consequently, DIC image intensity can be substituted for Raman microspectroscopy to extract values of Tlag and Trelease for large numbers of spores simultaneously. As noted above, while spores' DIC image intensity falls significantly when CaDPA is released, this accounts for only ∼70% of the loss in spores' DIC image intensity during germination. The remaining ∼30% of the decrease takes place following CaDPA release and is due to the hydrolysis of the spore cortex PG paralleled by core swelling and additional core water uptake (5, 6, 22).
RESULTS
Nutrient and nonnutrient germination of populations of spores of strains with alterations in cortex structure or degradation.
The germination of spore populations can easily be assessed by monitoring the release of spores' large CaDPA depot (30, 31). As found previously (22, 25, 31), following nutrient addition, the kinetics of CaDPA release for a spore population generally shows a period of little CaDPA release, termed the lag, followed by rapid CaDPA release. The maximal rate of CaDPA release can be determined from the slope of the rate of CaDPA release versus time (Fig. 1) (31). This analysis was then carried out on populations of spores of a number of B. subtilis strains with significant alterations in either cortex PG structure (Table 1) or cortex degradation during spore germination. Strain PS2066 lacks dacB, a low-molecular-weight penicillin-binding protein that exhibits high sequence similarity to known d,d-carboxypeptidases that modify the degree of cross-linking of glycan strands in PG. Compared to the PG of wild-type spores, cortical PG from dacB spores exhibits a 3- to 4-fold increase in the amount of cross-linking of glycan strands and ∼2-fold-decreased levels of muramic acid–l-alanine (15) (Table 1). Strain PS2422 (dacB dacF) lacks both DacB and another likely sporulation-specific d,d-carboxypeptidase, DacF, which both act to modify cortex PG structure (15). The dacB dacF spores' cortical PG has even higher levels of glycan strand cross-linking, even lower levels of muramic acid–l-alanine, and higher levels of muramic acid with tetrapeptides and tripeptides attached (15) (Table 1). Strain PS2307 (cwlD) lacks the enzyme needed for MAL formation in PG (16). Consequently, PS2307 spores' cortical PG lacks MAL and has elevated levels of muramic acid attached to either l-alanine or tri- or tetrapeptides and 2-fold-elevated levels of glycan strand cross-linking (18) (Table 1). The cortex of the cwlD spores is also not degraded during spore germination, because the specificity determinant for CLEs, MAL, is not present in these spores' PG (16, 18). Strain PS2422 (cwlD dacB) lacks both CwlD and DacB, so these spores' cortical PG lacks MAL, and this PG has low levels of muramic acid–l-alanine, elevated levels of muramic acid with tripeptides and especially tetrapeptide, which comprises ∼80% of all muramic acid residues, and the highest levels of glycan strand cross-linking (18) (Table 1). Previous work has found that the spores of these mutant strains all do germinate, and no major differences were seen other than that the cwlD and dacB cwlD spores appeared to release DPA slightly more slowly than wild-type spores (16, 18).
Table 1.
Cortex peptidoglycan structure in spores of various B. subtilis strainsa
| Strain | % of muramic acid with side chain ofb |
% glycan chain cross-linking | |||
|---|---|---|---|---|---|
| MAL | l-Ala | TetraP | TriP | ||
| Wild type | 50 | 26 | 23 | 1 | 4 |
| PS2066 (dacB) | 46 | 12 | 43 | 2 | 14 |
| PS2421 (dacB dacF) | 40 | 6 | 57 | 5 | 20 |
| PS2307 (cwlD) | 0 | 33 | 58 | 6 | 8 |
| PS2422 (cwlD dacB) | 0 | 13 | 80 | 5 | 32 |
To complement the analysis of the germination of spores with alterations in cortex PG structure, we also examined the germination of spores lacking one or both of spores' redundant CLEs, CwlJ and SleB. Previous work has found that cwlJ and cwlJ sleB spores germinate more slowly than wild-type spores with nutrient germinants and in particular take significantly longer to release CaDPA, while sleB spores germinate essentially identically to wild-type spores (7, 13, 20). However, the germination of CLE-deficient spores with the nonnutrient germinant dodecylamine has not been studied.
