Abstract
Anaerobic ammonium-oxidizing (anammox) bacteria play an important role in the biogeochemical cycling of nitrogen. They derive their energy for growth from the conversion of ammonium and nitrite into dinitrogen gas in the complete absence of oxygen. Several methods have been used to detect the presence and activity of anammox bacteria in the environment, including 16S rRNA gene-based approaches. The use of the 16S rRNA gene to study biodiversity has the disadvantage that it is not directly related to the physiology of the target organism and that current primers do not completely capture the anammox diversity. Here we report the development of PCR primer sets targeting a subunit of the hydrazine synthase (hzsA), which represents a unique phylogenetic marker for anammox bacteria. The tested primers were able to retrieve hzsA gene sequences from anammox enrichment cultures, full-scale anammox wastewater treatment systems, and a variety of freshwater and marine environmental samples, covering all known anammox genera.
INTRODUCTION
For a long time, the anaerobic oxidation of ammonium (anammox) was assumed to be impossible biochemistry, until its occurrence was predicted by Broda (2) and the process was discovered in a wastewater treatment plant 20 years later in Delft, The Netherlands (27). The observed ammonium and nitrate losses under anoxic conditions were the starting point for elucidating the key player of this process (42) and revealing the contribution of anammox to the global nitrogen cycle (for a review, see reference 13). Enrichments in sequencing batch reactors followed by 16S rRNA analyses showed that all anammox bacteria belong to the same monophyletic order, named the Brocadiales, and are related to the Planctomycetales (14). Both 16S rRNA and lipid biomarkers were developed to detect anammox bacteria in the environment and wastewater treatment systems (21, 29, 33, 36, 37). Consequently, anammox bacteria have been observed in wetland, freshwater, hot spring, terrestrial, and man-made ecosystems (10, 11, 29, 49). A successful enrichment was achieved using a nitrogen-loaded peat soil as an inoculum (8). The first evidence of the anammox process in a marine environment came from the studies of Skagerrak/Aarhus Bay sediment (45) and the Black Sea, the world's largest anoxic basin (21). Later, studies of oxygen minimum zones showed the presence of anammox bacteria in the Arabian Sea and the Benguela and Peru upwelling zones (6, 12, 22, 23). Furthermore, the anammox process has been detected at high temperatures in deep-sea hydrothermal vents on the Mid-Atlantic Ridge (3). These studies indicated that denitrification is not the only process playing a role in the loss of fixed nitrogen (1). The sampling of many ecosystems have yielded, so far, five “Candidatus” genera within the order Brocadiales, family Brocadiaceae: “Candidatus Brocadia,” “Candidatus Kuenenia,” “Candidatus Scalindua,” “Candidatus Anammoxoglobus,” and “Candidatus Jettenia” (14, 16, 30, 33, 35, 42), with 16S rRNA gene sequence identities ranging between 87 and 99%. These anammox bacteria are all able to combine ammonium and nitrite to form dinitrogen gas under anoxic conditions.
As mentioned above, several methods are being used to detect the presence and activity of anammox bacteria in the environment and wastewater treatment systems, such as fluorescence in situ hybridization (FISH) (29, 33, 34, 36), real-time PCR (46), tracer experiments with 15N-labeled NH4+, which reacts with 14NO2− to form 29N2 (31), and the use of the unique ladderane lipids as a biomarker (40, 41). However, when using a 16S rRNA gene-based approach on environmental samples, anammox bacteria may be underrepresented in general 16S rRNA clone libraries since the widely used “universal” primer set for 16S rRNA gene amplification has several mismatches. The use of more-specific primers in combination with a general eubacterial reverse primer was shown to increase relative amounts of planctomycete and/or anammox 16S rRNA gene sequences (28, 33, 37). The use of the 16S rRNA gene to study biodiversity has the disadvantage that it is not related to the physiology of the target organism and that current anammox primers do not capture all diversity (36). Since certain microorganisms performing a common physiological function may not be numerically dominant, the use of functional gene markers provides a good alternative. In a recent review, Junier et al. (15) summarized the state of the art for aerobic ammonium-oxidizing organisms from the domains Bacteria and Archaea and for anammox bacteria. Genes encoding hydroxylamine/hydrazine oxidoreductase (HAO/HZO) proteins are potential targets for molecular ecological studies of both aerobic and anaerobic ammonium-oxidizing bacteria (7, 24, 30, 38). But the presence of eight highly divergent genes in the genome of “Candidatus Kuenenia stuttgartiensis” (43) makes interpretation of the retrieved sequences complex. Recently, very specific primers targeting the nitrite reductase of marine anammox bacteria were designed and used to study biodiversity in the Peruvian oxygen minimum zone (23, 25).
The genome sequence of “Candidatus Kuenenia stuttgartiensis” was assembled from a metagenome obtained from a complex microbial community grown in a sequencing batch reactor (SBR) in which “Ca. Kuenenia stuttgartiensis” made up 74% of the microbial population (43). Based on this genome, a hypothetical metabolic pathway was proposed: first, nitrite is reduced to nitric oxide by a cd1-type nitric oxide/nitrite oxidoreductase (NirS), and then a hydrazine synthase produces hydrazine from nitric oxide and ammonium, and finally hydrazine is oxidized to produce dinitrogen gas by a hydrazine oxidoreductase (HZO). A nitrate/nitrite oxidoreductase (NarGH) oxidizes part of the nitrite to nitrate and generates electrons for the carbon dioxide fixation by the acetyl-coenzyme A (CoA) pathway. Recently, experimental evidence was published that fully supported the proposed metabolism (18). The hydrazine synthase protein encoded by the hzsC, hzsB, hzsA gene cluster (kuste2859-kuste2861) was successfully purified and shown to form an N-N bond from nitric oxide and ammonium (18). This gene cluster is not present in other genomes sequenced so far and might thus be a very good biomarker for anammox bacteria.
