Abstract
Recovery of spores from environmental surfaces varies due to sampling and analysis methods, spore size and characteristics, surface materials, and environmental conditions. Tests were performed to evaluate a new, validated sponge wipe method using Bacillus atrophaeus spores. Testing evaluated the effects of spore concentration and surface material on recovery efficiency (RE), false-negative rate (FNR), limit of detection (LOD), and their uncertainties. Ceramic tile and stainless steel had the highest mean RE values (48.9 and 48.1%, respectively). Faux leather, vinyl tile, and painted wood had mean RE values of 30.3, 25.6, and 25.5, respectively, while plastic had the lowest mean RE (9.8%). Results show roughly linear dependences of RE and FNR on surface roughness, with smoother surfaces resulting in higher mean REs and lower FNRs. REs were not influenced by the low spore concentrations tested (3.10 × 10−3 to 1.86 CFU/cm2). Stainless steel had the lowest mean FNR (0.123), and plastic had the highest mean FNR (0.479). The LOD90 (≥1 CFU detected 90% of the time) varied with surface material, from 0.015 CFU/cm2 on stainless steel up to 0.039 on plastic. It may be possible to improve sampling results by considering surface roughness in selecting sampling locations and interpreting spore recovery data. Further, FNR values (calculated as a function of concentration and surface material) can be used presampling to calculate the numbers of samples for statistical sampling plans with desired performance and postsampling to calculate the confidence in characterization and clearance decisions.
INTRODUCTION
Following the intentional, mail-borne anthrax contamination of several U.S. facilities in 2001, questions have been raised concerning the reliability of surface-sampling techniques for detecting anthrax contamination indoors. According to the Centers for Disease Control and Prevention (CDC) (8), over 125,000 samples from the contaminated buildings were taken during the 2001 incident and processed by the Laboratory Response Network (LRN)—an integrated network of state and local public health, federal, military, and international laboratories that can respond to bioterrorism, chemical terrorism, and other public health emergencies. However, results from surface samples were inconsistent. In some cases, contamination at a given location within a facility was not detected with initial samples, and subsequent samples were required to detect the contamination (19). A Government Accountability Office (GAO) investigation following the 2001 anthrax incident concluded that validated sampling methods and statistical sampling designs were needed to provide confidence that there is no contamination when all sample results are negative (17, 18). This conclusion strongly reinforces the need for validated sampling methods to effectively respond to biothreats and ensure public safety.
Following the 2001 anthrax incident, several research teams developed and investigated (in laboratory studies) the performance of sampling methods using swab, wipe, and vacuum collection devices for Bacillus anthracis or surrogate contaminants on different surfaces (1, 2, 3, 4, 5, 6, 7, 13, 14, 15, 16, 22, 23, 25, 28, 34, 38, 40, 44, 45). In addition, the CDC has conducted formal validation studies on two methods for sampling nonporous surfaces: macrofoam swabs (23) and cellulose sponge wipes (39).
A review of the cited laboratory studies identified numerous gaps in the data on method performance (30). For example, none of the studies quantified the false-negative rate (FNR) for the sampling and analysis methods investigated. The term “false negative” refers to a failure to detect contamination from a sample collected at a contaminated location. A false negative can occur because of inefficiencies (i.e., biases) and uncertainties (i.e., imprecision) at any step of the sampling and analysis process (sample collection, storage/transportation, processing/extraction, and analytical). False negatives can occur during preliminary screening or characterization sampling at low contamination levels and during clearance sampling following the decontamination process. A better understanding of FNRs and how they are influenced by surface materials and contaminant concentrations is critical in addressing the GAO concerns about method validation and increasing the confidence in negative results. Further, environmental sample collection and analysis for microbial contamination requires a sensitive (low limit of detection [LOD] and FNR) methodology to ensure public safety.
Another gap was a lack of wide testing of the sponge wipe sampling method. The food industry has used sponge wipe methods for decades (12), and the CDC-validated sponge wipe sampling and analysis method (39) is expected to have extensive use in environmental sampling. However, this method has not been tested at lower contaminant concentrations that may yield false negatives.
