Abstract
Approaches to control vector-borne diseases rarely focus on the interface between vector and microbial pathogen, but strategies aimed at disrupting the interactions required for transmission may lead to reductions in disease spread. We tested if the vector transmission of the plant-pathogenic bacterium Xylella fastidiosa was affected by three groups of molecules: lectins, carbohydrates, and antibodies. Although not comprehensively characterized, it is known that X. fastidiosa adhesins bind to carbohydrates, and that these interactions are important for initial cell attachment to vectors, which is required for bacterial transmission from host to host. Lectins with affinity to substrates expected to occur on the cuticular surface of vectors colonized by X. fastidiosa, such as wheat germ agglutinin, resulted in statistically significant reductions in transmission rate, as did carbohydrates with N-acetylglucosamine residues. Presumably, lectins bound to receptors on the vector required for cell adhesion/colonization, while carbohydrate-saturated adhesins on X. fastidiosa's cell surface. Furthermore, antibodies against X. fastidiosa whole cells, gum, and afimbrial adhesins also resulted in transmission blockage. However, no treatment resulted in the complete abolishment of transmission, suggesting that this is a complex biological process. This work illustrates the potential to block the transmission of vector-borne pathogens without directly affecting either organism.
INTRODUCTION
Vector-borne pathogens cause a wide range of diseases in animals and plants. Traditionally, most control strategies focus on the pathogen or the vector rather than targeting the interactions between them. In addition to the fact that it is conceptually easier to suppress vector populations or target pathogens after host infection, little is known about vector-pathogen interfaces for most of these systems. Insect-borne plant pathogens, which are of significant economical and ecological relevance, may circulate within the vector's body after acquisition and eventually be inoculated into new hosts during insect salivation events (circulative pathogens) (22). Alternatively, these pathogens may attach to various sections of the foregut of vectors, which is structurally part of the exoskeleton, without host internalization (22). Molecular interactions in both of these models determine transmission success (8, 12), highlighting the possibility that the disruption of such interactions leads to the blockage of transmission. In the case of circulative viruses, it has been shown that recombinant capsid proteins or peptides that bind to receptors on midgut epithelial cells of insects result in decreased transmission efficiency, presumably by masking receptors so that pathogens cannot attach to vectors (5, 10, 19, 32). A similar approach should also work for noncirculative pathogens, where there generally is more information available on vector-pathogen interactions. Most noncirculative plant pathogens are viruses that bind to the cuticular surface of the foregut (often maxillary stylets), are not persistent, and do not multiply within vectors (23). The bacterium Xylella fastidiosa is an exception, in that it colonizes (i.e., is persistent and multiplies) the foregut of its leafhopper vectors (11, 15, 24). Despite differences in biology, the disruption of the vector-pathogen interface also should be possible for noncirculative systems.
Xylella fastidiosa is a xylem-limited bacterium that causes disease in various hosts of economic importance, such as grape, almond, citrus, and coffee (13). In addition, it colonizes a wide range of plant species as an apparently harmless endophyte (6). Vectors of X. fastidiosa are xylem-sap sucking insects, a group that includes sharpshooter leafhoppers (Hemiptera, Cicadellidae) and spittlebugs (Hemiptera, Cercopidae) (1). Although the X. fastidiosa colonization of vectors is persistent for life in adults, nymphs lose infectivity when molting, as the cuticular lining of the foregut is part of the exoskeleton and is shed at each molt, in which case individuals must reacquire X. fastidiosa in their next life stage to be infective (3, 24). The surface colonized by X. fastidiosa in insects is not well characterized, but the nature of cell-vector interactions has been demonstrated to depend on carbohydrate-protein interactions (15). Cell surface proteins mediated X. fastidiosa attachment to various substrates, including leafhopper foregut extracts and hindwings. In addition, adhesion decreased when certain carbohydrates were added to suspensions in adhesion assays, indicating that carbohydrate-binding proteins on the cell surface are substrate specific and that the saturation of these proteins affects adhesion. Much like a biofilm, however, X. fastidiosa colonization of vectors is likely a complex multistep process (2, 15) in which different factors are important for each step of biofilm formation, from initial cell adhesion to colony maturation. Work on X. fastidiosa-vector interactions has focused primarily on the early stages of biofilm formation, i.e., initial cell adhesion.
Because of the protein-carbohydrate nature of the X. fastidiosa-vector interface, it should be possible to disrupt transmission by saturating carbohydrate-binding proteins on the cell surface; alternatively, lectins (carbohydrate-binding proteins) could be used to mask carbohydrate-coated surfaces on the foregut of leafhoppers. In addition, antibodies against X. fastidiosa could reduce transmission if they were to bind to proteins on the cell surface that are involved in vector adhesion. We performed a series of experiments testing different approaches to block the leafhopper transmission of X. fastidiosa to plants.