Here we describe the development of PCR primer sets to target this new unique functional hzsA biomarker for anammox bacteria. The primers were successfully tested on sequencing batch reactor enrichment cultures, full-scale anammox wastewater treatment systems, and a variety of environmental samples and yielded a broad diversity of hzsA sequences from these systems.
MATERIALS AND METHODS
Lab reactor samples.
To obtain DNA for metagenome sequencing and to evaluate the specificity of the designed primers, several anammox enrichment cultures in bioreactors from our laboratory were sampled: “Ca. Kuenenia stuttgartiensis,” “Ca. Brocadia fulgida,” “Ca. Brocadia anammoxidans,” “Ca. Scalindua” T23, and “Ca. Anammoxoglobus propionicus.” Biomass from “Candidatus Jettenia asiatica” was obtained from the anammox granular sludge reactor described previously (30). Furthermore, a peat soil anammox enrichment reactor (9) and a reactor with a coculture of “Candidatus Methylomirabilis”-like bacteria and anammox bacteria were sampled (26).
Samples from wastewater treatment plants.
Samples from three wastewater treatment plants in The Netherlands were investigated: the Rotterdam, Olburgen, and Lichtenvoorde full-scale Anammox reactors (47) (http://www.paques.nl/?pid=46). In addition, sludge samples from eight nitrogen removal reactors from China were available (8).
Environmental samples.
Samples from the Barents Sea and Northeast Greenland were available from previous studies (for details see the work of Rysgaard et al. [32] and Schmid et al. [37]). Freshwater sediment samples were obtained from two ditches in the Ooijpolder near Nijmegen, The Netherlands (51°50′40″N, 5°54′44″E): Boerenlandpad (BL) and Leeuwsweidje (L).
DNA isolation.
DNA isolation was performed following a slightly modified version of the protocol described by Kowalchuk et al. (20): 1 ml of sample was resuspended in 120 mM sodium phosphate buffer and treated by bead beating for 1 min. Cells lysis was performed by adding 10% sodium dodecyl sulfate solution; 1.6 M NaCl with 30% polyethylene glycol 6000 was used to precipitate the DNA, and 7.5 M ammonium acetate solution served for protein precipitation; DNA precipitation was done with absolute ethanol for 1 h at −20°C. Finally, the extracted DNA was resuspended in 50 μl of sterile Milli-Q water. DNA quality and quantity were checked by 1% agarose gel electrophoresis with ethidium bromide and Nanodrop analysis. Isolation of DNA from environmental samples was done using the PowerSoil DNA isolation kit (MO BIO Laboratories Inc.) according to the manufacturer's protocol.
PCR amplification and sequence analysis of the hydrazine synthase gene.
PCR amplifications were performed in a total volume of 25 μl containing 12.5 μl of a PCR premix (PerfeCTa SYBR green FastMix; Quanta BioScience Inc., Gaithersburg, MD), 1 μl of forward primer (20 pmol/μl), 1 μl of reverse primer (20 pmol/μl), 1 or 2 μl of template DNA (10 ng/μl, final concentration), and Milli-Q water. The thermal profile used for the amplification of the hydrazine synthase gene started with a denaturation step of 5 min at 96°C, followed by 30 cycles of denaturation (1 min at 96°C), primer annealing (1 min), and extension (1.5 min at 72°C), and finally a last extension step of 5 min at 72°C. The amplified products were analyzed by electrophoresis on 0.8% agarose gels. one microliter of 10-times-diluted DNA (10 ng/μl, final concentration) was used for the amplification of DNA extracted from anammox enrichment cultures, from three wastewater treatment plants, and also from marine environments. Primers used were hzsA_526F and the hzsA_1857R (Table 1), with an annealing temperature of 54°C. Two microliters of 10-times-diluted DNA was used in a PCR with the primer set hzsA_382F/hzsA_2390R with an annealing temperature of 56°C for the amplification of DNA extracted from the Ooijpolder freshwater ditches. This PCR was followed by a nested PCR: 1 μl of the previous PCR product was used as a template with the primers hzsA_526F and hzsA_1857R with an annealing temperature of 54°C.
Table 1.
