Abstract
Plasmalogens are ether-linked lipids that may influence oxidative stress resistance of eukaryotic cell membranes. Since bacterial membrane composition can influence environmental stress resistance, we explored the prevalence of plasmalogens in the cytoplasmic membrane of Bifidobacterium animalis subsp. lactis. Results showed plasmalogens are a major component of the B. animalis subsp. lactis membrane.
TEXT
Evidence suggests several species of bifidobacteria have probiotic properties (18), and two commercially important species are Bifidobacterium longum and Bifidobacterium animalis subsp. lactis. One of the challenges associated with the use of bifidobacteria probiotics involves the loss of viability due to the unfavorable environmental conditions that are encountered during the manufacture and storage of most food-based delivery systems (19). One potential mechanism to enhance cell survival involves manipulation of bacterial cell membrane fatty acid (CMFA) composition (12, 22). Several studies have shown that CMFA composition influences membrane fluidity, proton permeability, and the activity of a variety of transport proteins (6, 10, 21, 26). Cell exposure to acidic pH, for example, can trigger an increase in the level of saturated, branched, or cyclopropane fatty acids in the cell membrane (1a, 2, 4, 7, 9, 11). These changes render the cell membrane more rigid and result in greater cell membrane stability in acidic environments. Among bifidobacteria, a bile salt-resistant B. animalis subsp. lactis mutant has been shown to increase the unsaturated/saturated fatty acid ratio of its composition during bile salt exposure, whereas wild-type cells showed a decrease in the unsaturated/saturated fatty acid ratio (20). This study also showed that in both strains there was a large decrease in the amount of C19:0 cyclopropyl fatty acid in response to bile salt exposure.
Plasmalogens are phospholipids that contain a vinyl ether bond at the SN1 position instead of an ester bond, and they display physical properties distinct from those of diacyl analogs. Plasmalogens are widespread among eukaryotes, accounting for up to one-fifth of the total phospholipid pool of humans (24). In eukaryotes, cells with high CMFA plasmalogen content are associated with oxidative environments and display lower membrane ion permeability and surface potential and an increase in cell membrane fluidity (24). Additionally, the vinyl ether bond is more easily oxidized than the carbon-carbon double bond of unsaturated fatty acids, and in contrast to oxidized unsaturated fatty acids, plasmalogens do not propagate free radicals in response to peroxides (3, 15). Because of these characteristics, plasmalogens have been proposed to act as antioxidants in membrane physiology via protection of unsaturated fatty acids and membrane proteins from harmful oxidation (5, 27). Several Clostridium, Mycobacterium, and methanogenic archaea species have also been found to possess vinyl ether-linked lipids in their membrane, but little is known about the role of these lipids (8, 11, 13, 16, 25). Very few studies on the membrane composition of bifidobacteria have noted vinyl ether-linked lipids (1); more commonly, these lipids are grouped with their esterified analogs (20). Because of the unique properties of vinyl ether-linked lipids, plasmalogen content should be considered in research that seeks to explore the role of CMFA composition in environmental stress resistance among bifidobacteria. Here, we use a previously described and simple methodology for derivatization of plasmalogens to isolate these lipids and provide mass spectra lacking in the literature but necessary for identification. Results reveal that plasmalogens are a significant component of the cytoplasmic membrane of B. animalis subsp. lactis.
Two industrially important B. animalis subsp. lactis strains, DSM10140 and BL-04 (1b), were maintained as glycerol freezer stocks at −80°C, and working cultures were prepared by two successive transfers (1% inoculum, vol/vol) into peptonized milk medium (MP5) (17) and incubated at 37°C for 18 h in anaerobic chambers (Becton Dickinson Microbiology Systems, Cockeysville, MD). Batch cultures of each strain were prepared by dilution of the working culture to an absorbance at 600 nm (A600) of 1.0 in MP5 medium, inoculated at 1% (vol/vol) into 1 liter of MP5 in a New Brunswick BioFlo III fermentor (New Brunswick Scientific, Edison, NJ), and finally incubated at 37°C with an agitation rate of 100 rpm to prevent sedimentation. A gas mixture of 5% CO2 and 95% N2 was continuously passed over the headspace of the fermentor to achieve anaerobic conditions, and the pH was maintained at 6.5 by automatic addition of 15% (vol/vol) NH4OH. The cultures were incubated until the cells reached early stationary phase (approximately 12 h) (17).
