Abstract
Infections caused by biofilms are abundant and highly persistent, displaying phenotypic resistance to high concentrations of antimicrobials and modulating host immune systems. Tuberculosis (TB), caused by Mycobacterium tuberculosis, shares these qualities with biofilm infections. To identify genetic determinants of biofilm formation in M. tuberculosis, we performed a small-scale transposon screen using an in vitro pellicle biofilm assay. We identified five M. tuberculosis mutants that were reproducibly attenuated for biofilm production relative to that of the parent strain H37Rv. One of the most attenuated mutants is interrupted in pks1, a polyketide synthase gene. When fused with pks15, as in some M. tuberculosis isolates, pks1 contributes to synthesis of the immunomodulatory phenolic glycolipids (PGLs). However, in strains such as H37Rv with split pks15 and pks1 loci, PGL is not produced and pks1 has no previously defined role. We showed that pks1 complementation restores biofilm production independently of the known role of pks1 in PGL synthesis. We also assessed the relationship among biofilm formation, the pks15/1 genotype, and M. tuberculosis phylogeography. A global survey of M. tuberculosis clinical isolates revealed surprising sequence variability in the pks15/1 locus and substantial variation in biofilm phenotypes. Our studies identify novel M. tuberculosis genes that contribute to biofilm production, including pks1. In addition, we find that the ability to make pellicle biofilms is common among M. tuberculosis isolates from throughout the world, suggesting that this trait is relevant to TB propagation or persistence.
INTRODUCTION
Microbial species in nature often grow as self-assembled, organized, multicellular, and matrix-encapsulated structures of diverse architecture that range from surface-attached colonies to pellicles at the air-fluid interface. These structures are collectively called biofilms (30). Microbial biofilms are formed through genetically controlled developmental stages that include attachment at the solid or liquid surface and synthesis of extracellular polymeric substances (EPS) (10, 15, 31). Biofilms have high structural integrity, and their constituent microbes acquire a unique stress-tolerant phenotype, perhaps by growing in heterogeneous microenvironments within the biofilm that are absent from their single-cell planktonic state (12, 28). Up to 80% of bacterial infections are caused by biofilms, including conditions such as gingivitis, infectious kidney stones, bacterial endocarditis, and inner ear infections, along with many hospital-acquired infections from catheters and ports (8, 31). Biofilm infections are highly persistent, display phenotypic resistance to antimicrobials, and show a propensity to modulate the host immune system (10, 11, 23, 31).
Mycobacterium tuberculosis shares several characteristics with organisms that produce biofilms during infection. For example, infections caused by M. tuberculosis are highly persistent, display phenotypic resistance to antimicrobials, and show a propensity to modulate the host immune system (1, 2, 17, 34, 35). In addition, M. tuberculosis has microbiological properties reminiscent of biofilms. It is well known for “clumping” or “cording” in detergent-free broth culture, and in certain conditions, it forms pellicles on the air-medium interface (18, 19, 22, 28). Formation of these structures requires specific genetic inputs and follows a distinct developmental pattern that is not associated with single-cell planktonic growth (27). Furthermore, these cells secrete a lipid-rich extracellular matrix, producing structures with substantial tensile integrity and a highly drug-tolerant phenotype (28, 26). Recent publications refer to these structures as biofilms (26–28).
Evidence suggests these structures may be relevant to tuberculosis (TB) disease. The ability to form cords in vitro has long been associated with M. tuberculosis virulence in animal models (24), and cords have been visualized in vivo during Mycobacterium marinum infection of zebrafish (5, 39). Such structures could potentially be formed at the air-fluid interface inside cavities of human granulomas (19), a site of localized infection that also provides surfaces for possible attachment.
The M. tuberculosis protein polyketide synthase Pks15/1 is necessary for the production of phenolic glycolipid (PGL), an immunomodulatory lipid virulence factor found in some M. tuberculosis isolates, including a subset of W Beijing strains (7). Pks15/1 is a multidomain, modular polyketide synthase in which the Pks1 and Pks15 units occur as one or two open reading frames (ORFs), depending on the mycobacterial strain. The five domains of Pks1, annotated as acyl transferase (AT), dehydrogenase (DH), enoyl reductase (ER), ketoreductase (KR), and acyl carrier protein (ACP), and the single domain of Pks15, annotated as a keto-acyl synthase (KS), catalyze the elongation of p-hydroxybenzoic acid with malonyl coenzyme A (CoA) units to form p-hydroxyphenylalkanoic acid intermediate of the PGL backbone (4). However, in many M. tuberculosis strains—including H37Rv, the background strain of these studies—pks15/1 is split into two ORFs, pks15 and pks1, due to a frameshift at base pair 1461, causing a nonsense mutation (7). Strains like H37Rv with separate pks15 and pks1 loci do not make PGL (7, 33), suggesting that all 6 domains function together in production of this lipid. However, retention of pks1 as a distinct open reading frame suggests that the remaining five domains of Pks1 could have some distinct but unknown function.