Sequences of new hydrazine synthase primers targeting the hzsA subunit of anammox bacteriaa
| Primerb | Position (bp) | Sequence |
|---|---|---|
| Forward | ||
| hzsA_370F | 370–389 | 5′-CACAAGAADGGYGGYGGDTG-3′ |
| hzsA_382F | 382–400 | 5′-GGYGGDTGYCAGATATGGG-3′ |
| hzsA_526F | 526–543 | 5′-TAYTTTGAAGGDGACTGG-3 |
| hzsA_757F | 757–773 | 5′-AGTTCNAAYTWTGATCC-3′ |
| hzsA_757F Scalindua | 757–773 | 5′-AGTTCNAAYTWTGACCC-3′ |
| hzsA_862F | 862–878 | 5′-GTDGAYAACTGGGAYGG-3′ |
| hzsA_1318F | 1318–1338 | 5′-TATCAGCCRTTTGAYCAGGTG-3′ |
| hzsA_1597F | 1597–1615 | 5′-WTYGGKTATCARTATGTAG-3′ |
| hzsA_1600F | 1600–1617 | 5′-GGKTATCARTATGTAGAG-3′ |
| hzsA_1600F Scalindua | 1600–1620 | 5′-GGKTATCARTATGTAGAAG-3′ |
| Reverse | ||
| hzsA_887R | 868–887 | 5′-GGATAHGCRCCRTCCCAGTT-3′ |
| hzsA_1353R | 1333–1353 | 5′-ATAHCCTTCMACHTTCACCTG-3′ |
| hzsA_1829R | 1813–1829 | 5′-TCATACCACCARTTGTA-3′ |
| hzsA_1829R Scalindua | 1813–1829 | 5′-CTGAACCACCARTTGTA-3′ |
| hzsA_1857R | 1838–1857 | 5′-AAABGGYGAATCATARTGGC-3′ |
| hzsA_2210R | 2191–2210 | 5′-GGRTTDACATAYTTWCCRCC-3 |
| hzsA_2390R | 2371–2390 | 5′-ATRTTRTCCCAYTGYGCHCC-3′ |
The positions indicated refer to the “Ca. Kuenenia stuttgartiensis” hzsA gene (kuste2861).
Several primer set were tested, but generally the primer set hzsA_526F/hzsA_1857R performed best for most of the samples. In addition, for the Ooijpolder freshwater ditches, a nested PCR with the primer set hzsA_382F/hzsA_2390R followed by the primer set hzsA_526F/hzsA_1857R or a direct PCR with the primer set hzsA_526F/hzsA_1829R was used. For the RT-PCR and qPCR, primer set hzsA_1597F/hzsA1857R is recommended. PCR products of the expected size have been obtained for Northeast Greenland and from the Barents Sea samples, using the primer combination hzsA_526F/hzsA_1829R. The primers with “Scalindua” added to their names were designed specifically to target marine “Ca. Scalindua” species.
Transcription analysis.
RNA was extracted using the Ribopure bacterial kit (Ambion, Foster City, CA) according to the manufacturer's instructions. First-strand cDNA was synthesized with random primers using the RevertAid H Minus First Strand cDNA synthesis kit, and the second strand was synthesized using DNA polymerase and the manufacturer's instructions (Fermentas, Vilnius, Lithuania). Quantitative real-time PCR was performed using the MyiQ detection system and IQ SYBR green supermix (Bio-Rad, Laboratories Inc., Veenendaal, The Netherlands). Reactions were carried out in a total volume of 25 μl containing 12.5 μl of supermix, 0.5 μl of forward primer (20 pmol/μl), 0.5 μl of reverse primer (20 pmol/μl), 1 μl of template DNA, and Milli-Q water. For hzsA analysis, the primers hzsA_1597F and hzsA1857R (Table 1) were used. For nirS, a forward primer (5′-AAATTACTGGCCTCCAAGC-3′) and reverse primer (5′-TCCACAAGCAGGATGAGTC-3′) were designed based on the “Ca. Kuenenia stuttgartiensis” genome sequence (43). In controls, the reverse transcriptase step was omitted. The thermal profile used for reverse transcriptase quantitative PCR (RT-qPCR) started with an initialization step of 3 min at 96°C, followed by 40 cycles of denaturation (30 s at 96°C), primer annealing (30 s at 55°C), and extension (30 s at 72°C), and finally a last extension step of 5 min at 72°C.
Cloning and sequence analysis.
Hydrazine synthase and 16S rRNA clone libraries were constructed after PCR amplifications. The PCR products were ligated into the pGEM-T Easy cloning vector and cloned according to manufacturer's instructions (Promega). Plasmids were isolated from randomly selected clones for each PCR product with the GeneJet Miniprep kit (Fermentas, Lithuania). Cloned inserts were checked by electrophoresis on a 1% agarose gel after 2 h of restriction digestion at 37°C using 1 μl of EcoRI enzyme (10 U/μl), 1 μl of enzyme buffer, and 6 μl of Milli-Q water for 2 μl of plasmid DNA. Sequence analysis was performed at the DNA Diagnostics Center of the Nijmegen University Medical Center using the forward and reverse primers previously used for the PCR amplification.
Phylogenetic analysis.
Alignments and phylogenetic analyses were performed using the Mega 5.0 software program (44), and a BLAST search was performed to search for related sequences in GenBank.
Nucleotide sequence accession numbers.
The hzsA gene sequences obtained in this study are deposited in the GenBank database under accession numbers JN703678 to JN703724.
RESULTS AND DISCUSSION
The hydrazine synthase gene as a functional marker.