Analysis of the germination of populations of the spores of these various strains with either l-valine via the GerA GR or AGFK via the GerB and GerK GRs acting together revealed that spores lacking DacB or DacB and DacF germinated more rapidly than wild-type spores and had shorter lag periods prior to rapid CaDPA release (Table 2). In contrast, sleB spores had a germination rate and lag period almost identical to those of wild-type spores, while cwlJ, and cwlJ sleB spores exhibited germination rates and lag periods longer than those of wild-type spores, and cwlD, and cwlD dacB spores' germination parameters were similar to those of wild-type spores.
Table 2.
Maximum germination rates and lags for populations of spores of various B. subtilis strains germinating with nutrient germinantsa
| Strain | Maximum germination rate (lag [min]) with germinant |
|
|---|---|---|
| l-Valine | AGFK | |
| PS533 (wild type) | 100b (10) | 100c (7) |
| PS2066 (dacB) | 187 (3.5) | 200 (3.5) |
| PS2307 (cwlD) | 99 (14) | 109 (12) |
| PS2421 (dacB dacF) | 215 (2.5) | 204 (3) |
| PS2422 (cwlD dacB) | 95 (8.5) | 92 (8) |
| FB111 (cwlJ) | 51 (24) | 54 (27) |
| FB112 (sleB) | 96 (14) | 81 (6) |
| FB113 (cwlJ sleB) | 39 (28) | 49 (29) |
Maximum rates and lags of the germination of spore populations were determined as described in Materials and Methods and shown in Fig. 1; all values are ±<13%.
Value set at 100.
Value set at 100 but is actually ∼30% of that of PS533 spores germinating with l-valine.
The germination of the spores of these various strains with the nonnutrient germinant dodecylamine exhibited no major differences in lag periods (Table 3). However, loss of DacB and in particular both DacB and DacF resulted in much higher germination rates with dodecylamine, while loss of CwlJ resulted in decreased spore germination rates.
Table 3.
Maximum germination rates and lags for populations of spores of various B. subtilis strains germinating with dodecylaminea
| Strain | Maximum germination rate | Lag (min) |
|---|---|---|
| PS533 (wild type) | 100b | 14 |
| PS2066 (dacB) | 430 | 16 |
| PS2307 (cwlD) | 108 | 14 |
| PS2421 (cwlD dacB) | 355 | 16 |
| PS2422 (dacB dacF) | 1,060 | 18 |
| FB111 (cwlJ) | 36 | 15 |
| FB112 (sleB) | 85 | 13 |
| FB113 (cwlJ sleB) | 32 | 15 |
Populations of spores were germinated with dodecylamine, and maximum rates and lags were determined as described in Materials and Methods and shown in Fig. 1. All values are ±<15%.
This value was set at 100 and is actually ∼10-fold lower than the rate of PS533 spore germination with 10 mM l-valine.
Kinetic parameters of nutrient and nonnutrient germination of multiple individual spores of various strains.
Analysis of the kinetics of germination of populations of spores with alterations in cortex structure or degradation did provide some insight into the effects of such changes on kinetic parameters of germination. However, it is known that these parameters vary significantly between individual spores in populations, in particular in values for the lag period prior to initiation of rapid CaDPA release (6). Consequently, the germination of multiple individual spores of these strains with both a nutrient and a nonnutrient germinant was also measured as described in Materials and Methods and shown in Fig. 2. This analysis allowed the determination of the times for the initiation and completion of rapid CaDPA release (Tlag and Trelease, respectively), as well as the time for complete CaDPA release (ΔTrelease, calculated as Trelease − Tlag) (6, 13, 32, 33), and analysis of multiple individual spores from two independent preparations of spores of the various strains gave generally quite similar results (Tables 4 and 5). With l-valine as the germinant, the mean Tlag values for multiple individual dacB, dacB dacF, cwlD, and cwlD dacB spores were 3- to 10-fold lower than those for wild-type spores, with dacB dacF spores having the lowest Tlag values (Table 4). However, cwlJ, sleB, and cwlJ sleB spores all had Tlag values essentially identical to those of wild-type spores. Values of ΔTrelease were lowered slightly by loss of DacB or DacB and DacF, lowered more by lack of CwlD, and lowered most (∼10-fold) by loss of CwlJ, while loss of SleB alone had no effect (Table 4). The effects of CwlJ and SleB on the ΔTrelease values of individual B. subtilis spores germinating with nutrient germinants have been seen previously (7, 13, 20). However, it was also observed with wild-type spores that a high EDTA concentration lowered the ΔTrelease values of individual spores germinating with l-valine almost 3-fold (Table 4). This is consistent with a role for CaDPA released from an individual spore in triggering cortex hydrolysis by CwlJ and thus further stimulating the rate of CaDPA release from that spore, since DPA is not as effective in activating CwlJ as CaDPA (7, 11, 13, 20).