Here we report results from a small-scale transposon screen for mutants deficient in forming pellicle biofilms. We identified five M. tuberculosis mutants with reproducibly reduced pellicle production relative to that of the parent strain H37Rv. One of the most attenuated mutants was interrupted in pks1. We show that pks1 complementation restores biofilm production and that the effect is independent of the known role of pks1 in PGL synthesis. Further, analysis of the pks1 locus and production of extracellular material in M. tuberculosis clinical isolates revealed substantial variability. Our studies identify novel M. tuberculosis genes that contribute to biofilm production, including pks1. In addition, we find that the ability to make these structures is common among M. tuberculosis isolates from throughout the world, suggesting that this trait is relevant to TB propagation or persistence.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
H37Rv (ATCC 27294) was utilized as the wild-type strain for these studies. Planktonic cultures were grown in Middlebrook 7H9 medium, 0.2% glycerol, and 0.05% Tween 80, supplemented with albumin, dextrose, and catalase (Becton, Dickinson, and Company). Liquid cultures were aerated by shaking at 37°C. When needed, cells were plated on Middlebrook 7H10 medium supplemented with oleic acid, albumin, dextrose, and catalase (Becton, Dickinson, and Company). Kanamycin (30 μg/ml) or hygromycin (50 μg/ml) was used as needed. All M. tuberculosis cultures were handled within a biosafety level 3 (BL3) laboratory. Cloning was performed in Escherichia coli DH5α, and isolates were selected on LB plates with hygromycin (200 μg/ml).
Pellicle biofilm growth conditions.
M. tuberculosis biofilms were generated by modifying published protocols (28) in either 96-well or 12-well polystyrene plates. Cells from a 7H9 medium-aerated culture (optical density at 600 nm [OD600], ∼1) were diluted 1:100 (vol/vol) in Sauton's medium without detergent and added to each well. The plates were wrapped twice in Parafilm and incubated at 37°C for 5 weeks without shaking.
Transposon mutagenesis.
Mutagenesis was conducted as described previously (36). In brief, H37Rv cells were grown to an OD600 of 1, washed twice, concentrated 10 times, and resuspended in 1 ml MP (50 mM Tris-HCl [pH 7.6], 150 mM NaCl, 2 mM CaCl2, 10 mM MgCl2) buffer. Approximately 1010 MycoMarT7 phagemids were added to ∼109 cells and incubated for 3 h at 37°C. The suspension was plated on 7H10 medium with kanamycin. Plates were incubated at 37°C for 18 days and then picked into 7H9 medium with kanamycin in 96-well plates. Plates were stored at −80°C.
Pellicle biofilm screen.
Members of a transposon mutant library were picked into 96-well plates with 7H9 medium containing kanamycin. Plates were incubated for 1 week at 37°C to induce replication. Biofilms were seeded by adding 2 μl of growing transposon mutants to 198 μl of Sauton's medium without detergent in new 96-well plates. Plates were sealed 2 times with Parafilm and incubated at 37°C for 5 weeks. The wells were visually inspected; pictures were taken weekly to document the progression of biofilm formation.
Mutants with defects in biofilm formation were picked and grown in 7H9 medium to an OD600 of 1 for retesting. These mutants were diluted 1:100 in Sauton's medium without detergent in 12-well plates. Plates were sealed twice with Parafilm and incubated at 37°C. The isolates were then assessed visually for their ability to form biofilms after 5 weeks of culture. Mutants with obvious biofilm deficiencies in 2 or more of 3 wells were assayed further by crystal violet staining (see below). Mutants selected as consistently reduced in biofilm formation were tested in at least four independent biological assays.
Crystal violet staining of extracellular material.
Crystal violet staining was carried out by modifying methods previously described (3). The medium was removed from wells by pipetting underneath the biofilm at the interface. Biofilms were dried in a biosafety cabinet and incubated with 500 μl of 1% crystal violet for 10 min. Wells were washed three times with water and dried again. One milliliter of 95% ethanol was added to each well for 10 min. Then 3-fold serial dilutions were read at A600 on a spectrophotometer in a 96-well plate.
Identification of transposon insertion sites.
Planktonic cultures of each mutant were boiled at 105°C for 20 min under mineral oil and then removed from the BL3 laboratory. These samples were pelleted at the highest speed for 5 min in a tabletop microcentrifuge, and the supernatant was collected and used as a PCR template. We used an arbitrary PCR strategy (14). Briefly, primers K2 (GGCCAGCGAGCGAACGAGACNNNNGTTGC) and Tnout1.3 (CCCGAAAAGTGCCACCTAAATTGTAAGCG) were used in the first round of PCR, with 5 cycles of 30 s at 95°, 30 s at 30°C, and 1 min 30 s at 72°C. The next 25 cycles had an annealing temperature of 38°C instead of 30°C. This first PCR product was then diluted 1:10, and a nested primer pair, K3 (GGCCAGCGAGCGAACGAGAC) and K148 (CGCCTTCTTGACGAGTTCTTCTGA), was used in the second round of PCR, with 30 cycles of 30 s at 95°C, 30 s at 56°C, and 1 min 30 s at 72°C. DNA sequencing was then performed using K148 as the primer. Loci interrupted by the transposon were identified by using BLAST analysis software.