MATERIALS AND METHODS
Insects, plants, and bacteria.
A greenhouse population of the leafhopper Graphocephala atropunctata (Hemiptera, Cicadellidae) was initiated with field-collected insects from riparian plants at Wohler creek near Forestville, CA, in May and June 2009. Each colony consisted of 40 to 50 adult insects on a single basil plant (Ocimum basilicum, Lamiaceae) contained in a wood-framed mesh cage; plants were replaced every 2 to 3 weeks. Healthy dormant cuttings of grapevines, Vitis vinifera cv. Cabernet Sauvignon, were kindly provided by Foundation Plant Services at the University of California, Davis. Two-bud cuttings were planted in a mixture of perlite and vermiculite and placed on a mist bench. After root development (∼5 to 6 weeks), cuttings were transplanted into 1-gallon pots filled with Supersoil (Rod Mclellan Company, San Mateo, CA). The Temecula strain of X. fastidiosa (30) was used for all experiments. PWG medium was used to recover X. fastidiosa from infected plants (11).
Lectin toxicity.
Lectin toxicity to insects has been shown in previous studies. Thus, estimating nonlethal concentrations of lectins to G. atropunctata was important, because in this study they were used as transmission-blocking molecules. Using an artificial diet system to deliver lectins to insects (14), an experiment was conducted with three concentrations for each treatment: 0.01, 0.1, and 1% (vol/vol). Treatments included wheat germ agglutinin (WGA), concanavalin A (CoA), lentil lectin (LL), and peanut lectin (PL); bovine serum albumin (BSA) and ovalbumin (OV) were used as control treatments along with the diet solution alone. Insects (n = 12/treatment) were individually caged in the feeding chamber (described below) for 4 h. The 12 insects of the same treatment were caged on a grape cutting for 10 days. Insect survivorship was recorded every 12 h for 10 consecutive days. The effect of lectins is described in Results.
Transmission-blocking experiments: general design.
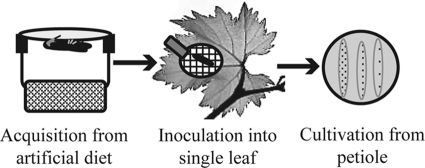
Xylella fastidiosa was grown in XFM-pectin for 10 days prior to experiments with the artificial diet system by following protocols previously described (14). The optical density (at 600 nm) of suspensions was adjusted to 0.4, which is equivalent to 109 cells/ml in the diet solution (l-glutamine, 0.7 mM; l-asparagine, 0.1 mM; sodium citrate, 1 mM; pH 6.4) for insect feeding. Molecules used as competitors that could disrupt X. fastidiosa transmission by affecting cell-vector interactions included carbohydrates, lectins (carbohydrate-binding proteins), and antibodies against X. fastidiosa. Individual leafhoppers were presented with a 100-μl suspension drop of cells mixed (or not, depending on the treatment) with the various competitor molecules. The suspension was placed between two layers of thinly stretched parafilm over a 14- by 20-mm (diameter by height) plexiglass feeding chamber. The acquisition access period (AAP), or the total time insects spent in the feeding chamber, was 4 h. At the end of the AAP, insects were removed and individually caged on a single leaf of a grape host using a small 2-cm (in diameter) clip cage (Fig. 1). Insects were allowed to feed on plants for 12 h (inoculation access period [IAP]). Once the 12-h IAP had elapsed, insects were removed and each corresponding leaf was marked and collected after 3 weeks. To estimate transmission rates, petioles were ground in buffer using a tissue homogenizer, and the presence of X. fastidiosa was evaluated by culturing the sample on PWG plates (11). Assays were performed in two time blocks, except for those with antibodies. In the competition assay with lectins, only for the second block, insects were collected after the IAP to quantify the number of cells acquired by insects using real-time PCR (15).
Fig 1.
Schematic representation of the protocol used to test transmission. After acquisition through an artificial diet system, insects were individually caged on one leaf; 3 weeks later, Xylella fastidiosa cells were recovered from that leaf's petiole by culturing on solid medium.
Competition assays with carbohydrates.