The study described in this article addresses these two gaps. The study evaluated the sponge wipe sampling method by testing very low concentrations of spores (Bacillus atrophaeus) deposited on coupons of a variety of nonporous surface materials, followed by surface sampling, extraction, and analysis. Test results were used to evaluate the effects of contaminant concentrations and surface materials on FNR, recovery efficiency (RE), and LOD, as well as the uncertainties of these values. The findings of this study provide new insights for interpreting negative results from sponge wipe surface samples. Also, the variation in RE and FNR for different surface materials will be of high interest in the field of public health.
MATERIALS AND METHODS
Test overview.
The study investigated the performance of the cellulose sponge wipe method for a range of low surface concentrations on coupons of six surface materials. The surface sampling procedure provides (i) a standardized method of processing cellulose sponge wipes of environmental surfaces to culture Bacillus spores and (ii) a semiquantitative estimate of the amount of contamination at the sampled location.
The sponge wipe method uses traditional culture methods because organism viability is important in an environmental investigation. Spores are removed from the wipe by mechanical extraction in phosphate-buffered saline with Tween 80 (PBST) as a surfactant. The eluted suspension is diluted in series (when necessary), and aliquots are inoculated onto aerobic growth plates, as well as onto membrane filters that are placed on tryptic soy agar (TSA) growth plates. The membrane filters maximize the detection of low numbers of spores. After a 48-h incubation, CFU are counted. The procedure was modified to reflect a biohazard level (BL) 1 for the test microorganism, B. atrophaeus, rather than a BL3, as is necessary for B. anthracis (43).
The experimental work was conducted in 16 test runs, with one surface concentration of B. atrophaeus investigated in each test run. In test runs 1 to 8 (block 1), target spore concentrations of 3.10 × 10−3, 7.70 × 10−3, 1.55 × 10−2, 2.33 × 10−2, 3.10 × 10−2, 1.55 × 10−1, and 1.86 CFU/cm2 were used on coupons of three materials (stainless steel, vinyl tile, and ceramic tile). In test runs 9 to 16 (block 2), target concentrations of 7.70 × 10−3, 1.55 × 10−2, 2.33 × 10−2, 3.10 × 10−2, 3.88 ×10−2, 5.43 × 10−2, 7.75 × 10−2, and 1.55 × 10−1 CFU/cm2 were used on coupons of three different materials (primed wood paneling, faux leather, and plastic [acrylic] lighting panels). The concentrations tested in block 2 were different than in block 1 to improve the profile of false-negative rates achieved in block 2. These concentrations were selected based on preliminary test results (data not shown). Within each block of eight test runs, the concentrations were tested in a randomized order. For each test run, 10 coupons of each of the appropriate three materials were assigned in a balanced way to the bench test locations (so that materials would appear roughly the same number of times in the possible bench locations over the course of the study). Three technicians were assigned to three steps of the sampling and analysis process (collecting, processing, enumerating) in a balanced way. These balanced assignments protected against confounding any effects of test locations and technicians with the primary test variables (i.e., contaminant concentration and surface material) (29).
Spore characteristics.
The surrogate organism for B. anthracis in these tests was B. atrophaeus spores, American Type Culture Collection (ATCC) no. 9372 (formerly Bacillus subtilis var. Niger), from Apex Laboratories (Apex, NC). The material was lot no. 718701-E9 with a mean population of 3.5 × 109 CFU/ml in water, post-heat shocked at 80 to 85°C for 10 min. These spores ranged in size from about 0.9 to 1.1 μm. Spore stock solutions were made in PBS with 0.01% Tween buffer. PBS was autoclaved and then sterile filtered after the addition of Tween. For each test, the spore concentration was carefully adjusted to the target value and tested for accuracy by plate counts. The concentrations were also verified periodically (throughout the duration of the corresponding test run) by plating eight replicate samples per concentration.
Coupon characteristics.