Genetic complementation.
Primers used to amplify sequences for complements were specific for ∼10 bp upstream and downstream of the target sequence, with HindIII sequences on the 5′ end of each primer. The PCR products and the vector pJC49 were cut with HindIII, gel extracted or PCR purified, and ligated together. The ligation reaction was transformed into E. coli DH5α, and isolates were selected on LB plates with hygromycin (200 μg/ml). Plasmid DNA was isolated using a miniprep kit (Qiagen) and then electroporated into glycerol-washed H37Rv. Transformants were selected on hygromycin-containing 7H10 medium plates (50 μg/ml) and incubated at 37°C for 3 weeks.
LC-MS analysis of clinical isolate lipid extracts.
Liquid chromatography-mass spectrometry (LC-MS) analyses were carried out using an Agilent Technologies 6520 Accurate-Mass Q-TOF and a 1200 Series high-performance liquid chromatography (HPLC) system with a Varian Monochrome diol column (3 mm by 150 mm by 2 mm) as previously described (22a). Briefly, 50 μg of lipid extract was resuspended at 0.5 mg/ml in solvent A (hexane/isopropanol ratio of 70:30 [vol/vol], 0.02% [mass/vol] formic acid, 0.01% [mass/vol] ammonium hydroxide). Ten μg was injected, and the column was eluted at 0.15 ml/min with a binary gradient of 0% to 100% solvent B (isopropanol/methanol ratio of 70:30 [vol/vol], 0.02% [mass/vol] formic acid, 0.01% [mass/vol] ammonium hydroxide), corresponding to the following time periods: 0 to 10 min, 0% B; 17 to 22 min, 50% B; 30 to 35 min, 100% B; 40 to 44 min, 0% B, followed by an additional 6 min of 0% B postrun. PGLs were detected at 4 min.
RESULTS
Twelve M. tuberculosis mutants attenuated in pellicle biofilm formation in vitro.
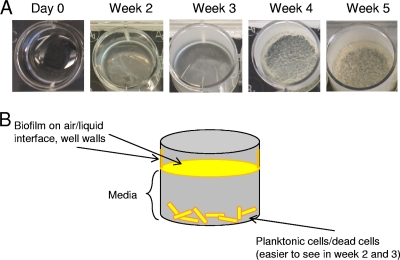
We devised a genetic screen to begin identifying genes with roles in M. tuberculosis biofilm formation. Initially, we assayed 352 randomly selected transposon mutants by seeding them in 96-well polystyrene plates in Sauton's medium without detergent. Biofilm formation and progression were visually inspected weekly for 5 weeks. During weeks 1 and 2, H37Rv forms thin, loosely associated aggregations of cells at the bottom of the well. During weeks 2 and 3, however, a thin pellicle comprised of both live cells and extracellular material forms on the air-surface interface. Throughout weeks 4 and 5, the pellicle at the interface thickens, developing a thick mat of cording and reticulations. In addition, extracellular material from the M. tuberculosis biofilms adheres to and climbs up the walls of the well during weeks 2 through 5 (Fig. 1A and B).
Fig 1.
M. tuberculosis biofilm formation. (A) Time course of H37Rv biofilm formation. (B) Schematic of mature M. tuberculosis biofilm.
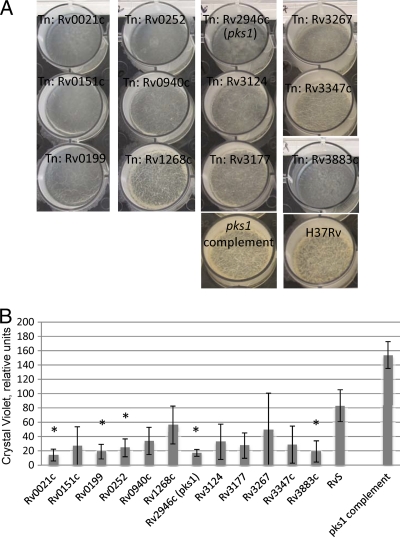
From the initial screen, 39 mutants with apparent biofilm defects were selected for further study. These mutants were tested in an inoculum-controlled, 12-well plate model that more quantitatively evaluates biofilm formation. All mutants produced at least some extracellular material in the secondary assay, but the structures produced by 12 mutants were incompletely formed. They lacked the thickness and reticulations of wild-type biofilms, indicating defects in biofilm maturation (Fig. 2A). Due to the high tensile strength and structural integrity of the pellicles, we were unable to disperse cells well and could not reliably determine the numbers of live bacteria within these structures. However, these mutants displayed wild-type growth rates in liquid broth and wild-type growth/colony morphologies on solid medium (data not shown), suggesting that the lack of biofilm maturation is not due to inherently poor growth. The large number of attenuated mutants we obtained from a small-scale genetic screen suggests that biofilm formation in M. tuberculosis is a complex, multigenic process, as has been shown in other bacteria (15).
Fig 2.