Fig 2.
Kinetics of germination of multiple individual spores of several strains. Heat-activated spores of B. subtilis strains PS533 (wild type) (A and C) and FB111 (cwlJ) (B and D) were allowed to adhere to a microscope slide and germinated with l-valine, and individual spores' CaDPA levels and DIC image intensities (in arbitrary units [a.u.]) were determined by Raman microspectroscopy and DIC microscopy as described in Materials and Methods. Data for five individual spores are shown in each panel, with each symbol representing the germination of one spore; this is the same spore in panels A and C and in panels B and D. In panels A and B, arrows indicate Tlag and Trelease for one spore, and Trelease for all spores is indicated by the dotted lines. In panels B and D, the final DIC image intensities were subtracted from the DIC image intensities at various times, and these values were then normalized to the values at 0 min.
Table 4.
Values of Tlag, Trelease, and ΔTrelease of multiple individual spores of various B. subtilis strains germinating with l-valinea
| Strain | Preparation | Mean value (min) ± standard deviation of |
No of spores |
Observation period (min) | |||
|---|---|---|---|---|---|---|---|
| Tlag | Trelease | ΔTrelease | Examined (% germinated) | Counted | |||
| 533 | Prep 1 | 25.4 ± 14.6 | 27.9 ± 14.8 | 2.6 ± 1.0 | 335 (95) | 145 | 90 |
| Prep 2 | 21.9 ± 12.0 | 24.4 ± 12.2 | 2.5 ± 0.9 | 341 (97) | 217 | 90 | |
| 533b | Prep 1 | 14.3 ± 15.8 | 21.5 ± 16.5 | 7.2 ± 4.7 | 217 (94) | 70 | 120 |
| 2066 | Prep 1 | 4.9 ± 2.7 | 10.3 ± 3.4 | 5.4 ± 1.9 | 413 (99) | 126 | 90 |
| Prep 2 | 8.5 ± 5.1 | 13.8 ± 5.6 | 5.4 ± 1.7 | 238 (100) | 116 | 90 | |
| 2421 | Prep 1 | 2.6 ± 1.0 | 7.4 ± 2.3 | 4.8 ± 2.1 | 329 (100) | 135 | 60 |
| Prep 2 | 2.7 ± 0.9 | 8.3 ± 2.7 | 5.6 ± 2.2 | 249 (100) | 123 | 60 | |
| 2307 | Prep 1 | 8.6 ± 8.9 | 23.4 ± 11.6 | 14.9 ± 8.5 | 286 (99) | 115 | 90 |
| Prep 2 | 8.7 ± 6.4 | 22.4 ± 8.8 | 13.7 ± 5.7 | 337 (99) | 105 | 90 | |
| 2422 | Prep 1 | 4.7 ± 5.1 | 15.8 ± 6.4 | 11.1 ± 4.2 | 293 (99) | 84 | 90 |
| Prep 2 | 4.9 ± 5.3 | 18.9 ± 8.1 | 14.0 ± 6.3 | 204 (97) | 134 | 90 | |
| 111 | Prep 1 | 20.8 ± 15.5 | 46.6 ± 23.1 | 25.8 ± 12.8 | 306 (96) | 101 | 120 |
| Prep 2 | 19.8 ± 12.7 | 47.4 ± 16.4 | 27.6 ± 8.8 | 252 (99) | 94 | 120 | |
| 112 | Prep 1 | 26.9 ± 16.8 | 29.6 ± 17.3 | 2.7 ± 1.1 | 233 (96) | 138 | 90 |
| Prep 2 | 29.2 ± 17.1 | 32.4 ± 17.4 | 3.1 ± 1.4 | 447 (96) | 154 | 90 | |
| 113 | Prep 1 | 19.3 ± 16.4 | 40.8 ± 21.1 | 21.5 ± 12.4 | 290 (99) | 81 | 120 |
| Prep 2 | 26.8 ± 13.4 | 50.5 ± 18.4 | 23.7 ± 13.5 | 409 (98) | 110 | 120 | |
Heat-activated spores adhering to a microscope slide were germinated with l-valine, and the kinetic parameters of spore germination were determined as described in Materials and Methods and the legend to Fig. 2.