Because X. fastidiosa cells have been shown to bind to various carbohydrates, and because cell adhesion to surfaces such as insects wings can be reduced by the addition of N-acetylglucosamine (GlcNAc) (15), we tested if the addition of various saccharides to the diet being provided to leafhoppers affected the efficiency of transmission to plants. This assay was conducted in two time blocks (block 1, n = 12; block 2, n = 22). The carbohydrates used were the monosaccharides glucose, galactose, mannose, and GlcNAc and chitobiose (GlcNAc)2 and chitotriose (GlcNAc)3; three concentrations of each were used: 0.1, 0.25, and 0.5 M. No toxic effect on insects was observed with any of the concentrations. The hypothesis was that these saccharides saturate potential carbohydrate-binding proteins on the surface of X. fastidiosa cells.
Competition assays with lectins.
In contrast to the hypothesis tested with the carbohydrates, lectins could bind to receptors on the surface of insects, thereby prohibiting cells from attaching to vectors and reducing transmission rates. In this case, lectins and controls assayed for insect toxicity were tested at a concentration of 0.1% (vol/vol). This assay consisted of two time blocks with 22 individuals per lectin treatment. The four lectins tested had different affinities, which can be summarized as follows: WGA-GlcNAc, the core of N-linked oligosaccharides; CoA-branched mannose, carbohydrates with terminal mannose or glucose; LL-branched mannose with fucose linked α(1,6) to the GlcNAc; and PL-terminal β-galactose.
Competition assays with antibodies against X. fastidiosa.
Polyclonal antibodies against X. fastidiosa whole cells, extracellular gum, and various cell surface proteins were used as blocking agents. We hypothesized that if specific targets on the cell surface could be masked, prohibiting them from interacting with insect vectors, transmission efficiency would be decreased when those targets were involved in initial attachment to vectors or foregut colonization. The antibodies used targeted whole cells (provided by B. C. Kirkpatrick, University of California, Davis), gum (26), afimbrial adhesins (XadA1 and XadA2 [4] and Hxfs [B. C. Kirkpatrick]), and type IV pili (PilA2 and PilC [4]). There were 25 insect replicates per antibody treatment. XadA1 and Hxf proteins have been shown to be secreted and cell bound, while XadA2 is not secreted (4, 31). PilA2 is a structural component of X. fastidiosa's type IV pilus, and it is found both membrane bound and away from cells, presumably on the pilus filament itself; PilC is a membrane-bound protein associated with pilus assembly. All antibodies were used at a 40 μg/ml concentration.
Quantitative PCR.
To quantify X. fastidiosa populations in insects used in different lectin treatments (n = 22/treatment), DNA was extracted from the dissected heads of leafhoppers as previously described (15). For the standard curve, X. fastidiosa DNA was extracted from a suspension of the cells cultured on PWG medium that was dilution plated to correlate the number of cells (i.e., CFU) with DNA content. The absolute quantification was performed using SYBR green on a real-time thermocycler (model 7500; Applied Biosystems).
Statistical analyses.
To compare the effect of lectins on insect survivorship, Kaplan-Meier survival analysis was performed with concentration and treatment as categorical variables and the number of days as the time factor. To evaluate the effects of treatment and time block on the probability of a successful transmission in experiments with lectins, a binary logistic regression model was used. Preliminary analysis revealed no significant effect of the block (X12 = 0.214 by Wald test; P = 0.644); therefore, it was removed from the final model. A binary logistic regression also was used to analyze competition assays with carbohydrates. Initial analyses revealed no effect of either block (X12 = 0.425 by Wald test; P = 0.51) or concentration (X22 = 1.31 by Wald test; P = 2.14), thus they were removed from the final model in a stepwise approach. For the competition experiment with antibodies, treatment was the sole factor in the model. Pairwise comparisons of transmission rates were performed with chi-square tests. Univariate analysis of variance (ANOVA) was used to compare estimated bacterial populations in insect heads in the competition experiments with lectins. Bacterial populations were log10 transformed to meet the normality assumption of the general linear model (P = 0.102 by Shapiro-Wilk test). The lectin group was treated as the categorical variable in the model. Post hoc pairwise comparisons between treatments were performed with the Tukey honestly significant different test. Statistical analyses were performed in IBM SPSS Statistics, version 19 (IBM SPSS, Armonk, NY).
RESULTS
Lectins affect vector survivorship.
We detected a significant among-treatment variation in insect survivorship (log rank X62 = 165.63; P < 0.001), with LL and PL being the two treatments inflicting the highest mortality rate across all three concentrations over time. However, none of the treatments or concentrations tested caused insect mortality within the first 48 h of exposure. Following this verification of survivorship within the experimental time frame for transmission experiments (4 h of exposure followed by 12-h IAP on healthy grapevines), we proceeded to the competition assay with lectins using the 0.1% concentration. This intermediate concentration was selected to reduce potential short-term effects of higher concentrations on insect feeding while ensuring the presence of a minimum number of competitor molecules in suspensions for the competition experiments.