M. tuberculosis transposon mutants are deficient in biofilm formation after 5 weeks. (A) M. tuberculosis biofilms in 5-week assay. (B) Crystal violet quantification of biofilm mass in relative units. Values are the means of the results of three biological replicates. Identically treated planktonic M. tuberculosis cells were used to define the background in this assay as ∼0.2 relative units. An asterisk indicates a P value of <0.05 in Student's paired t test.
Assays to quantitate the biomass present in these structures using crystal violet (3, 9, 37) corroborated our visual observations. All 12 isolated mutants showed defects in biofilm formation, averaging between 17 and 41% of the biofilm mass made by the parent strain H37Rv (Fig. 2B). However, only five mutants met statistical criteria for attenuation (P < 0.05). These mutants, marked with asterisks in Fig. 2B and Table 1, produced 17 to 29% of the biofilm made by wild-type H37Rv.
Table 1.
Genes interrupted in transposon mutants with biofilm maturation deficiency
| Rv no.a | Gene name | Description | Functional category | Tn insertion (bp)b |
|---|---|---|---|---|
| Rv0021c* | Conserved hypothetical protein (possible 2-nitropropane dioxygenase) | Conserved hypotheticals | 147 | |
| Rv0151c | pe1 | PE family protein | PE/PPE | 837 |
| Rv0199* | Probable conserved membrane protein | Cell wall and cell processes | 126 | |
| Rv0252* | nirB | Probable nitrite reductase [NAD(P)H] large subunit (Fad flavoprotein) | Intermediary metabolism and respiration | 46 |
| Rv0940c | Possible oxidoreductase | Intermediary metabolism and respiration | 45 | |
| Rv1268c | Hypothetical protein | Conserved hypotheticals | 157 | |
| Rv2946c* | pks1 | Probable polyketide synthase | Lipid metabolism | 2,343 |
| Rv3124 | Probable transcriptional regulatory protein | Regulatory proteins | 31 | |
| Rv3177 | Possible peroxidase (nonheme peroxidase) | Virulence, detoxification, adaptation | 783 | |
| Rv3267 | Conserved hypothetical protein (CpsA-related protein) | Conserved hypotheticals | 1,497 | |
| Rv3347c | ppe55 | PPE family protein | PE/PPE | 5,354 |
| Rv3883c* | mycP1 | Membrane-anchored mycosin (serine protease) | Intermediary metabolism and respiration | Not determined |
*, statistically significant attenuation in M. tuberculosis biofilm assay (P < 0.5, Student's paired t test).
Tn insertion sites are reported relative to the first base of the coding sequence for each ORF.
Identification of genes contributing to M. tuberculosis pellicle biofilm formation and complementation.
To identify the loci interrupted in mutants with decreased biofilm formation, we used a modified nested PCR to amplify the DNA sequences flanking the transposon. BLAST searches of the sequenced products revealed the identities of the interrupted loci. The 12 disrupted genes map to different functional categories, including cell wall processes, intermediary metabolism, and regulatory proteins (Table 1). The five mutants with statistically significant attenuation were interrupted in the following genes: Rv0021c, a possible 2-nitropropane dioxygenase; Rv0199, a conserved hypothetical ORF with unknown function; Rv0252 (nirB), a nitrogen reductase; Rv2946c (pks1), a polyketide synthase; and Rv3883c (mycP1), a serine protease (Table 1). Biofilm formation of three mutants, Rv0252 (nirB), Rv2946c (pks1), and Rv3883c (mycP1), was able to be restored when a wild-type copy of the gene was integrated at a heterologous site in the M. tuberculosis genome (Fig. 2 and data not shown). Despite several cloning attempts, we were unable to generate complementing plasmids for either the Rv0021c or Rv0199 mutant.
pks1 and biofilm formation.
The pks15/1 locus is known to be required for production of the immunomodulatory, virulence-associated compound PGL (7, 33). The precise mechanism of PGL synthesis is not fully known, but homology analyses suggest that all six functional domains present in the two ORFs of the pks15/1 locus work together to make the phenol-phthiocerol core of PGL. However, in contrast to the extensively studied, PGL-replete HN878 and other W Beijing strains, the reference strain H37Rv is among those with pks15 and pks1 split into separate loci. Strains with separate pks15 and pks1 loci are incapable of producing PGL (7, 33), leaving it unclear if these enzymes retained any residual activity. No function has yet been ascribed to Pks1 acting alone in these strains.
As the pks1 mutant was among our most attenuated for biofilm formation, we chose to study it in more detail. Biofilms made by the pks1 mutant spanned across the well but did not thicken or develop cords and reticulations characteristic of mature wild-type biofilms after 5 weeks (Fig. 2A). The biofilm phenotype of the mutant was complemented by expressing pks1 at a heterologous site in the genome. The complement produced more biofilm than the wild type (Fig. 2A and B), demonstrating even more cording than the wild type after 5 weeks of growth. These findings indicate that Pks1 contributes to biofilm maturation. In addition, expression of pks1 alone, and not intact pks15/1, is sufficient to complement the attenuation in biofilm formation in this strain.