Coupons were cut to match the recommended size (39) for surface wipe samples, 25.4 by 25.4 cm (645.16 cm2). Sample surface materials selected for testing (stainless steel, vinyl tile, ceramic tile, faux leather, plastic light panel, and primed wood) were relatively nonporous and chosen to represent materials commonly found in buildings. Stainless steel served as the standard test surface, because the majority of previous sampling studies were performed on stainless steel. It represents a universally recognized carrier and also serves as a conservative proxy for building material roughness. The stainless steel coupons were cut from 1.2-mm-thick 316L stainless steel (Neeley Plastic Fabrication Inc., Albuquerque, NM). The vinyl tiles were Armstrong Excelon vinyl composition tile (no. 51830; Armstrong World Industries Inc., Lancaster, PA). The glazed ceramic tiles were from Dal-Tile Corp. (Dallas, TX). The faux leather is a fabric made of polyester yarn with a vinyl surface finished with a urethane topcoat (Spradling International, Inc., Pelham, AL). The plastic light cover was an acrylic, cracked-ice ceiling light panel (Plaskolite, Inc., Columbus, OH). The wood panel was primed with acrylic paint (DPI Decorative Panels Intl., Toledo, OH).
Prior to spore deposition, the stainless steel, ceramic tile, vinyl tile, and plastic light cover coupons were washed with Alcojet powdered detergent (Alconox Inc., New York, NY), rinsed, treated with 10% bleach (30-min contact time), rinsed in deionized water, and air dried. The faux leather and primed wood coupons were treated with 10% bleach (30-min contact time), wiped with sodium thiosulfate to neutralize the bleach, and then air dried. There was no measurable chlorine found in control samples. This modification to the coupon cleaning treatment was necessary to avoid damaging these two materials. Precleaned coupons were placed on clean laboratory tabletops in predetermined locations. Each was wiped with 95% ethanol using a sterile gauze wipe, and the right corner of each coupon (outside the sampling area) was labeled with a unique number linked to the sample's type, number, and placement.
The surface roughness of coupon materials was measured using a surface roughness tester (SRG-1000; Phase II Machine & Tool Inc.). Roughness is a measure of vertical deviations of the surface material. The tester uses a piezoelectric pickup stylus with diamond tip to measure a range between 0.05 and 10.0 μm.
Spore deposition.
Spores were deposited onto coupons using a liquid inoculation technique. Sampling method performance studies have primarily employed protocols that use spores suspended in aqueous buffers to prepare test surfaces (35, 36, 42). Liquid inoculation methods allow relatively easy control of the number of spores and the contaminated area. Liquid deposition was used in this study because some concentrations to be tested were well below the sample method's LOD and preliminary testing showed that such concentrations could not be accurately achieved when using dry aerosol deposition methods.
The spores in buffer were dropped onto the cleaned and sterilized 25.4- by 25.4-cm coupons. Each spore stock solution was stirred constantly using a stir plate (F37017-0000 battery-powered magnetic stirrer; Scienceware). From the spore stock solution, a 1-ml pipettor (Thermo Electron 4500120, calibrated following manufacture's recommendations) was used to deposit 20 droplets (0.05 ml each) on each coupon in a pattern from left to right. During the tests, the temperature was maintained at 25 ± 2°C and the relative humidity was maintained between 30 and 45%. The inoculated coupons were dried for approximately 2 h.
Positive and negative controls.
A positive control (reference) sample was colocated with each test coupon (i.e., 30 test coupons and 30 colocated positive-control samples per test). A 1-ml sample of the spore titer solution was directly inoculated onto Petrifilm aerobic count plates (3M, St. Paul, MN). The reference plates were incubated for 48 h; the results were counted and recorded on an automatic plate count reader (Petrifilm plate reader, model 6499; 3M, St. Paul, MN), and the counts were manually verified. The counts of reference plates were used to verify the concentrations of each spore stock solution and served as the reference value for RE calculations. Reference and test samples were processed using the same extraction and analytical method.
For each of the 16 tests, 16% of the samples (5 samples per 30 test samples) were laboratory process controls and 25% of the samples (8 medium samples per 30 test samples) were analyzed as material and medium (culture and buffer) control samples. All laboratory material and medium control samples were negative for spore growth.
Wipe sampling and analysis method.
This study used the sampling procedure to collect samples on hard nonporous surfaces that may occur in both indoor and outdoor environments (9). Using a sterile technique and a sterile, premoistened sponge wipe, each test coupon was wiped using an overlapping S pattern with horizontal strokes. The wipe was then rotated, and the coupon was wiped using vertical S strokes. The sample area was then wiped using diagonal S strokes, and the process was concluded by wiping the edges of the coupon.