These spores were germinated with 25 mM EDTA also present.
Table 5.
Mean values and standard deviations of Tlag, Trelease, and ΔTrelease of multiple individual spores of various B. subtilis strains germinating with dodecylaminea
| Strain | Preparation | Mean value (min) ± standard deviation of |
No. of spores |
Observation time (min) | |||
|---|---|---|---|---|---|---|---|
| Tlag | Trelease | ΔTrelease | Examined (% germinated) | Counted | |||
| 533 | Prep 1 | 80.9 ± 43.0 | 83.3 ± 43.5 | 2.3 ± 1.4 | 288 (51) | 90 | 180 |
| Prep 2 | 68.1 ± 47.5 | 69.8 ± 47.7 | 1.7 ± 0.8 | 259 (60) | 98 | 180 | |
| 2066 | Prep 1 | 28.1 ± 24.4 | 33.7 ± 24.6 | 5.6 ± 4.5 | 500 (98) | 85 | 180 |
| Prep 2 | 58.9 ± 40.0 | 65.6 ± 41.2 | 6.7 ± 4.5 | 332 (87) | 96 | 180 | |
| 2421 | Prep 1 | 5.4 ± 4.6 | 8.7 ± 5.1 | 3.4 ± 1.5 | 312 (99) | 100 | 180 |
| Prep 2 | 15.6 ± 20.5 | 20.1 ± 21.3 | 4.5 ± 3.9 | 311 (94) | 100 | 180 | |
| 2307 | Prep 1 | 50.3 ± 41.8 | 63.0 ± 42.0 | 12.7 ± 7.7 | 254 (81) | 94 | 180 |
| Prep 2 | 58.7 ± 46.3 | 71.5 ± 50.6 | 12.8 ± 7.8 | 377 (79) | 71 | 180 | |
| 2422 | Prep 1 | 16.9 ± 11.2 | 24.3 ± 11.1 | 7.4 ± 2.3 | 282 (99) | 72 | 180 |
| Prep 2 | 25.1 ± 27.1 | 33.1 ± 28.6 | 8.1 ± 4.4 | 300 (99) | 105 | 180 | |
| 111 | Prep 1 | 78.4 ± 38.0 | 97.6 ± 39.1 | 19.2 ± 11.0 | 454 (20) | 69 | 180 |
| Prep 2 | 73.8 ± 44.6 | 90.7 ± 47.0 | 16.8 ± 7.7 | 345 (47) | 70 | 180 | |
| 112 | Prep 1 | 78.8 ± 36.4 | 82.4 ± 36.4 | 3.6 ± 2.0 | 574 (26) | 75 | 180 |
| Prep 2 | 74.1 ± 45.8 | 76.5 ± 46.6 | 2.4 ± 1.7 | 374 (41) | 93 | 180 | |
| 113 | Prep 1 | 61.3 ± 38.2 | 75.6 ± 38.4 | 14.3 ± 7.6 | 505 (29) | 61 | 180 |
| Prep 2 | 62.9 ± 49.1 | 74.7 ± 53.8 | 11.8 ± 8.2 | 373 (33) | 72 | 180 | |
Spores of various strains adhering to a microscope slide were germinated with dodecylamine, and the kinetic properties of spore germination were determined as described in Materials and Methods and in the legend to Fig. 2.
Effects of cortex structure or degradation on kinetic parameters of individual spores' germination with dodecylamine (Table 5) were generally similar to those with l-valine. Loss of CwlD or CwlJ caused the largest increases in ΔTrelease, although loss of DacB or DacB plus DacF had at most a small effect on ΔTrelease. In addition, loss of DacB alone or in combination with loss of CwlD or DacF reduced mean Tlag values substantially, consistent with the faster germination of populations of spores of these strains with dodecylamine (Table 3).