Clinical strains with distinct pks15 and pks1 loci make pellicle biofilms in vitro.
Having observed the biofilm attenuation of the pks1 mutant in H37Rv, we sought to determine if the pks15/1 genetic structure of M. tuberculosis clinical isolates also affected their ability to form biofilms. The TB Database (http://tbdb.org) currently has information available for 37 M. tuberculosis strains, most of which are clinically derived (32). We seeded 15 clinical strains of various pks15/1 organizations in our biofilm assay. We included strains from all four M. tuberculosis lineages previously described (6, 16).
The clinical isolates produced a range of biofilm phenotypes (Fig. 3). At least one strain from each M. tuberculosis lineage demonstrated the ability to form biofilms in this assay, suggesting that biofilm formation is a general characteristic of M. tuberculosis and not specific to particular lineages. H37Rv made the thickest, most mature biofilms, while some strains failed to produce biofilm at all. Some strains also grew slowly in planktonic culture (Table 2), possibly explaining their reduced biofilm formation over 5 weeks. However, 12 clinical strains grew as well as or faster than H37Rv in liquid culture yet were attenuated for biofilm formation after 5 weeks. Three of these isolates with planktonic growth rates comparable to that of H37Rv (00_1695, M4100A, and T85) failed to produce any biofilm at all in 5 weeks, suggesting either that biofilm formation is not a requirement for virulence or that these strains require different conditions for biofilm formation. Interestingly, none of these strains have a split pks15/1 locus (Fig. 4), and all belong to clade 2. However, other clinical strains with intact pks15/1 loci and others in clade 2 form biofilms over the same period of time.
Fig 3.
M. tuberculosis clinical strains present a variety of biofilm phenotypes after 5 weeks. Fifteen clinical M. tuberculosis strains and reference strain H37Rv were seeded in biofilms for 5 weeks. Pink, M. tuberculosis clade 1; blue, M. tuberculosis clade 2; green, M. tuberculosis clade 3; orange, M. tuberculosis clade 4.
Table 2.
M. tuberculosis clinical strains in this study
| Strain | M. tuberculosis clade | Growth speeda | Genotype | PGL/PDIM expressionb |
|---|---|---|---|---|
| 95_0545 | 1 | Slower | pks15/1 | NA |
| T17 | 1 | Same | pks15/1 | −/+ |
| T83 | 1 | Same | pks15/1 | NA |
| T92 | 1 | Slower | pks15/1 | NA |
| 00_1695 | 2 | Same | pks15/1 | NA |
| 02_1987 | 2 | Faster | pks15/1 | −/+ |
| 98_1833 | 2 | Slower | pks15/1 | −/+ |
| M4100A | 2 | Same | pks15/1 | −/+ |
| T67 | 2 | Faster | pks15/1 | −/+ |
| T85 | 2 | Same | pks15/1 | NA |
| HN878 | 2 | faster | pks15/1 | +/+ |
| 91_0079 | 3 | Slower | pks15/1 | NA |
| SG1 | 3 | Faster | pks15/1 | −/+ |
| CDC1551 | 4 | Faster | pks15 pks1 | −/+ |
| GM_1503 | 4 | Faster | pks15 pks1 | NA |
| RvS | 4 | Same | pks15 pks1 | −/+ |
Growth was determined in aerated, planktonic culture, and the speed is in comparison to that of H37Rv.
PGL and PDIM expression was determined using positive-mode, high-accuracy mass spectrometry. NA, not assayed.
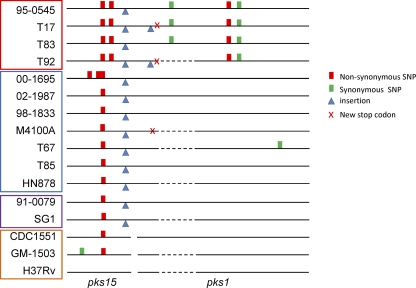
Fig 4.
Polymorphisms in pks15/1 loci of M. tuberculosis clinical strains and H37Rv. Sequences are compared to the published H37Rv sequence. Separate pks15 and pks1 loci are represented by a break in the black line. Dashed lines show regions that were unable to be sequenced in this study. Strains are highlighted to represent different M. tuberculosis clades: red, clade 1; blue, clade 2; purple, clade 3; orange, clade 4.
Clinical strains exhibit a variety of genotypes in the pks15/1 locus.
While a split pks15/1 locus was not strictly required for biofilm formation, we reasoned that other genetic variations in this region could potentially contribute to the varied biofilm phenotypes of clinical strains. To assess this possibility and resolve some ambiguities in the publicly available sequences, we resequenced the 6.5-kb pks15/1 locus of 15 clinical strains and compared their sequences with that of H37Rv. While M. tuberculosis is known for its stable genome (13), we found 9 different genotypes of the pks15/1 locus, formed by various combinations of synonymous and nonsynonymous single nucleotide polymorphisms (SNPs), insertions, and deletions, among 16 sequences (Fig. 4). There were also four different protein lengths, due to premature stop codons generated by nonsynonymous SNPs or deletion events. Variation was high even among strains of the same lineage. For example, H37Rv, CDC1551, and GM-1503, all from clade 4, had distinct genotypes, though all three share the split pks15 pks1 genetic structure. In clade 2, four genotypes were evident among seven sequenced strains.