Lectins reduce transmission rate.
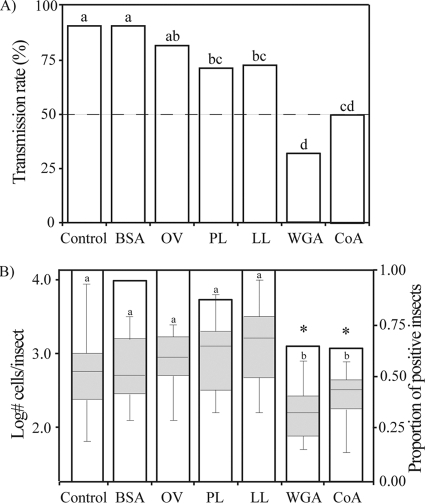
The probability of a successful transmission event was significantly affected by lectin treatments (X62 = 44.84 by Wald test; P < 0.001) (Fig. 2A). Compared to the sharpshooters fed the control diet, insects belonging to all lectin treatments showed a significant reduction in transmission rate (by Wald test): PL, X12 = 4.93 (P = 0.026); LL, X12 = 5.15 (P = 0.023); WGA, X12 = 23.67 (P < 0.001); and CoA, X12 = 11.71 (P < 0.001). The Xylella fastidiosa transmission rate by leafhoppers fed on diets with either BSA or OV was not different from that of the control (BSA, X12 = 0.01 [P = 0.975]; OV, X12 = 1.49 [P = 0.221]). The transmission rate of the insects treated with WGA was significantly lower than those of all other treatments except for CoA. Insects treated with CoA also had lower transmission rates than both OV and BSA treatments but not those of peanut and lentil lectins (Fig. 2A).
Fig 2.
Lectins affect vector transmission of Xylella fastidiosa to plants. (A) Lectins, BSA, and OV were provided to insects through diet solutions containing X. fastidiosa cells; the control contained cells only. Treatments with different letters on bars indicate statistically different transmission rates. (B) X. fastidiosa retention and populations within vectors after the inoculation access period on plants. The y axis on the right represents the proportion of insects that were positive for X. fastidiosa (empty bars); WGA and CoA were the only treatments statistically different from the control (marked with asterisks). The y axis on the left shows bacterial populations within insects as measure by quantitative PCR; boxes show the interquartile range, including 50% of results, and midhorizontal line represents the median; different letters on top of error bars represent statistically significant different treatments.
There was a significant variation in acquisition and retention rates among the insects treated with different lectins (X62 = 27.70; P < 0.001) (Fig. 2B), which was measured by quantitative PCR after the 12-h IAP on plants. The proportion of leafhoppers that acquired the pathogen in WGA and CoA treatments was significantly lower than that of the control (P < 0.003) (Fig. 2B). The acquisition rate for the other treatments was not statistically different from that of the control (P > 0.232) (Fig. 2B). These differences likely are not associated with feeding deterrence due to the presence of lectins in the diet, as LL and PL were the lectins that were toxic to leafhoppers, not WGA or CoA. Similarly to the acquisition rates, the number of bacterial cells acquired and retained by the experimental insects varied significantly among treatments (F6, 126 =7.71 and P < 0.001 by ANOVA) (Fig. 2B). Further pairwise comparisons indicated that the number of cells recovered from the insect heads in the WGA treatment was significantly lower than those of all other treatments (P < 0.043 by Tukey's test), with the exception of CoA (P = 0.54 by Tukey's test).
Carbohydrates affect transmission rate.
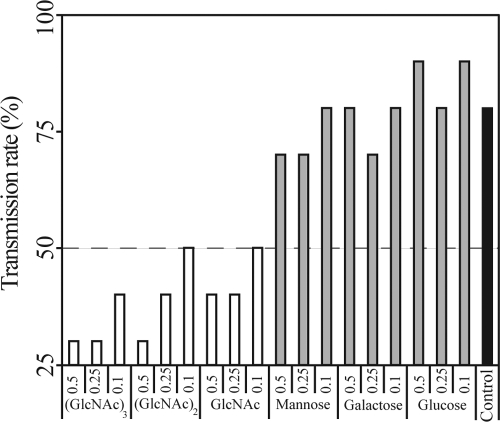
Overall, GlcNAc, (GlcNAc)2, and (GlcNAc)3 significantly reduced X. fastidiosa transmission rate by vectors (Fig. 3). For the insects treated with glucose, galactose, or mannose, the rate of successful transmission events was not statistically different from that of the control (P > 0.60 by binary logistic regression). However, transmission rates varied significantly between vectors treated with GlcNAc, (GlcNAc)2, (GlcNAc)3, and the control (P < 0.001). Transmission rates were not different among vectors treated with glucose, galactose, and mannose (X22 = 2.61; P = 0.12). Likewise, no difference in transmission rates was detected among insects treated with GlcNAc, (GlcNAc)2, and (GlcNAc)3 (X22 = 1.73; P = 0.42).