This study presents the design of new degenerate oligonucleotide primers that are suitable for detecting the hzsA gene, which encodes one of the subunits of hydrazine synthase of anammox bacteria (18, 43). Hydrazine synthase, presently thought to be unique to anammox metabolism, catalyzes the synthesis of hydrazine from nitric oxide and ammonium. In contrast to hydroxylamine and hydrazine oxidoreductases, only one gene cluster encoding the 3 subunits is present in the genome of “Ca. Kuenenia stuttgartiensis” (kuste2959-kuste2861). BLAST searches in GenBank with the “Ca. Kuenenia stuttgartiensis” hzsA gene (kuste2861) sequence resulted in only one significant hit (AB365070) (39), a gene from an anammox bacterium named strain KSU-1 (5). This DNA sequence is 79% identical to kuste2861. To obtain more hydrazine synthase sequences from other anammox bacteria, metagenome sequencing of DNA extracted from enrichment cultures dominated by “Ca. Jettenia asiatica” (30), “Ca. Brocadia fulgida” (17), and “Ca. Scalindua” T23 (48) was done. The raw data were assembled into contigs, and these were used in a BlastX search against kuste2861 (hzsA) of “Ca. Kuenenia stuttgartiensis.” To confirm the identities of the dominant anammox bacteria in the different enrichments, the 16S rRNA sequences were used. Finally, this resulted in 4 new and different hzsA gene sequences, which were aligned by ClustalW together with the gene kuste2861 (2,430 bp) from “Ca. Kuenenia stuttgartiensis” (see Fig. S1 in the supplemental material). Identities at the DNA and protein levels varied from 62 to 92.8% and from 61.5 to 97%, respectively (see Table S1). “Ca. Scalindua” T23 hzsA had the most different sequence, which is in agreement with 16S rRNA gene phylogeny (38).
Primer design and testing of their specificities in hzsA PCR.
Based on the alignment of identified hzsA genes (see above), we manually designed 10 forward and 7 reverse (degenerate) primers at conserved regions. Their sequences and positions on the gene (numbered according to the alignment) are shown in Table 1 and in Fig. S1 in the supplemental material.
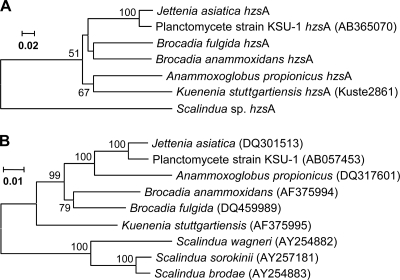
Several combinations of the newly designed primers were extensively tested in PCRs with DNA extracted from available anammox enrichment cultures as a template. The cultures were each dominated at 70 to 95% by single anammox species, e.g., Kuenenia stuttgartiensis, “Ca. Scalindua” T23, Brocadia “Ca. Brocadia fulgida,” and “Ca. Jettenia asiatica.” Although several PCR products of the expected sizes were obtained when using different combinations of forward and reverse primers, the primer set hzsA_526F/hzsA_1857R (at annealing temperature 54°C and with 2 to 2.5 mM MgCl2) was the best by far. High intensities of a single specific band (1,331 nucleotides [nt]; 1,327 nt for “Ca. Scalindua” species) without by-products could be observed on agarose gels (see Fig. S2 in the supplemental material). The PCR products were cloned, sequenced, and compared to the original sequences used to design the primers. Translated protein sequences were 100% identical to the GenBank entries (accession no. CAJ73613 and BAF98477) and metagenome sequence data. During the study, two more DNA samples of, e.g., “Ca. Anammoxoglobus propionicus” (16) and “Ca. Brocadia anammoxidans” (42) became available and were used to obtain hzsA sequences, and subsequently a phylogenetic tree of all the retrieved hzsA sequences was calculated (Fig. 1A). The hzsA phylogeny was largely congruent with that of the 16S rRNA gene sequences of the anammox sequences present in the databases (Fig. 1B).
Fig 1.
(A) Phylogenetic analysis of hzsA gene sequences from established species of anammox bacteria used to design primers. The evolutionary history was inferred using the neighbor-joining method. The bootstrap consensus tree inferred from 500 replicates is taken to represent the evolutionary history of the taxa analyzed. Branches corresponding to partitions reproduced in less than 50% bootstrap replicates are collapsed. The percentages of replicate trees in which the associated taxa clustered together in the bootstrap test (500 replicates) are shown next to the branches. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Jukes-Cantor method and are shown as numbers of base substitutions per site. Codon positions included were first plus second plus third. All ambiguous positions were removed for each sequence pair. There were a total of 2,433 positions in the final data set. (B) For comparison, a 16S rRNA gene-based phylogenetic tree is shown (derived from reference 38).
Anammox species present in (full-scale) nitrogen removal reactors.
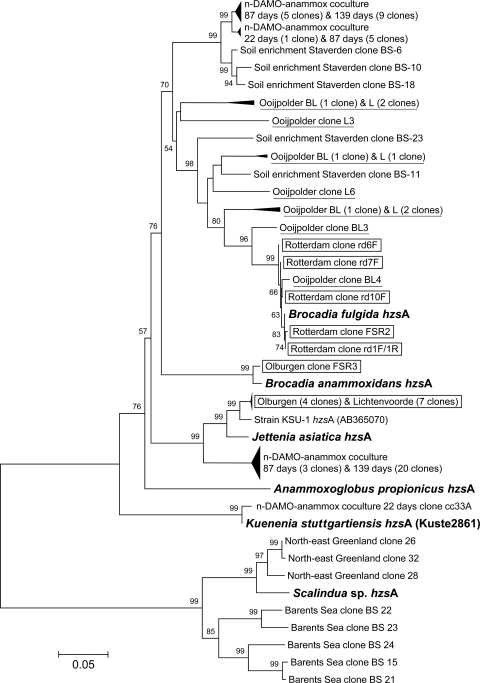
Biomass was sampled from three Dutch full-scale Anammox wastewater treatment plants in Rotterdam, Olburgen, and Lichtenvoorde (47) (http://www.paques.nl/?pid=46). DNA extracted from these biomass samples was used as a template in a PCR with the primer set hzsA_526F/hzsA_1857R and resulted in a specific band of the expected size. After cloning and sequence analysis, all (18 out of 18) clones appeared to represent hzsA genes; no false positives were detected. Phylogenetic analysis of the hzsA genes (Fig. 2) showed the presence of sequences related to “Ca. Brocadia fulgida” as the only hzsA sequence present in the Rotterdam full-scale reactor sample. One hzsA clone sequence from the Olburgen wastewater treatment plant was closely related to the sequence of “Ca. Brocadia anammoxidans,” while four others clustered together with hzsA from “Ca. Jettenia asiatica” and strain KSU-1. The hzsA sequences from the Lichtenvoorde reactor all clustered together with the hzsA sequences of “Ca. Jettenia asiatica” and strain KSU-1. Although preliminary fluorescence in situ hybridization analysis was described previously for the Rotterdam treatment plant (47), thus far no detailed molecular analyses of these wastewater treatment systems have been described.