Due to the variety of pks15/1 genotypes in this sample, the PGL expression status of several strains was also examined by positive-mode, high-accuracy mass spectrometry for an alkane series containing an ion m/z of 1886.59 and retention time of 3.9 to 4.0 min, as previously described (22a). Triglycosylated PGL abundance was estimated by the ion chromatograms for 10 strains (see Fig. S1 in the supplemental material). Whereas the majority of strains had an intact pks15/1 locus, as defined by the presence of 7 additional base pairs at nucleotide 1461 of pks15, only HN878 expressed triglycosylated PGL and nine other strains did not. Three strains, 98-1833, 02-1987, and SG1, share the same pks15/1 genotype as HN878 without producing PGL. This result is consistent with the findings of others showing that the intact pks15/1 locus is necessary but not sufficient for PGL production (7). Strains lacking PGL showed a variety of biofilm phenotypes, including none (M4100A), moderate (T83), or robust (H37Rv), while the PGL-replete HN878 is a good biofilm producer (Fig. 3). Thus, PGL is not required for and does not correlate with biofilm formation, indicating that pks1 influences biofilm formation in a manner that does not depend on PGL. In addition, we found that all strains examined were positive for the PGL-related polyketide phthiocerol dimycocerosate (PDIM), the expression of which is not affected by the genetic structure of pks15/1 (Table 2).
DISCUSSION
Biofilms occur naturally and are estimated to be responsible for up to 80% of bacterial infections (31). Biofilms vary widely but share general features, including the requirement for both external cues and genetic input for development, attachment on a surface or at the air-liquid interface, involvement of both live cells and extracellular material to form biomass of high structural integrity, and elaboration of a drug-tolerant phenotype that is distinctly different from the same cells in their planktonic state. All these features are also found in M. tuberculosis pellicles, arguing that these structures are closely related to the biofilms formed by other organisms.
We sought to identify genes that contribute to pellicle biofilm formation in M. tuberculosis. In a small-scale genetic screen, we identified five loci that are important in biofilm maturation in a static in vitro system. Consistent with findings in other microorganisms (15), these results suggest that many additional genes likely contribute to pellicle biofilm formation in M. tuberculosis. The mutants highlight a variety of functions important to biofilm production and maintenance, including nitrogen metabolism (Rv0021c and nirB), cell surface protease activity (mycP1), and complex lipid biosynthesis (pks1). The fifth ORF identified in our screen, Rv0199, carries a gene of unknown function.
Polyketide synthases produce a diversity of lipids, many of which, such as sulfatides, phenolic glycolipid (PGL), and PDIM, play a role in M. tuberculosis virulence. Polyketide synthases are large, modular, multidomain enzymatic machines, producing an array of biologically active molecules called polyketides (20, 21). In other organisms, polyketides fill a variety of roles, functioning as antibiotics, toxins, immunosuppressants, and antitumor compounds (29, 41).
The M. tuberculosis polyketide synthase gene pks15/1 is necessary for the production of phenolic glycolipid, an immunomodulatory lipid virulence factor (7). It was previously unclear if pks1 is functional in strains where pks15 and pks1 are split. However, given the modular nature of polyketide synthases (4) and the widespread conservation of the reading frame even in mutants within this region (Fig. 4), it seems plausible that pks1, which encodes 5 of 6 domains of the Pks15/1 machinery, has a biological function even when physically separated from pks15. Indeed, our work here shows that pks1 inactivation results in attenuated biofilm production and that this deficiency can be complemented by a functional pks1 allele (Fig. 2). Pks1 may be functional on its own or as a heteroprotein, combining with another polyketide synthase. For example, some polyketide synthases lack an acyl transferase domain, which is provided in trans (25, 38). In addition, FadD proteins, of which there are 34 in the M. tuberculosis genome, often present substrates to polyketide synthases, raising the possibility that another protein may present Pks1 with its substrate (40).
The relationship between M. tuberculosis phylogeography and biofilm formation is complex. All four M. tuberculosis clades worldwide include strains that can produce biofilm material (Fig. 3). However, we find that biofilm production is not universal among clinical isolates, and there is substantial variation in this trait both within and between clades. For example, our analysis of clade 2 includes three strains (00_1695, M4100A, and T85) that grow well in planktonic culture but fail to produce detectable biofilm. In contrast, all three clade 4 strains that we analyzed were good to excellent biofilm producers.