Fig 3.
N-Acetylglucosamine, chitobiose, and chitotriose decrease the efficiency of Xylella fastidiosa vector transmission to plants. Three concentrations (0.1, 0.25, and 0.5 M) were used for each saccharide tested in this competition assay. No differences among concentrations for any treatment were statistically significant. The control included only X. fastidiosa cells. Transmission rates for treatments with GlcNAc, (GlcNAc)2, and (GlcNAc)3 were not statistically different from each other (white bars), but all were different from glucose, mannose, and galactose, which also did not differ from each other (gray bars).
Antibody-mediated blocking of cell surface reduces transmission rate.
There was a significant variation in transmission efficiencies among vectors treated with different antibodies (X72 = 31.22 by Wald test; P < 0.001) (Fig. 4). With the exception of PilA2 (P = 0.068) and PilC (P = 0.111), the two type IV pilus antibodies, all of the remaining antibody treatments significantly reduced the transmission rate (P < 0.007). A separate chi-square analysis revealed no variation in transmission rates of insects treated with antibodies against gum, whole cells, and the three different afimbrial adhesins (X42 = 4.53; P = 0.34).
Fig 4.
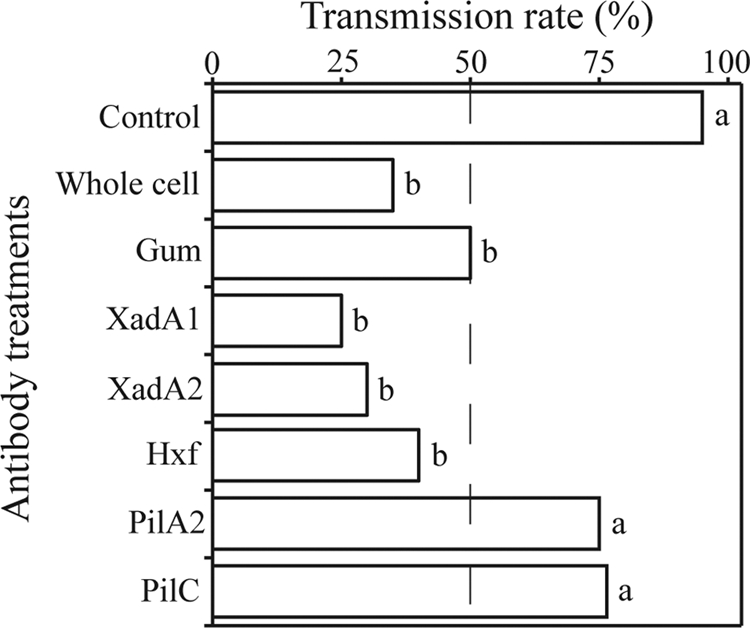
Antibodies against Xylella fastidiosa cell surface reduce transmission to plants. Various antibodies were added to the diet solution containing X. fastidiosa; different letters on bars indicate statistically different results. Antibodies prepared against whole cells, afimbrial adhesins (XadA1, XadA2, and Hxf), and gum (EPS) all reduced transmission, while those against type IV pilus proteins did not.
DISCUSSION
We tested three distinct approaches to determine whether X. fastidiosa transmission efficiency could be reduced by competitor molecules based on the fact that carbohydrate-protein interactions appear to be essential in the pathogen-vector interface (14, 15). Competition for receptors on the vector's foregut was performed using lectins with affinity for GlcNAc, which resulted in reduced transmission rates. Likewise, the saturation of carbohydrate-binding proteins on the cell surface with GlcNAc-containing sugars reduced the rate of successful transmission events. Lastly, the use of various antibodies had a similar effect. Thus, all three approaches significantly reduced the vector transmission of X. fastidiosa; however, no treatment completely abolished transmission. Different concentrations of lectins must be used to determine when the complete saturation of putative binding sites on vectors occurs; the same is true for the blocking assays with antibodies. Similar results were observed with circulative viruses (10, 32). Due to the biological complexity of biofilm establishment and development, it was expected that no individual treatment would result in the total blockage of transmission.