Laboratory processing methods.
A modified LRN procedure for recovering spores from wipes (39) was used. Spore extraction from the sponge wipe was accomplished by transferring each sponge wipe to the stomacher bag (Stomacher 400 Circulator, no. 0400/001/AJ; closure bags, no. BA6141/CLR; both from Seward) using sterile forceps. PBST (90 ml) was added to the bag and the stomacher was set to 260 rpm for 1 min. The wipe was squeezed and moved to the top of the bag and then removed with sterile forceps. The elution suspension was mixed and then centrifuged at 3,500 × g for 15 min. The 90-ml elution suspension was concentrated to 6 ml. The concentrated suspension was vortexed for 30 s and sonicated at 40 kHz in a sonicator bath (Branson ultrasonic cleaner, model 1510, no. 952-116; Process Equipment and Supply, Inc.) for 30 s; sonication and vortexing steps were repeated twice in order to evenly disperse the spores. The final volume of suspension was measured. Aliquots of the spore elution were serially diluted in Butterfield buffer using standard methods. One milliliter of the dilution was plated on aerobic growth medium in triplicate.
If there was no growth on a plate, then an aliquot (1 ml) of the remaining spore suspension was filtered (vacuum filtration manifold and MicroFunnel filter funnels; Fisher Scientific) using microfunnel membranes (0.45-μm-pore-size MCE membrane, no. 28143-544; VWR) and the membrane was cultured on TSA plates.
Recovery efficiency, false-negative rate, and uncertainties.
REs were calculated as
| (1) |
where REhijk is the RE of the sponge wipe method for the kth coupon of the jth material with the ith concentration in the hth block of tests; Rhijk is the CFU recovered from the kth coupon of the jth material with the ith concentration in the hth block; and Chi is the mean CFU from the positive controls for the ith concentration in the hth block. The mean and standard deviation (SD) of RE over the nhij (usually 10) coupons of the jth material with the ith concentration in the hth block were calculated (using standard formulas) and are denoted as REhij and SD(REhij). The standard error (SE) of REhij was calculated as
| (2) |
and the percent relative standard deviation (%RSD) was calculated as
| (3) |
The notation FNRhijk represents the FNR of the sponge wipe method for the kth coupon of the jth material with the ith concentration in the hth block. The mean and standard deviation of these FNRhijk values for each “hij” combination were calculated (using the standard formulas) over the nhij test coupon samples and are denoted as FNRhij and SD(FNRhijk), respectively. The standard error of FNRhij was calculated as
| (4) |
Below the contaminant concentration at which false negatives first begin to occur for the sponge wipe method, the FNR generally increases as concentration decreases. Using statistical methods to develop equations that relate experimentally determined FNRs to the concentration of the contaminant was therefore of interest. Such equations allow predicting the FNR at any concentration within the range that false negatives occur. Further, statistical methods enable calculating the uncertainty in the predicted FNR at a given concentration. The three-coefficient, cumulative-distribution form of the Johnson SB equation (20, 26)
| (5) |
was used to relate FNR to contaminant concentration for each of the six surface materials (j = 1, 2, …, 6). FNRhijk and C̄hi were defined previously, where 0 ≤ C̄hi ≤ λj, Φ is the standard normal (Gaussian) cumulative distribution function, and γj, δj (>0), and λj (>0) are three coefficients that depend on surface material and were estimated from the experimental data by nonlinear weighted-least-squares regression (41).
Limits of detection and uncertainties.
The LOD of the sponge wipe method for a given surface material was defined and estimated in two ways. LOD90 is the lowest concentration reliably detected (i.e., at least one CFU > 90% of the time) (21). LOD95 is the contaminant concentration for which there is a 95% probability of correct detection (PCD). An estimate of the LOD95 is calculated for a given surface material using the corresponding FNR-concentration equation of the form in equation 5. Specifically, the LOD95 is the concentration at which the equation predicts FNR = 0.05 (i.e., the PCD = 0.95). The statistical bootstrap method (11) was used to calculate a 95% confidence interval for the LOD95 of each surface material.