DISCUSSION
The work in this communication allows a number of new conclusions about the process of B. subtilis spore germination. First, the absence of the CLE CwlJ alone resulted in a large increase in the ΔTrelease value in spore germination with the nutrient germinant l-valine. While this observation has been made previously (13, 20), we found that the lack of CwlJ also resulted in a high ΔTrelease value during germination with the nonnutrient germinant dodecylamine, and this is a new result. Since the action of dodecylamine as a germinant does not require GRs, this result suggests that CwlJ directly or indirectly affects CaDPA release specifically. This is consistent with the suggestion made previously (13, 20) that the initial release of CaDPA from a spore activates that spore's CwlJ, which is located at the outer edge of the spore cortex (2), and the resultant initiation of cortex degradation further increases the rate of CaDPA release somehow. That it is CaDPA which is most effective in activating cortex hydrolysis and thus accelerating CaDPA release is further supported by the almost 3-fold increase observed in the ΔTrelease times for individual spores during l-valine germination with EDTA also present to chelate Ca2+. While DPA and K+-DPA can also trigger spore germination by activating CwlJ, CaDPA is significantly more effective (11). Presumably the ∼3-fold increase in the ΔTrelease time for spores germinating with l-valine plus EDTA, rather than the almost 10-fold increase with CwlJ spores germinating with l-valine alone, is due to the partial stimulation of CwlJ activity by DPA alone.
The conclusion noted above strongly suggests that the precise structure of the spore cortex has a significant effect on the rate of CaDPA release during spore germination. This suggestion is further supported by the results in this work showing that the precise cortex PG structure has a significant effect on ΔTrelease. Thus, the absence of MAL in cortical PG increased the average ΔTrelease values in both l-valine and dodecylamine germination ≥5-fold. Alterations in cortex cross-linking and levels of various muramic acid side chains also increased ΔTrelease values, although not as much as the absence of MAL. These results indicate that the precise cortex structure can significantly alter rates of ΔTrelease and, further, that these effects are generally similar for both GR-dependent and GR-independent germination. Thus, these effects must be largely on CaDPA release itself, and not on the mechanism whereby GRs transmit a signal to the CaDPA release mechanism following nutrient germinant-GR binding. The major question, of course, is how cortex PG structure influences CaDPA release rates, and given the lack of precise understanding of the mechanism of CaDPA release during germination, at present we cannot answer this question. However, it appears likely that the SpoVA proteins thought to be involved in CaDPA release are distributed relatively uniformly throughout the spores' inner membrane, in contrast to GRs that are localized in one discrete inner membrane location (3). Since the likely CaDPA release channels appear to be located throughout the inner membrane and thus are surrounded by the cortex, it is certainly possible that the precise structure of the cortex and perhaps its physical effects due to contractile or other properties of the cortical PG sacculus (22, 24) would affect the ease of opening of CaDPA channels.
Another new conclusion is that cortex PG structure also has large effects on the overall rate of spore germination, as measured either with populations or with individual spores. However, the effects on individual spores were larger and easier to document. In particular, loss of DacB with or without loss of DacF, with the attendant increases in cortex glycan chain cross-linking and tetrapeptides attached to muramic acid, increased rates of germination of spore populations and resulted in large decreases in Tlag, indicative of faster germination. The absence of MAL in cortex PG also decreased Tlag, and the effects of MAL loss were increased by concomitant loss of DacB, perhaps because of the effects of the greatly increased cortex cross-linking in cwlD dacB spores. In contrast, the absence of CwlJ and/or SleB had very little effect on Tlag, and presumably wild-type, cwlJ, sleB, and cwlJ sleB spore cortical PGs all have the same structure. Strikingly, these effects were generally identical with both nutrient and nonnutrient germination. The latter finding again suggests that the effects of cortex PG structure on CaDPA release in germination are not due to some influence of cortex structure on GR activity or signaling. Rather, these results suggest that the effects of cortex structure on the germination process are largely on the CaDPA release process itself. As noted above, how such effects are achieved is not clear. However, one can imagine two opposing possibilities: (i) cortex structure exerts some influence directly on the CaDPA release mechanism, or (ii) rates of cortex degradation vary significantly depending on the precise cortex PG structure. As a corollary to the latter explanation, perhaps a more highly cross-linked cortex is more easily cleaved to trigger the initial CaDPA release, which then triggers even more rapid CaDPA release and thus ultimately more rapid completion of germination. Perhaps spores with a more highly cross-linked cortex are more “susceptible” to cortex lysis even if minimal CLEs are actually activated. One way to test if the latter explanation is correct would be to measure the rate of productive cortex hydrolysis, i.e., cortex hydrolysis that leads to core swelling and the completion of the fall in spores' DIC image intensity. However, analysis of the rate of this process in dacB dacF spores showed that the rate of loss of the final ∼30% of these spores' DIC image intensity during l-valine germination, i.e., the change in DIC image intensity due to cortex hydrolysis and core swelling, was essentially identical to that of wild-type spores (data not shown). In addition, the effects of changes in cortex PG structure on Tlag values were also seen with both cwlD and cwlD dacB spores, in which the cortex is not degraded by either CwlJ or SleB, due to the absence of MAL. Consequently, we currently favor a model in which the structure of the cortex PG exerts some direct or indirect effect on the CaDPA release mechanisms, such that higher degrees of cortex cross-linking result in a CaDPA release process that has a lower threshold for its activation in germination. However, how this is achieved is a matter for future investigation.