Twenty milliliters of cells was centrifuged and washed twice with phosphate-buffered saline (PBS). Total fatty acids were extracted from cell pellets by acid hydrolysis and methylation according to the MIDI laboratory protocol described by Sasser (23). To inhibit oxidation, butylated hydroxytoluene was added to each sample, and the GC vial headspace was flushed with nitrogen gas. The samples were then analyzed on a GCMS-QP2010S (Shimadzu Scientific Instruments, Columbia, MD) mass spectrometer equipped with a flame ionization detector and fitted with a 10-m guard column and a 30-m DB5 capillary column. The injector temperature was held at 250°C, and 1 μl of the sample was injected splitless. The temperature of the oven was held at 50°C for 1 min, increased to 150°C at 20°C/min, and then increased to 250°C at 4°C/min, with the final temperature of 250°C held for 1 min. Helium was used as the carrier gas at a column flow rate of 1.79 ml/min. Electron impact ionization at 70 eV was used for fragmentation. A bacterial acid methyl ester mix (Supelco, Bellefonte, PA) was used as a standard to identify derivatized methyl esters in the samples. The data were normalized, and the percentage of the total membrane composition was determined for each fatty acid. Because ion fingerprints for plasmalogens are not available in the standard or the literature, samples containing suspected plasmalogens were collected as described and sent to the University of California, Riverside, Analytical Chemistry Instrumentation Facility for GC-accurate mass measurements of ionized lipid fragments. Samples were also sent to the University of Utah Mass Spectrometry and Proteomics Core Facility for precise mass measurements of the intact parent species using GC-electrospray ionization accurate mass measurements on a Waters GCT Premier lock mass.
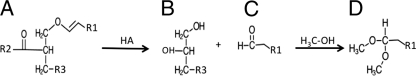
The vinyl ether bond of plasmalogens is easily hydrolyzed under acidic conditions to form an aldehyde, which rapidly reacts to form dimethyl acetals (DMA) in the presence of methanol (Fig. 1) (14). The isolated DMA elute at a lower rate on the GC column than their methyl ester analogs, allowing separation. Figure 2 shows a chromatogram of the membrane fatty acids of B. animalis subsp. lactis strains DSM10140 and BL-04. After analysis and comparison to the standard, peaks of interest were identified based on their elution time and the presence of a strong ion peak of 75 (Fig. 3). Under electron impact ionization, DMA fragments extensively, with the most abundant ion (m/z = 75) resulting from the loss of the DMA head group. This ion peak is the principal identifier of DMA and is unique to these molecules (25). The extracted mass spectrum of each peak (Fig. 3) was analyzed to determine its empirical formula.
Fig 1.
Acid hydrolysis of plasmalogens and derivatization to dimethyl acetals. A, plasmalogen; B, phosphoglycerol; C, fatty aldehyde; D, dimethyl acetal. HA, Bronsted acid; R1/R2, fatty acid carbon tail; R3, phospholipid head group.
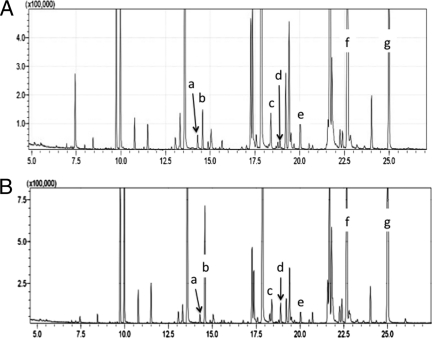
Fig 2.
Gas chromatogram of cytoplasmic membrane lipid extracts from Bifidobacterium animalis subsp. lactis DSM10140 (A) and BL-04 (B) showing peaks for dimethyl acetal-derived plasmalogens. Peaks of interest include C14:1 (a), C14:0 (b), C16:1 (c), C16:0 (d), C17:0 cyclopropyl (e), C18:1 (f), and C19:0 cyclopropyl (g). y axis, signal intensity; x axis, time (min).