Fig 2.
Phylogenetic analysis of hzsA gene sequences from several anammox bacteria (in bold), reactor samples, and environmental samples (93 in total). Clones from full-scale Anammox wastewater treatment plants are shown in boxes, and clones from the agricultural land ditches are underlined. Bootstrap values of >50 (500 replicates) are shown at the branches. The neighbor-joining tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Jukes-Cantor method and are in the units of the number of base substitutions per site. The bar represents 5% sequence divergence. Codon positions included were first plus second plus third. All ambiguous positions were removed for each sequence pair. There were a total of 2,433 positions in the final data set.
In addition to these full-scale reactors, we also used biomass from eight Chinese anammox reactors (8). In a previous study, the biodiversity of anammox bacteria in these reactors was studied by 16S rRNA gene analysis and fluorescence in situ hybridization (8). Quantification was performed by quantitative PCR (qPCR) targeting a small fragment of the 16S rRNA gene. For comparison, qPCR using the primer set hzsA_1597F/hzsA_1857R, targeting a 260-nt fragment of the hzsA gene, was performed (Table 2). The hzsA gene copy numbers were more or less similar to the 16S rRNA gene copy numbers (8) in two reactors, but in the others, hzsA gene copy numbers were 3- to 6-fold lower. The unique functional hzsA gene may be more suitable for qPCR quantification, since the 16S rRNA gene primers are known to amplify nonanammox Planctomycetes-derived 16S rRNA genes (32, 36). To check the anammox biodiversity, qPCR products of hzsA obtained using the DNA template from the different reactors were cloned and sequenced (35 clones in total). Phylogenetic analysis (see Fig. S3) revealed that sequences from reactors A2 and A6 all clustered with “Ca. Kuenenia stuttgartiensis.” Those from reactor A3 were restricted to the B. anammoxidans cluster. The sequences from the other reactors all showed more biodiversity, with reactors A1 and A4 being the most diverse. This finding is in good agreement with the 16S rRNA gene phylogeny reported previously (8). We did not obtain any “Ca. Scalindua”-like hzsA sequence, which is not unexpected, since “Ca. Scalindua” species were enriched mainly from marine ecosystems (19, 48) and were shown to be the dominant anammox bacteria in these ecosystems (37).
Table 2.
Copy numbers of 16S rRNA and hzsA genes in Chinese anammox reactorsa
| Reactor | Gene copy no. per ml of reactor vol |
Ratio | |
|---|---|---|---|
| 16S rRNA gene | hzsA gene | ||
| A1 | 3.7 × 107 | 2.9 × 107 | 1.3:1 |
| A2 | 1.3 × 109 | 1.0 × 109 | 1.2:1 |
| A3 | 1.2 × 109 | 2.4 × 108 | 4.9:1 |
| A4 | 4.0 × 109 | 1.4 × 109 | 2.9:1 |
| A5 | 2.2 × 109 | 7.9 × 108 | 2.8:1 |
| A6 | 8.6 × 109 | 3.1 × 109 | 2.8:1 |
| A7 | 5.9 × 109 | 6.0 × 108 | 9.8:1 |
| A8 | 1.7 × 109 | 3.4 × 108 | 5.2:1 |
qPCR was performed with primer sets Amx694F-Amx960R and hzsA_1597F-hzsA_1857R (this study), targeting the 16S rRNA and hzsA genes. 16S rRNA qPCR data were extracted from the work of Hu et al. (8).
As a proof of principle, it was shown that the primer pair hzsA_1597F/hzsA_1857R, described above for qPCR, could also be successfully used in a real time RT-PCR to analyze the mRNA expression level of the hzsA gene in “Ca. Kuenenia stuttgartiensis” (see Fig. S4 in the supplemental material). For comparison, a primer pair targeting the nirS gene was included. The expression levels of both genes are in good agreement with transcriptome data reported before (18).
Phylogenetic analysis of environmental hzsA gene sequences.
DNA extracted from samples from the Ooijpolder agricultural land in The Netherlands, from Northeast Greenland, and from the Barents Sea has been used to detect hzsA genes in the environment. PCR products of the expected size have been obtained for Northeast Greenland and from the Barents Sea samples, using the primer combination hzsA_526F/hzsA_1829R. The phylogenetic analysis showed that the partial hzsA sequences amplified from these two marine samples were closely related to “Ca. Scalindua” species (Fig. 2). The hydrazine synthase sequences from the Barents Sea were more closely related to the “Ca. Scalindua” T23 sequence, and the ones from the Northeast Greenland seem to represent a new species within the “Ca. Scalindua” cluster. Recently, a broad distribution of anammox bacteria with a highly niche-specific community structure within several marine ecosystems using hzo gene-specific primers was reported (24). It would be interesting to use the DNA from these sites to amplify the more unique hzsA genes (no multiple copies present) from these environments for comparison.