RESULTS
Recovery efficiencies and uncertainties.
Table 1 summarizes the mean recovery efficiencies (REhij) and uncertainties [%RSD(REhij)] for the sponge wipe method for each combination of concentration and surface material. Because a positive control was paired with each of the 30 test coupons in each test run, the option existed to calculate RE using the results from each pair of test coupon and positive control. However, analysis of the positive-control data revealed no significant differences in results by bench location or technician. Hence, REs were calculated using equation 1, the denominator of which is the mean contaminant concentration of the 30 positive-control samples in a given test run.
Table 1.
Performance measures of sponge wipes with liquid-deposited Bacillus atrophaeus spores on coupons of six surface materials
| Test coupons |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive control concn (CFU/cm2) |
Recovery concn (CFU/cm2) |
Recovery efficiency (%) |
FNR |
|||||||||
| Target deposition, CFU/coupona (CFU/cm2) | Test no. | Blockb | Meanc | %RSDc | Surface materiald | No. of test coupons | Meane | %RSDe | Meanf | %RSDf | Meang | SDg |
| 2 (0.00310) | 3 | 1 | 0.00248 | 58.3 | S | 10 | 0.00155 | 105.41 | 62.5 | 105.94 | 0.600 | 0.322 |
| V | 10 | 0.00031 | 316.23 | 12.5 | 316.41 | 0.933 | 0.211 | |||||
| C | 10 | 0.00093 | 161.02 | 37.5 | 161.37 | 0.800 | 0.322 | |||||
| 5 (0.00775) | 5 | 1 | 0.00677 | 25.9 | S | 10 | 0.00372 | 76.58 | 55.0 | 76.72 | 0.367 | 0.367 |
| V | 10 | 0.00217 | 96.42 | 32.1 | 96.54 | 0.600 | 0.344 | |||||
| C | 10 | 0.00217 | 96.42 | 32.1 | 96.54 | 0.567 | 0.387 | |||||
| 9 | 2 | 0.00775 | 15.8 | L | 10 | 0.00031 | 316.23 | 4.0 | 316.62 | 0.933 | 0.211 | |
| W | 10 | 0.00124 | 129.10 | 16.0 | 129.13 | 0.733 | 0.344 | |||||
| P | 10 | 0.0 | 0.0 | 0.0 | 0.0 | 1.0 | 0.0 | |||||
| 10 (0.01550) | 4a | 1 | 0.01669 | 10.5 | S | 10 | 0.00961 | 32.08 | 57.6 | 32.14 | 0.033 | 0.105 |
| V | 10 | 0.01085 | 43.12 | 65.0 | 43.16 | 0.0 | 0.0 | |||||
| C | 10 | 0.00899 | 59.62 | 53.9 | 59.65 | 0.100 | 0.161 | |||||
| 4b | 1 | 0.01519 | 14.3 | S | 10 | 0.00961 | 28.25 | 63.3 | 28.37 | 0.0 | 0.0 | |
| V | 10 | 0.00713 | 21.00 | 46.9 | 21.16 | 0.0 | 0.0 | |||||
| C | 10 | 0.01147 | 42.35 | 75.5 | 42.43 | 0.0 | 0.0 | |||||
| 15 | 2 | 0.01535 | 15.3 | L | 10 | 0.00651 | 41.70 | 42.4 | 41.79 | 0.100 | 0.316 | |
| W | 10 | 0.00279 | 81.98 | 18.2 | 82.03 | 0.500 | 0.360 | |||||
| P | 10 | 0.00031 | 316.23 | 2.0 | 316.24 | 0.933 | 0.211 | |||||
| 15 (0.02325) | 7 | 1 | 0.02253 | 12.6 | S | 10 | 0.01116 | 14.34 | 49.5 | 14.53 | 0.0 | 0.0 |