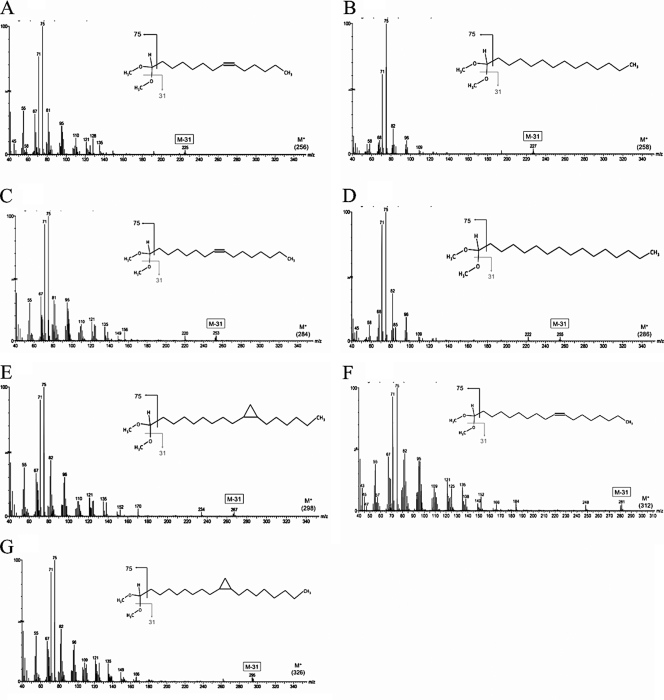
Fig 3.
Mass spectra of dimethyl acetal-derived plasmalogens. Spectra are shown for C14:1 (A), C14:0 (B), C16:1 (C), C16:0 (D), C17:0 cyclopropyl (E), C18:1 (F), and C19:0 cyclopropyl (G). y axis, relative abundance; x axis, mass-to-charge ratio (m/z).
Another important fragmentation product is the parent ion molecular mass minus 31 (M−31) that results from the loss of a methoxy group (Fig. 3). These peaks reveal the numbers of carbons and levels of saturation in the alkenyl moieties of the DMA. Table 1 shows the measured accurate mass-to-charge ratio (m/z) of M−31 for each peak and compares them to the calculated m/z. A parts-per-million (ppm) error was calculated for each m/z, with each error under the threshold value for significance (5 ppm). Under electron impact ionization, these molecules fragment easily and do not produce a detectable parent ion. Electrospray ionization was used to measure the m/z of the parent ion peaks to confirm the empirical formulas of our samples. This is a soft ionization method which allows for the addition of an electron without fragmenting the molecule. The measured accurate m/z of the parent ion peaks were also compared to the calculated m/z, with all having a ppm error of less than 5 (Table 1). Together, these mass spectrometry (MS) data provide positive identification of the DMA in B. animalis subsp. lactis membrane samples.
Table 1.
Measured m/z of DMA-derived plasmalogens
| Fatty acid | Empirical formula | M−31 peaks |
Parent ion peaks |
||
|---|---|---|---|---|---|
| Calculated m/z | Measured accurate m/z | Calculated m/z | Measured accurate m/z | ||
| C14:1 | C16H32O2 | 225.2062 | 225.2216a | 256.2402 | 256.2396a |
| C14:0 | C16H34O2 | 227.2219 | 227.2373a | 258.2559 | 258.2481a |
| C16:1 | C18H36O2 | 253.2375 | 253.2547a | 284.2715 | 284.2697a |
| C16:0 | C18H38O2 | 255.2532 | 255.2679a | 286.2872 | 286.3019a |
| C17:0 cyclopropyl | C19H38O2 | 267.4760 | 267.2675a | 298.5100 | 298.3925a |
| C18:1 | C20H40O2 | 281.2688 | 281.2837a | 312.3028 | 312.3028a |
| C19:0 cyclopropyl | C21H42O2 | 295.2845 | 295.2551a | 326.3185 | 326.3138a |
Measured mass with error of less than 5 ppm.