In addition to the marine samples, two different ditches (i.e., BL [Boerenlandpad] and L [Leeuwsweidje]) from the agricultural land of the Ooijpolder were sampled for DNA extraction. A PCR with the primer set hzsA_382F/hzsA_2390R (at 56°C), followed by nested PCR with the primer set hzsA_526/hzsA_1857, was needed to obtain a specific 1,331-bp product. After cloning and sequence analysis of these PCR products, all sequences resembled hzsA genes clustering with the Brocadia species (Fig. 2). Some sequences from the BL ditch seem to be closely related to “Ca. Brocadia fulgida,” but most of the sequences of the Ooijpolder ditches grouped between “Ca. Brocadia fulgida” and “Ca. Brocadia anammoxidans” and may represent a new Brocadia species. To test if direct amplification would be possible, primer hzsA_1829R was used in combination with hzsA_526F. This reverse primer hzsA_1829R excludes “Ca. Scalindua” species (see Fig. S1 in the supplemental material). This primer pair yielded a specific product of 1,303 bp, avoiding the second nested PCR. Upon cloning and sequencing, the same biodiversity, all “Ca. Brocadia”-like sequences, was obtained (data not shown).
hzsA genes from a peat soil enrichment and a coculture.
To demonstrate the contribution of anammox bacteria to nitrogen cycling in natural and agricultural soils, an enrichment with nitrogen-loaded peat soil as an inoculum was described (9). After 8 months of incubation, anammox bacteria constituted 40 to 50% of the cells present in this culture. To test hzsA as a functional gene marker, we used DNA from this enrichment in a PCR with the primer set hzsA_526F/hzsA_1857R. After cloning and sequencing, two dominant anammox phylotypes were found, one affiliated with “Ca. Brocadia fulgida” and the other most likely representing a novel Brocadia species. This is in good agreement with the 16S rRNA sequences reported before (9).
Recently, a stable coculture of nitrite-dependent anaerobic methane oxidizing bacteria (n-DAMO) and anammox bacteria was obtained by feeding ammonium to a stable enrichment culture of “Candidatus Methylomirabilis oxyfera” bacteria (4, 26). After about 160 days of incubation, “Ca. Methylomirabilis oxyfera” and anammox bacteria were shown to be present in equal amounts. The anammox biodiversity was analyzed using 16S rRNA gene amplification (36). PCR sequences obtained from DNA extracted after 87 and 139 days of cocultivation revealed the presence of “Ca. Jettenia”- and “Ca. Brocadia”-like sequences, while on day 22, some clones also resembled the 16S rRNA gene of “Ca. Kuenenia stuttgartiensis” (26). To evaluate the potential application of hzsA in coculture experiments, the DNA samples from days 22, 87, and 139 were used as a template with two primer sets, hzsA_526F/hzsA_1829R and hzsA_526F/hzsA_1857R. The clone sequences obtained were aligned and included in the hzsA phylogenetic tree (Fig. 2). The results perfectly matched those obtained with the 16S rRNA gene (26).
In the present study, we have shown the suitability of using the hydrazine synthase gene as a unique functional marker for the detection of the anammox process. The hydrazine synthase enzyme catalyzes the synthesis of hydrazine from nitric oxide and ammonium (18). Since this enzyme has so far been identified and purified only for anammox bacteria, the gene encoding hydrazine synthase is a perfect candidate for design of anammox-specific PCR primers. In total, we designed 10 forward and 7 reverse primers and tested them in different combinations. The amplifications of the hydrazine synthase gene from various enrichment cultures of anammox demonstrated the specificity of these primers, and so far no false positives were obtained. Then, we successfully amplified the hydrazine synthase gene from environmental samples. Phylogenetic analyses of the hzsA gene are consistent with the phylogeny of the 16S rRNA gene. The hzsA primers are more specific than the primers targeting the hydrazine oxidoreductase (hzo) or nitrite reductase (nirS) genes, because these genes are also present in other bacteria (24, 38). Furthermore, the hzsA gene provides unambiguous functional information, since so far no false positives have been obtained. This makes the hzsA PCR more suited for the study of anammox biodiversity than 16S rRNA, hzo, or nirS gene analysis, which may produce many nonanammox sequences. In conclusion, the hzsA gene, encoding a subunit of the specific anammox enzyme hydrazine synthase, is a valuable molecular marker for detecting anammox bacteria in the environment.
Supplementary Material
ACKNOWLEDGMENTS
We thank our laboratory colleagues for their insights and advice and Wim Geerts and Katinka van de Pas for technical assistance.
The work was partly funded by ERC grant no. 2322937 to Mike Jetten and Mathilde Le Roy.