Some plant lectins are known to negatively affect various life history traits of insects, such as survival, feeding ability, fecundity, growth, and emergence (20), as they vary in their molecular structure and specificity (29). It has been previously documented that PL increased the mortality of the stem-borer larvae of Chilo partellus (Lepidoptera: Pyralidae) and caused the insect to stop feeding within 3 days of exposure (18). Likewise, LL also may increase insect mortality, as has been shown for the pea aphid Acyrthosiphon pisum (Hemiptera, Aphididae) (25). Here we observed increased leafhopper mortality in PL and LL treatments; this effect, however, did not appear in the first 48 h of the toxicity assay. It is possible that insect behavior was modified following the acquisition of these molecules, which could have resulted in a small but significant decrease in X. fastidiosa transmission for those two treatments (PL and LL) compared to the control, despite the fact that they were not expected to have much affinity for carbohydrates on the foregut surface. This possibility is highlighted by the fact that vector colonization by X. fastidiosa did not appear to be affected by PL or LL, and we interpret the small differences in transmission as being a consequence of changes in vector feeding behavior. CoA and WGA, on the other hand, were lectins that resulted in significant decreases in bacterial populations in insects, a smaller proportion of positive individuals, and a decrease in transmission efficiency; neither was detrimental to insect survivorship. Although the number of vector colonization events is unknown when the artificial diet system employed here is used for pathogen acquisition, we expect that multiple colonization events occurred as insects were exposed to high densities of adhesive cells (14). Moreover, acquisition through artificial systems occurs in a less turbulent environment than that of acquisition from plants (1). We interpret the results as a significant reduction in X. fastidiosa initial adhesion to vectors, leading to fewer colonization events, as observed by a smaller proportion of positive insects. However, the smaller population in insects would be due to the fact that fewer cells were able to colonize the foregut, not an effect on those that successfully colonized that surface.
Pathogen adhesion to carbohydrates functioning as host receptors for attachment are common. For example, a streptococcal adhesin that recognizes and binds to a galactosyl-α 1-4-galactose-containing glycoconjugate host receptor has been described recently (17). Likewise, Vibrio cholerae surface proteins mediate cell attachment to chitin and the surface of copepods (7, 27, 33). In addition, the presence of chitin may induce the expression of proteins with strong affinity for chitin and chito-oligomers (9). Thus, lectin-carbohydrate interactions mediate the binding of several pathogens to their hosts; interfering with these interactions may lead to the development of novel disease control strategies.
Xylella fastidiosa colonizes carbohydrate-rich surfaces in both plants and insects; therefore, it was not surprising that it adheres to such molecules (15). We have previously shown that cell adhesion to insect hindwings is reduced with the addition of GlcNAc, indicating that X. fastidiosa carbohydrate-binding proteins are saturated by the presence of this monosaccharide (15). These in vitro findings were confirmed and successfully applied in multiple biological assays in the present study. GlcNAc-containing sugars significantly reduced transmission efficiency to plants, whereas mannose, galactose, and glucose had no effect. This finding suggests that these carbohydrates saturate adhesins on the cell surface, and that the cuticular surface of leafhopper vectors is rich in these molecules. The recent finding that X. fastidiosa can process chitin and use it as its sole carbon source is further evidence that GlcNAc is important for vector colonization (16).
Although assays based on competitor molecules, such as those with lectins and carbohydrates, provide general information about the nature of X. fastidiosa-vector interactions, they do not address the role of specific components of the cell's surface that may be required for vector colonization. Targeting specific genes via knockout mutants is one approach to identify factors required for transmission, such as work performed with the afimbrial adhesins HxfA and HxfB (15). An alternative approach is to use antibodies against the same targets, where the masking of Hxf proteins, for example, would reduce transmission if they were involved in that process. Indeed, we found that antibodies generated against whole cells, gum, and afimbrial adhesins (XadA1, XadA2, and Hxf) all reduced the vector transmission of X. fastidiosa to plants. HxfA and HxfB have been shown to be involved in transmission (15), but no data are available for the other adhesins or gum. We propose that XadA1 and XadA2 also are involved in vector transmission. It is possible that gum is involved in initial attachment to vectors. It is also plausible that gum is evenly distributed on the cell surface, and that these antibodies have resulted in the inadvertent masking of adhesins actually involved in vector early colonization. This interpretation does not mean that gum is not required for successful vector colonization, but it may not be as important for initial adhesion. Lastly, PilC is involved in type IV pilus assembly and appears to be bound to the cell membrane (4), therefore it does not appear to be located on the cell surface and should not affect cell attachment. PilA2 is a rod-forming unit and was observed both in cell membranes and outside cells, presumably on pili (4). The fact that the PilA2 antibody did not reduce transmission efficiency suggests that type IV pili are not involved in the initial colonization of vectors. Twitching motility is mediated by type IV pili in X. fastidiosa and is involved in cell movement within plants (21).