| V | 10 | 0.00341 | 67.08 | 15.1 | 67.12 | 0.367 | 0.367 | |||||
| C | 10 | 0.01085 | 27.77 | 48.2 | 27.86 | 0.0 | 0.0 | |||||
| 11a | 2 | 0.02356 | 10.1 | L | 10 | 0.00837 | 30.49 | 35.5 | 30.55 | 0.033 | 0.105 | |
| W | 10 | 0.00713 | 21.00 | 30.3 | 21.08 | 0.0 | 0.0 | |||||
| P | 10 | 0.00155 | 105.41 | 6.6 | 105.43 | 0.67 | 0.351 | |||||
| 11b | 2 | 0.02289 | 11.6 | L | 10 | 0.00682 | 28.75 | 29.8 | 28.83 | 0.033 | 0.105 | |
| W | 10 | 0.00589 | 29.88 | 25.7 | 29.95 | 0.077 | 0.141 | |||||
| P | 10 | 0.00031 | 316.23 | 1.4 | 316.23 | 0.933 | 0.211 | |||||
| 20 (0.03100) | 1 | 1 | 0.03064 | 10.4 | S | 10 | 0.01581 | 19.50 | 51.6 | 19.59 | 0.0 | 0.0 |
| V | 9 | 0.00586 | 31.81 | 19.1 | 31.87 | 0.074 | 0.147 | |||||
| C | 10 | 0.01581 | 28.41 | 51.6 | 28.48 | 0.033 | 0.104 | |||||
| 13 | 2 | 0.03276 | 9.8 | L | 10 | 0.00899 | 25.44 | 27.4 | 25.51 | 0.033 | 0.105 | |
| W | 10 | 0.00341 | 51.60 | 10.4 | 51.64 | 0.333 | 0.272 | |||||
| P | 10 | 0.00093 | 161.02 | 2.8 | 161.03 | 0.800 | 0.322 | |||||
| 25 (0.03875) | 2 | 1 | 0.03725 | 9.4 | S | 10 | 0.01333 | 22.06 | 35.8 | 22.13 | 0.0 | 0.0 |
| V | 10 | 0.00682 | 28.75 | 18.3 | 28.80 | 0.067 | 0.141 | |||||
| C | 9 | 0.01584 | 18.16 | 42.5 | 18.24 | 0.0 | 0.0 | |||||
| 16 | 2 | 0.03834 | 7.0 | L | 10 | 0.00930 | 35.14 | 24.3 | 35.16 | 0.033 | 0.105 | |
| W | 10 | 0.00930 | 31.43 | 24.3 | 31.45 | 0.0 | 0.0 | |||||
| P | 10 | 0.00527 | 39.70 | 13.7 | 39.72 | 0.200 | 0.322 | |||||
| 35 (0.05425) | 10 | 2 | 0.05430 | 6.6 | L | 10 | 0.01550 | 21.08 | 28.5 | 21.12 | 0.0 | 0.0 |
| W | 10 | 0.01798 | 15.84 | 33.1 | 15.89 | 0.0 | 0.0 | |||||
| P | 10 | 0.00651 | 35.14 | 12.0 | 35.16 | 0.100 | 0.161 | |||||
| 50 (0.07750) | 12 | 2 | 0.07905 | 4.4 | L | 10 | 0.06076 | 27.86 | 76.9 | 27.87 | 0.0 | 0.0 |
| W | 10 | 0.03906 | 16.39 | 49.4 | 16.41 | 0.0 | 0.0 | |||||
| P | 10 | 0.01395 | 24.00 | 17.6 | 24.02 | 0.0 | 0.0 | |||||
| 100 (0.15500) | 6 | 1 | 0.15629 | 3.7 | S | 10 | 0.07194 | 14.34 | 46.0 | 14.35 | 0.0 | 0.0 |
| V | 9 | 0.03548 | 18.07 | 22.7 | 18.08 | 0.0 | 0.0 | |||||
| C | 10 | 0.07037 | 12.29 | 45.0 | 12.31 | 0.0 | 0.0 | |||||
| 14 | 2 | 0.15371 | 3.0 | L | 10 | 0.08339 | 18.99 | 54.3 | 19.00 | 0.0 | 0.0 | |
| W | 10 | 0.05642 | 18.82 | 36.7 | 18.83 | 0.0 | 0.0 | |||||
| P | 10 | 0.03968 | 22.04 | 25.8 | 22.04 | 0.0 | 0.0 | |||||
| 1,200 (1.86000) | 8 | 1 | 1.85380 | 2.9 | S | 10 | 0.97402 | 8.84 | 52.5 | 8.86 | 0.0 | 0.0 |
| V | 10 | 0.46934 | 10.55 | 25.3 | 10.56 | 0.0 | 0.0 | |||||
| C | 10 | 0.99355 | 6.56 | 53.6 | 6.58 | 0.0 | 0.0 | |||||
Target number of spores deposited per 25.4-cm by 25.4-cm (645.16 cm2) coupon.