While many other loci are clearly also involved, pks15/1 genetic structure has a strong influence on pellicle biofilm formation. All three strains with interrupted pks1 coding regions (T92, M4100A, and H37Rv:Tnpks1) that we analyzed were inhibited in biofilm production. Conversely, all strains with the split pks15 pks1 genotype were strong biofilm producers. Altogether, we noted 9 different genotypes, generated by different combinations of SNPs, insertions, and deletions, among the 16 strains analyzed here. In an organism known for its stable genome, this kind of variation at one locus is rare, suggesting that this genetic region and the trait(s) it controls may be under selective pressure. It is tempting to speculate that biofilm formation is advantageous for M. tuberculosis in certain circumstances or niches but potentially disadvantageous in others. Further investigations will help to determine the structure and effects of the Pks1 product and its role(s) in pellicle biofilm formation and M. tuberculosis pathogenesis.
Supplementary Material
ACKNOWLEDGMENTS
This research was funded by National Institute of Allergy and Infectious Diseases grants R01-AI071155 and R01-AI049313 (to D.B.M.) and by the Paul G. Allen Family Foundation.
We thank Eric Rubin for the gift of the MycoMarT7 plasmid and Sebastian Gagneux for access to clinical M. tuberculosis strains. In addition, we are grateful for the thoughtful discussions with Tom Hawn, Jerry Cangelosi, Carol Sibley, Colin Manoil, and Ted White. We also thank Jessica Winkler, Mark Hickey, and Reiling Liao for their technical expertise.
Footnotes
Published ahead of print 28 November 2011
Supplemental material for this article may be found at http://jb.asmusa.org/.
REFERENCES
- 1. Adams KN, et al. 2011. Drug tolerance in replicating mycobacteria mediated by a macrophage-induced efflux mechanism. Cell 145:39–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boshoff HI, Barry CE., III 2005. Tuberculosis—metabolism and respiration in the absence of growth. Nat. Rev. Microbiol. 3:70–80 [DOI] [PubMed] [Google Scholar]
- 3. Carter G, Wu M, Drummond DC, Bermudez LE. 2003. Characterization of biofilm formation by clinical isolates of Mycobacterium avium. J. Med. Microbiol. 52:747–752 [DOI] [PubMed] [Google Scholar]
- 4. Chopra T, Gokhale RS. 2009. Polyketide versatility in the biosynthesis of complex mycobacterial cell wall lipids. Methods Enzymol. 459:259–294 [DOI] [PubMed] [Google Scholar]
- 5. Clay H, Volkman HE, Ramakrishnan L. 2008. Tumor necrosis factor signaling mediates resistance to mycobacteria by inhibiting bacterial growth and macrophage death. Immunity 29:283–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Comas I, et al. 2010. Human T cell epitopes of Mycobacterium tuberculosis are evolutionarily hyperconserved. Nat. Genet. 42:498–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Constant P, et al. 2002. Role of the pks15/1 gene in the biosynthesis of phenolglycolipids in the Mycobacterium tuberculosis complex. Evidence that all strains synthesize glycosylated p-hydroxybenzoic methyl esters and that strains devoid of phenolglycolipids harbor a frameshift mutation in the pks15/1 gene. J. Biol. Chem. 277:38148–38158 [DOI] [PubMed] [Google Scholar]
- 8. Davies D. 2003. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2:114–122 [DOI] [PubMed] [Google Scholar]
- 9. Di Bonaventura G, et al. 2008. Influence of temperature on biofilm formation by Listeria monocytogenes on various food-contact surfaces: relationship with motility and cell surface hydrophobicity. J. Appl. Microbiol. 104:1552–1561 [DOI] [PubMed] [Google Scholar]
- 10. Donlan RM, Costerton JW. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15:167–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fedtke I, Gotz F, Peschel A. 2004. Bacterial evasion of innate host defenses—the Staphylococcus aureus lesson. Int. J. Med. Microbiol. 294:189–194 [DOI] [PubMed] [Google Scholar]
- 12. Fey PD. 2010. Modality of bacterial growth presents unique targets: how do we treat biofilm-mediated infections? Curr. Opin. Microbiol. 13:610–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ford CB, et al. 2011. Use of whole genome sequencing to estimate the mutation rate of Mycobacterium tuberculosis during latent infection. Nat. Genet. 43:482–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guinn KM, et al. 2004. Individual RD1-region genes are required for export of ESAT-6/CFP-10 and for virulence of Mycobacterium tuberculosis. Mol. Microbiol. 51:359–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hall-Stoodley L, Costerton JW, Stoodley P. 2004. Bacterial biofilms: from the natural environment to infectious diseases. Nat. Rev. Microbiol. 2:95–108 [DOI] [PubMed] [Google Scholar]