ACKNOWLEDGMENTS
This work was supported by a Multi-University Research Initiative award through the U.S. Army Research Laboratory and the U.S. Army Research Office under contract number W911NF-09-1-0286.
Footnotes
Published ahead of print 28 November 2011
REFERENCES
- 1. Atluri S, Ragkousi K, Cortezzo DE, Setlow P. 2006. Cooperativity between different nutrient receptors in germination of spores of Bacillus subtilis and reduction of this co-operativity by alterations in the GerB receptor. J. Bacteriol. 188:28–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bagyan I, Setlow P. 2002. Localization of the cortex lytic enzyme CwlJ in spores of Bacillus subtilis. J. Bacteriol. 184:1289–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Griffiths KK, Zhang J, Cowan AE, Yu J, Setlow P. 2011. Germination proteins in the inner membrane of dormant Bacillus subtilis spores colocalize in a discrete cluster. Mol. Microbiol. 81:1061–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kong L, Zhang P, Setlow P, Li Y-Q. 2011. Rapid confocal Raman imaging using a synchro multifoci-scan scheme for dynamic monitoring of single living cells. Appl. Phys. Lett. 98:213703 [Google Scholar]
- 5. Kong L, Zhang P, Setlow P, Li Y-Q. 2011. Multifocus confocal Raman microspectroscopy for rapid single-particle analysis. J. Biomed. Opt. Lett. 16:120503.1–120503.3 [DOI] [PubMed] [Google Scholar]
- 6. Kong L, et al. 2011. Phase contrast microscopy, fluorescence microscopy, Raman spectroscopy and optical tweezers to characterize the germination of individual bacterial spores. Nat. Protoc. 6:625–639 [DOI] [PubMed] [Google Scholar]
- 7. Kong L, Zhang P, Yu J, Setlow P, Li Y-Q. 2010. Monitoring the kinetics of uptake of a nucleic acid dye during the germination of single spores of Bacillus species. Anal. Chem. 82:8717–8724 [DOI] [PubMed] [Google Scholar]
- 8. Nicholson WL, Setlow P. 1990. Sporulation, germination and outgrowth, p. 391–450 In Harwood CR, Cutting SM. (ed.), Molecular biological methods for Bacillus. John Wiley and Sons, Chichester, United Kingdom [Google Scholar]
- 9. Paidhungat M, Ragkousi K, Setlow P. 2001. Genetic requirements for induction of germination of spores of Bacillus subtilis by Ca2+-dipicolinate. J. Bacteriol. 183:4886–4893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Paidhungat M, Setlow B, Driks A, Setlow P. 2000. Characterization of spores of Bacillus subtilis which lack dipicolinic acid. J. Bacteriol. 182:5505–5512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Paidhungat M, Setlow P. 2000. Role of Ger proteins in nutrient and nonnutrient triggering of spore germination in Bacillus subtilis. J. Bacteriol. 182:2513–2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Paredes-Sabja D, Setlow P, Sarker MR. 2011. Germination of spores of Bacillales and Clostridiales species: mechanisms and proteins involved. Trends Microbiol. 19:85–94 [DOI] [PubMed] [Google Scholar]
- 13. Peng L, Chen D, Setlow P, Li Y-Q. 2009. Elastic and inelastic light scattering from single bacterial spores in an optical trap allows monitoring of spore germination dynamics. Anal. Chem. 81:4035–4042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Popham DL. 2002. Specialized peptidoglycan of the bacterial endospore: the inner wall of the lockbox. Cell. Mol. Life Sci. 59:426–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Popham DL, Gilmore ME, Setlow P. 1999. Analysis of the roles of low-molecular weight penicillin-binding proteins in spore peptidoglycan synthesis and spore properties in Bacillus subtilis. J. Bacteriol. 