Block of testing (1, 2) where a and b denote replicate tests at a given concentration.
Mean and %RSD of concentrations, calculated over 30 positive controls for each target concentration in a block.
S, stainless steel; V, vinyl tile; C, ceramic tile; L, faux leather; W, painted wood; P, plastic.
Mean and %RSD of recovery concentrations calculated over the number of test coupons for a target concentration and surface material.
Mean and %RSD of recovery efficiencies calculated over the number of test coupons for a target concentration and surface material.
Mean and SD of FNRs calculated over the number of test coupons for a target concentration and surface material.
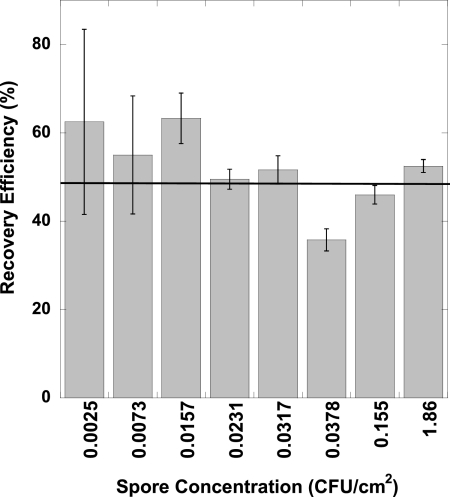
Over the range of B. atrophaeus concentrations tested (2.48 × 10−3 to 1.85 CFU/cm2, based on average concentrations of positive-control samples) there was no dependence of RE on spore concentration for any surface material. However, uncertainties in RE values tend to increase as the concentration decreases, as shown in Table 1. To illustrate these conclusions for stainless steel coupons, Fig. 1 displays the REhij values with ±1 SE(REhij) error bars. The REhij values for stainless steel range from 36 to 63% (average of 52%), while the SE(REhij) values ranged from 2 to 21%.
Fig 1.
Mean values of recovery efficiency for the sponge wipe method applied to stainless steel coupons plotted versus spore concentration from positive-control samples. The error bars are ±1 SE, as calculated by equation 2. The horizontal line across the figure shows the average recovery efficiency for all concentrations (52%).
A weighted analysis of variance with Tukey's multiple comparison procedure showed that the different surface materials tested have different mean RE values. Table 2 lists the mean RE (across all concentrations) for each surface material, noting the pairs of surface materials with statistically different RE mean values (P < 0.0001). Ceramic tile and stainless steel had the largest mean RE values (48.1 and 48.9%, respectively); these mean values were not statistically different. Faux leather, vinyl tile, and painted wood had mean RE values of 30.3, 25.6, and 25.5, respectively. Some, but not all, pairs of these materials had statistically different mean RE values (Table 2). Plastic had the lowest mean RE (9.8%), which is significantly lower than the mean REs of all other materials.
Table 2.
Recovery efficiency and false negative rate (averaged over all spore concentrations) for each surface material with the corresponding roughness index measurement
| Surface material | Recovery efficiency, mean (%) | Comparison of RE meansa | False-negative rate, mean | Roughness indexb (μm) |
|---|---|---|---|---|
| Stainless steel | 48.1 | A | 0.1229 | 0.13 |
| Ceramic tile | 48.9 | A | 0.1812 | 0.59 |
| Vinyl tile | 25.6 | B, D | 0.2551 | 1.63 |
| Faux leather | 30.3 | B, C | 0.1417 | 3.27 |
| Painted wood | 25.5 | C, D | 0.2000 | 4.11 |
| Plastic panel | 9.8 | 0.4792 | 5.88 |
All pairs of surface materials have statistically different mean RE values (P < 0.0001) except those pairs with the same letters (A, B, C, D), based on a weighted analysis of variance with Tukey's multiple comparison procedure (27). For example, stainless steel (A) and ceramic (A) are not statistically different from each other but are different from all other materials. No letter is shown for plastic panel because its mean RE was significantly lower than the mean REs of all other surface materials.