Footnotes
Published ahead of print 2 December 2011
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Brandes JA, Devol AH, Deutsch C. 2007. New developments in the marine nitrogen cycle. Chem. Rev. 107:577–589 [DOI] [PubMed] [Google Scholar]
- 2. Broda E. 1977. Two kinds of lithotrophs missing in nature. Z. Allg. Mikrobiol. 17:491–493 [DOI] [PubMed] [Google Scholar]
- 3. Byrne N, et al. 2009. Presence and activity of anaerobic ammonium-oxidizing bacteria at deep-sea hydrothermal vents. ISME J. 3:117–123 [DOI] [PubMed] [Google Scholar]
- 4. Ettwig KF, et al. 2010. Nitrite-driven anaerobic methane oxidation by oxygenic bacteria. Nature 464:543–548 [DOI] [PubMed] [Google Scholar]
- 5. Fujii T, Sugino H, Rouse JD, Furukawa K. 2002. Characterization of the microbial community in an anaerobic ammonium-oxidizing biofilm cultured on a nonwoven biomass carrier. J. Biosci. Bioeng. 94:412–418 [DOI] [PubMed] [Google Scholar]
- 6. Hamersley MR, et al. 2007. Anaerobic ammonium oxidation in the Peruvian oxygen minimum zone. Limnol. Oceanogr. 52:923–933 [Google Scholar]
- 7. Hirsch MD, Long ZT, Song B. 2011. Anammox bacterial diversity in various aquatic ecosystems based on the detection of hydrazine oxidase genes (hzoA/hzoB). Microb. Ecol. 61:264–276 [DOI] [PubMed] [Google Scholar]
- 8. Hu BL, et al. 2010. Identification and quantification of anammox bacteria in eight nitrogen removal reactors. Water Res. 44:5014–5020 [DOI] [PubMed] [Google Scholar]
- 9. Hu BL, et al. 2011. New anaerobic, ammonium-oxidizing community enriched from peat soil. Appl. Environ. Microbiol. 77:966–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Humbert S, et al. 2010. Molecular detection of anammox bacteria in terrestrial ecosystems: distribution and diversity. ISME J. 4:450–454 [DOI] [PubMed] [Google Scholar]
- 11. Jaeschke A, et al. 2009. 16S rRNA gene and lipid biomarker evidence for anaerobic ammonium-oxidizing bacteria (anammox) in California and Nevada hot springs. FEMS Microbiol. Ecol. 67:343–350 [DOI] [PubMed] [Google Scholar]
- 12. Jensen MM, et al. 2011. Intensive nitrogen loss over the Omani Shelf due to anammox coupled with dissimilatory nitrite reduction to ammonium. ISME J. 5:1660–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jetten MSM, et al. 2009. Biochemistry and molecular biology of anammox bacteria. Crit. Rev. Biochem. Mol. Biol. 44:65–84 [DOI] [PubMed] [Google Scholar]
- 14. Jetten MSM, Op den Camp HJM, Kuenen JG, Strous M. 2010. Description of the order Brocadiales, p 506–603 In Krieg NR, et al. (ed), Bergey's manual of systematic bacteriology, vol 4 Springer, Heidelberg, Germany [Google Scholar]
- 15. Junier P, et al. 2010. Phylogenetic and functional marker genes to study ammonia-oxidizing microorganisms (AOM) in the environment. Appl. Microbiol. Biotechnol. 85:425–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kartal B, et al. 2007. Candidatus “Anammoxoglobus propionicus” gen. nov., sp. nov., a new propionate oxidizing species of anaerobic ammonium oxidizing bacteria. Syst. Appl. Microbiol. 30:39–49 [DOI] [PubMed] [Google Scholar]
- 17. Kartal B, et al. 2008. Candidatus ‘Brocadia fulgida': an autofluorescent anaerobic ammonium oxidizing bacterium. FEMS Microbiol. Ecol. 63:46–55 [DOI] [PubMed] [Google Scholar]
- 18. Kartal B, et al. 2011. Molecular mechanism of anaerobic ammonium oxidation. Nature 479:127–230 [DOI] [PubMed] [Google Scholar]
- 19. Kawagoshi Y, et al. 2009. Enrichment culture of marine anaerobic ammonium oxidation (anammox) bacteria from sediment of sea-based waste disposal site. J. Biosci. Bioeng. 107:61–63 [DOI] [PubMed] [Google Scholar]
- 20. Kowalchuk GA, de Bruijn FJ, Head IM, Akkermans AD, van Elsas JD. 2004Molecular microbial ecology manual, 2nd ed, vol 1 Kluwer Academic Publishing, London, United Kingdom [Google Scholar]
- 21. Kuypers MMM, et al. 2003. Anaerobic ammonium oxidation by anammox bacteria in the Black Sea. Nature 422:608–611 [DOI] [PubMed] [Google Scholar]
- 22. Kuypers MMM, et al. 2005. Massive nitrogen loss from the Benguela upwelling system through anaerobic ammonium oxidation. Proc. Natl. Acad. Sci. U. S. A. 102:6478–6483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lam P, et al. 2009. Revising the nitrogen cycle in the Peruvian oxygen minimum zone. Proc. Natl. Acad. Sci. U. S. A. 106:4752–4757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li M, Hong Y, Klotz MG, Gu JD. 2010. A comparison of primer sets for detecting 16S rRNA and hydrazine oxidoreductase genes of anaerobic ammonium-oxidizing bacteria in marine sediments. Appl. Microbiol. Biotechnol. 86:781–790 [DOI] [PubMed] [Google Scholar]