Previous research in our laboratory (17) showed that B. animalis subsp. lactis BL-04 has significantly greater intrinsic resistance to H2O2 than strain DSM10140. Data collected in this study show that plasmalogens make up a significant proportion of the total membrane composition of B. animalis subsp. lactis DSM10140 and BL-04 (26.34% ± 4.73% and 30.35% ± 5.21%, respectively). Although the amounts of plasmalogens in DSM10140 and BL-04 as a percentage of total CMFA are not significantly different (P = 0.05), levels of C19:0 cyclopropyl vinyl ether lipids are significantly higher (P < 0.05) in strain BL-04 (15.48% ± 7.01%) than in DSM10140 (6.71% ± 1.74%). A previous study (1) reported that oxygen-tolerant fecal isolates of Bifidobacterium had a high content of plasmalogens in the CMFA, and data from that work also support a direct correlation between oxygen tolerance and higher CMFA concentrations of C19:0 cyclopropyl plasmalogens. Because plasmalogens have been shown to have physical attributes that affect membrane physiology differently from those of the ester-linked analogs, it is important to consider these lipids when characterizing the membrane composition of bifidobacteria. The high concentrations of plasmalogens in B. animalis subsp. lactis membranes, together with strain-specific differences in lipid species correlated with H2O2 sensitivity, suggest that these lipids may play an important role in environmental stress resistance. Further study is required to determine and understand the role of plasmalogens in membrane physiology and environmental stress adaptation of bifidobacteria.
ACKNOWLEDGMENTS
This project was supported by National Research Initiative grant no. 2006-35503-17194 from the USDA Cooperative State Research, Education, and Extension Service's Improving Food Quality and Value Program and by the Utah Agricultural Experiment Station.
Peggy Steele, a member of J. L. Steele's family, is employed by Danisco Inc., a supplier of bacterial cultures to the food industry.
Footnotes
Published ahead of print 2 December 2011
This article is contribution 8345 from the Utah Agricultural Experiment Station (UAES), Utah State University, Logan, Utah, USA.
REFERENCES
- 1. Ahn J, Hwang H, Park J. 2001. Physiological responses of oxygen-tolerant anaerobic Bifidobacterium longum under oxygen. J. Microbiol. Biotechnol 11:443–451 [Google Scholar]
- 1a. Annous B, Becker L, Bayles D, Labeda D, Wilkinson B. 1997. Critical role of anteiso-C-15:0 fatty acid in the growth of Listeria monocytogenes at low temperatures. Appl. Environ. Microbiol. 63:3887–3894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1b. Barrangou R, et al. 2009. Comparison of the complete genome sequences of Bifidobacterium animalis subsp. lactis DSM10140 and B1-04. J. Bacteriol. 191:4144–4151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brown J, Ross T, McMeekin T, Nichols P. 1997. Acid habituation of Escherichia coli and the potential role of cyclopropane fatty acids in low pH tolerance. Int. J. Food Microbiol. 37:163–173 [DOI] [PubMed] [Google Scholar]
- 3. Catalá A. 2009. Lipid peroxidation of membrane phospholipids generates hydroxy-alkenals and oxidized phospholipids active in physiological and/or pathological conditions. Chem. Phys. Lipids. 157:1–11 [DOI] [PubMed] [Google Scholar]
- 4. Corcoran BM, Stanton C, Fitzgerald GF, Ross RP. 2007. Growth of probiotic lactobacilli in the presence of oleic acid enhances subsequent survival in gastric juice. Microbiology 153:291–299 [DOI] [PubMed] [Google Scholar]
- 5. Engelmann B. 2004. Plasmalogens: targets for oxidants and major lipophilic antioxidants. Biochem. Soc. Trans. 32:147–150 [DOI] [PubMed] [Google Scholar]
- 6. Foster JW, Moreno M. 1999. Inducible acid tolerance mechanisms in enteric bacteria. Novartis Found. Symp. 221:55–69 [DOI] [PubMed] [Google Scholar]