The X. fastidiosa-vector interface appears to be different from those of other noncirculative insect-borne plant pathogens, which are dominated by viruses. Although different strategies are used by viruses to bind to the surface of vectors, it is clear that capsid proteins function as adhesins and that virus-encoded proteins that are not part of virions function as bridges between the capsid and the cuticle (8, 23). For viruses, however, one or few proteins are involved in this process, while in X. fastidiosa various carbohydrate-binding proteins appear to be associated with initial adhesion. Although limited information is available on receptors on the cuticle of vectors to which viruses would bind, recent work has identified cuticular proteins as receptors to the aphid-borne Cauliflower mosaic virus (28). Our results suggest that if proteins function as receptors to X. fastidiosa in the foregut of vectors, they are not as important as structural carbohydrates.
ACKNOWLEDGMENTS
We thank our laboratory colleagues for helpful discussions and comments on the manuscript. We also thank Bruce Kirkpatrick and Alessandra Souza for sharing X. fastidiosa antibodies that made this work possible.
Funding was provided by the California Department of Food and Agriculture Pierce's Disease Research Program.
Footnotes
Published ahead of print 18 November 2011
REFERENCES
- 1. Almeida RPP, Blua MJ, Lopes JRS, Purcell AH. 2005. Vector transmission of Xylella fastidiosa: applying fundamental knowledge to generate disease management strategies. Ann. Entomol. Soc. Am. 98:775–786 [Google Scholar]
- 2. Almeida RPP, Purcell AH. 2006. Patterns of Xylella fastidiosa colonization on the precibarium of sharpshooter vectors relative to transmission to plants. Ann. Entomol. Soc. Am. 99:884–890 [Google Scholar]
- 3. Almeida RPP, Purcell AH. 2003. Transmission of Xylella fastidiosa to grapevines by Homalodisca coagulata (Hemiptera: Cicadellidae). J. Econ. Entomol. 96:264–271 [DOI] [PubMed] [Google Scholar]
- 4. Caserta R, et al. 2010. Expression of Xylella fastidiosa fimbrial and afimbrial proteins during biofilm formation. Appl. Environ. Microbiol. 76:4250–4259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chaogang S, et al. 2003. Ectopic expression of the spike protein of Rice Ragged Stunt Oryzavirus in transgenic rice plants inhibits transmission of the virus to insects. Mol. Breed. 11:295–301 [Google Scholar]
- 6. Chatterjee S, Almeida RPP, Lindow S. 2008. Living in two worlds: the plant and insect lifestyles of Xylella fastidiosa. Annu. Rev. Phytopathol. 46:243–271 [DOI] [PubMed] [Google Scholar]
- 7. Chiavelli DA, Marsh JW, Taylor RK. 2001. The mannose-sensitive hemagglutinin of Vibrio cholerae promotes adherence to zooplankton. Appl. Environ. Microbiol. 67:3220–3225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Froissart R, Michalakis Y, Blanc S. 2002. Helper component-transcomplementation in the vector transmission of plant viruses. Phytopathology 92:576–579 [DOI] [PubMed] [Google Scholar]
- 9. Gildemeister O, Zhu B, Laine R. 1994. Chitovibrin: a chitin-binding lectin from Vibrio parahaemolyticus. Glyconconj. J. 11:518–526 [DOI] [PubMed] [Google Scholar]
- 10. Guoying Z, et al. 1999. Rice ragged stunt oryzavirus: role of the viral spike protein in transmission by the insect vector. Ann. Appl. Biol. 135:573–5798 [Google Scholar]
- 11. Hill BL, Purcell AH. 1995. Acquisition and retention of Xylella fastidiosa by an efficient vector, Graphocephala atropunctata. Phytopathology 85:209–212 [Google Scholar]
- 12. Hogenhout SA, Ammar ED, Whitfield AE, Redinbaugh MG. 2008. Insect vector interactions with persistently transmitted viruses. Annu. Rev. Phytopathol. 46:327–359 [DOI] [PubMed] [Google Scholar]
- 13. Hopkins DL, Purcell AH. 2002. Xylella fastidiosa: cause of Pierce's disease of grapevine and other emergent diseases. Plant Dis. 86:1056–1066 [DOI] [PubMed] [Google Scholar]