- 25. Li M, Gu J-D. 2011. Advances in methods for detection of anaerobic ammonium oxidizing (anammox) bacteria. Appl. Microbiol. Biotechnol. 90:1241–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Luesken FA, et al. 2011. Simultaneous nitrite-dependent anaerobic methane and ammonium oxidation processes. Appl. Environ. Microbiol. 77:6802–6807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mulder A, van de Graaf AA, Robertson LA, Kuenen JG. 1995. Anaerobic ammonium oxidation discovered in a denitrifying fluidized-bed reactor. FEMS Microbiol. Ecol. 16:177–183 [Google Scholar]
- 28. Penton CR, Devol AH, Tiedje JM. 2006. Molecular evidence for the broad distribution of anaerobic ammonium-oxidizing bacteria in freshwater and marine sediments. Appl. Environ. Microbiol. 72:6829–6832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pynaert K, et al. 2003. Characterization of an autotrophic nitrogen-removing biofilm from a highly loaded lab-scale rotating biological contactor. Appl. Environ. Microbiol. 69:3626–3635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Quan ZX, et al. 2008. Diversity of ammonium-oxidizing bacteria in a granular sludge anaerobic ammonium-oxidizing (anammox) reactor. Environ. Microbiol. 10:3130–3139 [DOI] [PubMed] [Google Scholar]
- 31. Risgaard-Petersen N, Nielsen LP, Rysgaard S, Dalsgaard T, Meyer RL. 2003. Application of the isotope pairing technique in sediments where anammox and denitrification coexist. Limnol. Oceanogr. Methods 1:63–73 [Google Scholar]
- 32. Rysgaard S, Glud RN, Risgaard-Petersen N, Dalsgaard T. 2004. Denitrification and anammox activity in arctic marine sediments. Limnol. Oceanogr. 49:1493–1502 [Google Scholar]
- 33. Schmid M, et al. 2000. Molecular evidence for genus level diversity of bacteria capable of catalyzing anaerobic ammonium oxidation. Syst. Appl. Microbiol. 23:93–106 [DOI] [PubMed] [Google Scholar]
- 34. Schmid M, Schmitz-Esser S, Jetten MSM, Wagner M. 2001. 16S-23S rDNA intergenic spacer and 23S rDNA of anaerobic ammonium oxidizing bacteria: implications for phylogeny and in situ detection. Environ. Microbiol. 3:450–459 [DOI] [PubMed] [Google Scholar]
- 35. Schmid MC, et al. 2003. Candidatus “Scalindua brodae”, sp. nov., Candidatus “Scalindua wagneri”, sp. nov., two new species of anaerobic ammonium oxidizing bacteria. Syst. Appl. Microbiol. 26:529–538 [DOI] [PubMed] [Google Scholar]
- 36. Schmid MC, et al. 2005. Biomarkers for the in situ detection of anaerobic ammonium oxidizing (anammox) bacteria. Appl. Environ. Microbiol. 71:1677–1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schmid MC, et al. 2007. Anaerobic ammonium-oxidizing bacteria in marine environments: widespread occurrence but low diversity. Environ. Microbiol. 9:1476–1484 [DOI] [PubMed] [Google Scholar]
- 38. Schmid MC, et al. 2008. Environmental detection of the octaheme cyctochrome c hydroxylamine/hydrazine oxidoreductase genes of aerobic and anaerobic ammonium-oxidizing bacteria. Environ. Microbiol. 10:3140–3149 [DOI] [PubMed] [Google Scholar]
- 39. Shimamura M, et al. 2008. Another multiheme protein, hydroxylamine oxidoreductase, abundantly produced in an anammox bacterium besides the hydrazine-oxidizing enzyme. J. Biosci. Bioeng. 105:243–248 [DOI] [PubMed] [Google Scholar]
- 40. Sinninghe Damsté JS, et al. 2002. Linearly concatenated cyclobutane lipids form a dense bacterial membrane. Nature 419:708–712 [DOI] [PubMed] [Google Scholar]
- 41. Sinninghe Damsté JS, et al. 2004. A mixed ladderane/n-alkyl glycerol diether membrane lipid in an anaerobic ammonium-oxidizing bacterium. Chem. Commun. (Camb.) 22:2590–2591 [DOI] [PubMed] [Google Scholar]
- 42. Strous M, et al. 1999. Missing lithotroph identified as new planctomycete. Nature 400:446–449 [DOI] [PubMed] [Google Scholar]
- 43. Strous M, et al. 2006. Deciphering the evolution and metabolism of an anammox bacterium from a community genome. Nature 440:790–794 [DOI] [PubMed] [Google Scholar]
- 44. Tamura K, et al. 2011. MEGA5: molecular Evolutionary Genetics Analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Thamdrup B, Dalsgaard T. 2002. Production of N2 through anaerobic ammonium oxidation coupled to nitrate reduction in marine sediments. Appl. Environ. Microbiol. 68:1312–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tsushima I, Kindaichi T, Okabe S. 2007. Quantification of anaerobic ammonium-oxidizing bacteria in enrichment cultures by real-time PCR. Water Res. 41:785–794 [DOI] [PubMed] [Google Scholar]
- 47. van der Star WRL, et al. 2007. Startup of reactors for anoxic ammonium oxidation: experiences from the first full-scale anammox reactor in Rotterdam. Water Res. 41:4149–4163 [DOI] [PubMed] [Google Scholar]
- 48. van de Vossenberg J, et al. 2008. Enrichment and characterization of marine anammox bacteria associated with global nitrogen gas production. Environ. Microbiol. 10:3120–3129 [DOI] [PubMed] [Google Scholar]
- 49. Zhu G, Jetten MSM, Kuschk P, Ettwig KF, Yin C. 2010. Potential roles of anaerobic ammonium and methane oxidation in the nitrogen cycle of wetland ecosystems. Appl. Microbiol. Biotechnol. 86:1043–1055 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.