181:126–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Popham DL, Helin J, Costello CE, Setlow P. 1996. Muramic lactam in peptidoglycan of Bacillus subtilis spores is required for spore outgrowth but not for spore dehydration or heat resistance. Proc. Natl. Acad. Sci. U. S. A. 93:15405–15410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Popham DL, Illades-Aguiar B, Setlow P. 1995. The Bacillus subtilis dacB gene, encoding penicillin-binding protein 5*, is part of a three gene operon required for proper spore cortex synthesis and spore core dehydration. J. Bacteriol. 177:4721–4729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Popham DL, Meador-Pardon J, Costello CE, Setlow P. 1999. Spore peptidoglycan structure in a cwlD dacB double mutant of Bacillus subtilis. J. Bacteriol. 181:6205–6209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Setlow B, Cowan AE, Setlow P. 2003. Germination of spores of Bacillus subtilis with dodecylamine. J. Appl. Microbiol. 95:637–648 [DOI] [PubMed] [Google Scholar]
- 20. Setlow B, et al. 2009. Characterization of the germination of Bacillus megaterium spores lacking enzymes that degrade the spore cortex. J. Appl. Microbiol. 107:318–328 [DOI] [PubMed] [Google Scholar]
- 21. Setlow B, Setlow P. 1996. Role of DNA repair in Bacillus subtilis spore resistance. J. Bacteriol. 178:3486–3495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Setlow P. 2003. Spore germination. Curr. Opin. Microbiol. 6:550–556 [DOI] [PubMed] [Google Scholar]
- 23. Setlow P. 2006. Spores of Bacillus subtilis: their resistance to radiation, heat and chemicals. J. Appl. Microbiol. 101:514–525. [DOI] [PubMed] [Google Scholar]
- 24. Setlow P, Johnson EA. Spores and their significance. In Doyle MP, Buchanan R. (ed.), Food microbiology, fundamentals and frontiers, 4th ed., in press ASM Press, Washington, DC. [Google Scholar]
- 25. Setlow P, Liu J, Faeder JR. Heterogeneity in bacterial spore populations. In Abel-Santos E. (ed.), Bacterial spores: current research and applications, in press Horizon Scientific Press, Norwich, United Kingdom [Google Scholar]
- 26. Tovar-Rojo F, Chander M, Setlow B, Setlow P. 2002. The products of the spoVA operon are involved in dipicolinic acid uptake into developing spores of Bacillus subtilis. J. Bacteriol. 184:584–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vepachedu VR, Setlow P. 2007. Role of SpoVA proteins in the release of dipicolinic acid during germination of Bacillus subtilis spores triggered by dodecylamine or lysozyme. J. Bacteriol. 189:1565–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang G, Yi X, Li Y-Q, Setlow P. 2011. Germination of individual Bacillus subtilis spores with alterations in the GerD and SpoVA proteins. J. Bacteriol. 193:2301–2311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang G, Zhang P, Setlow P, Li YQ. 2011. Kinetics of germination of wet heat-treated individual spores of Bacillus species as followed by Raman spectroscopy and differential interference contrast microscopy. Appl. Environ. Microbiol. 77:3368–3379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yi X, Bond C, Sarker MR, Setlow P. 2011. Multivalent cations including terbium (Tb3+) can efficiently inhibit the germination of coat-deficient bacterial spores. Appl. Environ. Microbiol. 77:5536–5539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yi X, Setlow P. 2010. Studies of the commitment step in the germination of spores of Bacillus species. J. Bacteriol. 192:3424–3433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang P, Garner W, Yi X, Yu J, Li Y-Q, Setlow P. 2010. Factors affecting the variability in the time between addition of nutrient germinants and rapid DPA release during germination of spores of Bacillus species. J. Bacteriol. 192:3608–3619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang P, Kong L, Wang G, Setlow P, Li Y-Q. 2010. Combination of Raman tweezers and quantitative differential interference microscopy for measurement of dynamics and heterogeneity during the germination of individual bacterial spores. J. Biomed. Opt. 15:056010. [DOI] [PubMed] [Google Scholar]