- 14. Killiny N, Almeida RPP. 2009. Host structural carbohydrate induces vector transmission of a bacterial plant pathogen. Proc. Natl. Acad. Sci. U. S. A. 106:22416–22420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Killiny N, Almeida RPP. 2009. Xylella fastidiosa afimbrial adhesins mediate cell transmission to plants by leafhopper vectors. Appl. Environ. Microbiol. 75:521–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Killiny N, Prado SS, Almeida RPP. 2010. Chitin utilization by the insect-transmitted bacterium Xylella fastidiosa. Appl. Environ. Microbiol. 76:6134–6140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kouki A, et al. 2011. Identification of a novel streptococcal adhesin P (SadP) recognizing galactosyl-α1-4-galactose-containing glycoconjugates: convergent evolution of bacterial pathogens to binding of the same host receptor. J. Biol. Chem. 286:38854–38864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Law IJ, Kfir R. 1997. Effect of mannose-binding lectin from peanut and pea on the stem borer Chilo partellus. Entomol. Exp. Appl. 82:261–265 [Google Scholar]
- 19. Liu S, Sivakumar S, Sparks WO, Miller WA, Bonning BC. 2010. A peptide that binds the pea aphid gut impedes entry of Pea enation mosaic virus into the aphid hemocoel. Virology 401:107–116 [DOI] [PubMed] [Google Scholar]
- 20. Machuka J, Van Damme EJM, Peumans WJ, Jackai LEN. 1999. Effect of plant lectins on larval development of the legume pod borer, Maruca vitrata. Entomol. Exp. Appl. 93:179–187 [Google Scholar]
- 21. Meng Y, et al. 2005. Upstream migration of Xylella fastidiosa via pilus-driven twitching motility. J. Bacteriol. 187:5560–5567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nault LR. 1997. Arthropod transmission of plant viruses: a new synthesis. Ann. Entomol. Soc. Am. 90:521–541 [Google Scholar]
- 23. Ng JCK, Falk BW. 2006. Virus-vector interactions mediating nonpersistent and semipersistent transmission of plant viruses. Annu. Rev. Phytopathol. 44:183–212 [DOI] [PubMed] [Google Scholar]
- 24. Purcell AH, Finlay A. 1979. Evidence for non-circulative transmission of Pierce's disease bacterium by sharpshooter leafhoppers. Phytopathology 69:393–395 [Google Scholar]
- 25. Rahbe Y, Sauvion N, Febvay G, Peumans WJ, Gatehouse AMR. 1995. Toxicity of lectins and processing of ingested proteins in the pea aphid Acyrthosiphon pisum. Entomol. Exp. Appl. 76:143–155 [Google Scholar]
- 26. Roper MC, Greve LC, Labavitch JA, Kirkpatrick BC. 2007. Detection and visualization of an exopolysaccharide produced by Xylella fastidiosa in vitro and in planta. Appl. Environ. Microbiol. 73:7252–7258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tarsi R, Pruzzo C. 1999. Role of surface proteins in Vibrio cholerae attachment to chitin. Appl. Environ. Microbiol. 65:1348–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Uzest M, et al. 2007. A protein key to plant virus transmission at the tip of the insect vector stylet. Proc. Natl. Acad. Sci. U. S. A. 104:17959–17964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vandenborre G, Smagghe G, Van Damme EJM. 2011. Plant lectins as defense proteins against phytophagous insects. Phytochemistry 72:1538–1550 [DOI] [PubMed] [Google Scholar]
- 30. Van Sluys MA, et al. 2003. Comparative analyses of the complete genome sequences of Pierce's disease and citrus variegated chlorosis strains of Xylella fastidiosa. J. Bacteriol. 185:1018–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Voegel TM, Warren JG, Matsumoto A, Igo MM, Kirkpatrick BC. 2010. Localization and characterization of Xylella fastidiosa haemagglutinin adhesins. Microbiology 156:2172–2179 [DOI] [PubMed] [Google Scholar]
- 32. Whitfield AE, et al. 2008. A soluble form of the Tomato spotted wilt virus (TSWV) glycoprotein G(N) [G(N)-S] inhibits transmission of TSWV by Frankliniella occidentalis. Phytopathology 98:45–50 [DOI] [PubMed] [Google Scholar]
- 33. Zampini M, et al. 2003. Role for mannose-sensitive hemagglutinin in promoting interactions between Vibrio cholerae El Tor and mussel hemolymph. Appl. Environ. Microbiol. 69:5711–5715 [DOI] [PMC free article] [PubMed] [Google Scholar]