- 7. Fozo E, Quivey R., Jr 2004. Shifts in the membrane fatty acid profile of Streptococcus mutans enhance survival in acidic environments. Appl. Environ. Microbiol. 70:929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guan Z, et al. 2011. Structural characterization of the polar lipids of Clostridium novyi NT. Further evidence for a novel anaerobic biosynthetic pathway to plasmalogens. Biochim. Biophys. Acta 1811:186–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guerzoni ME, Lanciotti R, Cocconcelli PS. 2001. Alteration in cellular fatty acid composition as a response to salt, acid, oxidative and thermal stresses in Lactobacillus helveticus. Microbiology 147:2255–2264 [DOI] [PubMed] [Google Scholar]
- 10. Hall HK, Karen KL, Foster JW. 1995. Molecular responses of microbes to environmental pH stress, p 229–272 In Poole RK. (ed), Advances in microbial physiology, vol 37 Academic Press, London, United Kingdom: [DOI] [PubMed] [Google Scholar]
- 11. Hutkins R, Nannen N. 1993. pH homeostasis in lactic acid bacteria. J. Dairy Sci. 76:2354–2365 [Google Scholar]
- 12. Jordan S, Hutchings MI, Mascher T. 2008. Cell envelope stress response in Gram-positive bacteria. FEMS Microbiol. Rev. 32:107–146 [DOI] [PubMed] [Google Scholar]
- 13. Koga Y, Nishihara M, Morii H, Akagawa-Matsushita M. 1993. Ether polar lipids of methanogenic bacteria: structures, comparative aspects, and biosyntheses. Microbiol. Rev. 57:164–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kresge AJ, Chiang Y. 1967. The hydrolysis of ethyl vinyl ether. Part 1. Reaction mechanism. J. Chem. Soc. B 1967:53–57 [Google Scholar]
- 15. Magnusson CD, Haraldsson GG. 2011. Ether lipids. Chem. Phys. Lipids 164:315–340 [DOI] [PubMed] [Google Scholar]
- 16. Matsumi R, Atomi H, Driessen AJM, van der Oost J. 2011. Isoprenoid biosynthesis in Archaea—biochemical and evolutionary implications. Res. Microbiol. 162:39–52 [DOI] [PubMed] [Google Scholar]
- 17. Oberg TS, et al. 2011. Intrinsic and inducible resistance to hydrogen peroxide in Bifidobacterium species. J. Ind. Microbiol. Biotechnol. 38:1947–1953. [DOI] [PubMed] [Google Scholar]
- 18. Parvez S, Malik K, Ah Kang S, Kim H-Y. 2006. Probiotics and their fermented food products are beneficial for health. J. Appl. Microbiol. 100:1171–1185 [DOI] [PubMed] [Google Scholar]
- 19. Ross RP, Desmond C, Fitzgerald GF, Stanton C. 2005. Overcoming the technological hurdles in the development of probiotic foods. J. Appl. Microbiol. 98:1410–1417 [DOI] [PubMed] [Google Scholar]
- 20. Ruiz L, Sãnchez B, Ruas-Madiedo P, De Los Reyes-Gavilã CGN, Margolles A. 2007. Cell envelope changes in Bifidobacterium animalis ssp. lactis as a response to bile. FEMS Microbiol. Lett. 274:316–322 [DOI] [PubMed] [Google Scholar]
- 21. Russell NJ. 1989. Functions of lipids: structural roles and membrane functions, p 279–365 In Hatiedge C, Wilkinson SC. (ed), Microbial lipids, vol 2 Academic Press, London, United Kingdom [Google Scholar]
- 22. Russell NJ, et al. 1995. Membranes as a target for stress adaptation. Int. J. Food Microbiol. 28:255–261 [DOI] [PubMed] [Google Scholar]
- 23. Sasser M. 1990. Identification of bacteria by gas chromatography of cellular fatty acids.Technical note 101; Midi, Inc, Newark, DE: http://www.microbialid.com/PDF/TechNote_101.pdf [Google Scholar]
- 24. Snyder F, Lee T, Wykle R. 2002. Ether-linked lipids and their bioactive species. New Compr. Biochem. 36:233–262 [Google Scholar]
- 25. Timmons MD, Knutson BL, Nokes SE, Strobel HJ, Lynn BC. 2009. Analysis of composition and structure of Clostridium thermocellum membranes from wild-type and ethanol-adapted strains. Appl. Microbiol. Biotechnol. 82:929–939 [DOI] [PubMed] [Google Scholar]
- 26. Vigh L, et al. 2005. The significance of lipid composition for membrane activity: new concepts and ways of assessing function. Prog. Lipid Res. 44:303–344 [DOI] [PubMed] [Google Scholar]
- 27. Wang G, Wang T. 2010. The role of plasmalogen in the oxidative stability of neutral lipids and phospholipids. J. Agric. Food Chem. 58:2554–2561 [DOI] [PubMed] [Google Scholar]