- 16. Hershberg R, et al. 2008. High functional diversity in Mycobacterium tuberculosis driven by genetic drift and human demography. PLoS Biol. 6:e311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hu Y, et al. 2000. Detection of mRNA transcripts and active transcription in persistent Mycobacterium tuberculosis induced by exposure to rifampin or pyrazinamide. J. Bacteriol. 182:6358–6365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hunter RL, Olsen M, Jagannath C, Actor JK. 2006. Trehalose 6,6′-dimycolate and lipid in the pathogenesis of caseating granulomas of tuberculosis in mice. Am. J. Pathol. 168:1249–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hunter RL, Olsen MR, Jagannath C, Actor JK. 2006. Multiple roles of cord factor in the pathogenesis of primary, secondary, and cavitary tuberculosis, including a revised description of the pathology of secondary disease. Ann. Clin. Lab. Sci. 36:371–386 [PubMed] [Google Scholar]
- 20. Jackson M, Stadthagen G, Gicquel B. 2007. Long-chain multiple methyl-branched fatty acid-containing lipids of Mycobacterium tuberculosis: biosynthesis, transport, regulation and biological activities. Tuberculosis (Edinb.) 87:78–86 [DOI] [PubMed] [Google Scholar]
- 21. Jenke-Kodama H, Dittmann E. 2009. Evolution of metabolic diversity: insights from microbial polyketide synthases. Phytochemistry 70:1858–1866 [DOI] [PubMed] [Google Scholar]
- 22. Kim KS, Salton MR, Barksdale L. 1976. Ultrastructure of superficial mycosidic integuments of Mycobacterium sp. J. Bacteriol. 125:739–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22a. Layre E, et al. A comparative lipidomics platform for chemotaxic analysis of Mycobacterium tuberculosis. Chem. Biol., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lewis K. 2008. Multidrug tolerance of biofilms and persister cells. Curr. Top. Microbiol. Immunol. 322:107–131 [DOI] [PubMed] [Google Scholar]
- 24. Middlebrook G, Dubos RJ, Pierce C. 1947. Virulence and morphological characteristics of mammalian tubercle bacilli. J. Exp. Med. 86:175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moss SJ, Martin CJ, Wilkinson B. 2004. Loss of co-linearity by modular polyketide synthases: a mechanism for the evolution of chemical diversity. Nat. Prod. Rep. 21:575–593 [DOI] [PubMed] [Google Scholar]
- 26. Ojha AK, Trivelli X, Guerardel Y, Kremer L, Hatfull GF. 2010. Enzymatic hydrolysis of trehalose dimycolate releases free mycolic acids during mycobacterial growth in biofilms. J. Biol. Chem. 285:17380–17389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ojha A, et al. 2005. GroEL1: a dedicated chaperone involved in mycolic acid biosynthesis during biofilm formation in mycobacteria. Cell 123:861–873 [DOI] [PubMed] [Google Scholar]
- 28. Ojha AK, et al. 2008. Growth of Mycobacterium tuberculosis biofilms containing free mycolic acids and harbouring drug-tolerant bacteria. Mol. Microbiol. 69:164–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Olano C, Mendez C, Salas JA. 2010. Post-PKS tailoring steps in natural product-producing actinomycetes from the perspective of combinatorial biosynthesis. Nat. Prod. Rep. 27:571–616 [DOI] [PubMed] [Google Scholar]
- 30. O'Toole G, Kaplan HB, Kolter R. 2000. Biofilm formation as microbial development. Annu. Rev. Microbiol. 54:49–79 [DOI] [PubMed] [Google Scholar]
- 31. Parsek MR, Singh PK. 2003. Bacterial biofilms: an emerging link to disease pathogenesis. Annu. Rev. Microbiol. 57:677–701 [DOI] [PubMed] [Google Scholar]
- 32. Reddy TB, et al. 2009. TB database: an integrated platform for tuberculosis research. Nucleic Acids Res. 37:D499–D508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reed MB, et al. 2004. A glycolipid of hypervirulent tuberculosis strains that inhibits the innate immune response. Nature 431:84–87 [DOI] [PubMed] [Google Scholar]
- 34. Russell DG, Barry CE, III, Flynn JL. 2010. Tuberculosis: what we don't know can, and does, hurt us. Science 328:852–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rustad TR, Sherrid AM, Minch KJ, Sherman DR. 2009. Hypoxia: a window into Mycobacterium tuberculosis latency. Cell. Microbiol. 11:1151–1159 [DOI] [PubMed] [Google Scholar]
- 36. Sassetti CM, Boyd DH, Rubin EJ. 2003. Genes required for mycobacterial growth defined by high density mutagenesis. Mol. Microbiol. 48:77–84 [DOI] [PubMed] [Google Scholar]
- 37. Sule P, et al. 2009. A combination of assays reveals biomass differences in biofilms formed by Escherichia coli mutants. Lett. Appl. Microbiol. 49:299–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tang GL, Cheng YQ, Shen B. 2004. Leinamycin biosynthesis revealing unprecedented architectural complexity for a hybrid polyketide synthase and nonribosomal peptide synthetase. Chem. Biol. 11:33–45 [DOI] [PubMed] [Google Scholar]
- 39. Tobin DM, et al. 2010. The lta4h locus modulates susceptibility to mycobacterial infection in zebrafish and humans. Cell 140:717–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Trivedi OA, et al. 2004. Enzymic activation and transfer of fatty acids as acyl-adenylates in mycobacteria. Nature 428:441–445 [DOI] [PubMed] [Google Scholar]
- 41. Weissman KJ, Leadlay PF. 2005. Combinatorial biosynthesis of reduced polyketides. Nat. Rev. Microbiol. 3:925–936 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.