Roughness parameter Ra (arithmetic average of absolute values) was computed to conform to ISO class 3.
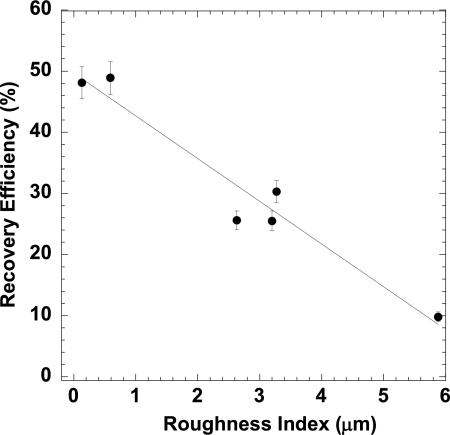
To further explore the dependence of RE on surface materials, mean RE values were compared to the roughness indices of the test materials. The mean RE (across all spore concentrations) for each surface material and the corresponding roughness index measurement are listed in Table 2. The roughness indices range from 0.13 to 5.88 μm. Figure 2 shows that there is a roughly linear dependence of RE on surface roughness, with the smoothest surfaces showing the largest mean RE values. The standard errors for the mean RE values shown in Fig. 2 were calculated using a bootstrap approach (11), accounting for two sources of variation (between and within concentrations).
Fig 2.
Mean percent recovery efficiency versus roughness index (μm) of the six material surfaces tested using the sponge wipe method (see Table 2 for the roughness index of surface materials). The error bars are ±1 SE.
FNRs and uncertainties.
FNRs were calculated using the positive-control data and test coupon data, as described previously. No false negatives occurred for positive-control samples, except for test 3 at a target concentration of 3.10 × 10−3 CFU/cm2 (i.e., 2 CFU/coupon). For that test, the FNR values for the 30 positive-control samples had mean = 0.133 and SE = 0.024.
For the test coupon data, Table 1 lists the mean (FNRhij) and standard deviation [SD(FNRhijk)] values of FNR for each combination of surface material and target concentration (ij). The FNRhij values range from 0 to 1. The SD(FNRhijk) values are relatively large (ranging up to 0.387) because they are the uncertainties in FNR values for a single test coupon. Although not shown in Table 1, the SE(FNRhij) values were calculated using equation 4; they range up to 0.122.
Table 3 shows the coefficients from fitting equation 5 to the FNR-concentration data for each of the six surface materials. The coefficients can be used to predict the FNR for a given concentration and surface material. For example, the FNR of the sponge wipe method for a concentration of 5.0 × 10−2 on vinyl tile is predicted by substituting the coefficients from Table 3 into equation 5, which yields 0.014. Figure 3 shows the mean FNR results from test coupons of the six surface materials (FNRhij) plotted against contaminant concentration (C̄hi) from the positive-control data. Figure 3 also shows the corresponding fitted equations from Table 3.
Table 3.
Coefficients and R2 values for the Johnson SB equation (equation 5)a
| Surface material | Value for coefficientb |
||
|---|---|---|---|
| γ | δ | λ | |
| Stainless steel | 3.205 | 0.958 | 0.079 |
| Ceramic tile | 4.736 | 1.898 | 0.079 |
| Vinyl tile | 1.705 | 0.929 | 0.079 |
| Faux leather | 5.873 | 3.483 | 0.079 |
| Painted wood | 3.506 | 1.838 | 0.079 |
| Plastic | 0.506 | 1.900 | 0.079 |
The Johnson SB equation relates false-negative rate to contaminant concentration for each surface material.
The coefficients γ and δ are two shape parameters, while λ is a scale parameter. For this application, λ essentially represents the concentration at which FNR reaches the zero asymptote. The regression estimates of γ and δ were highly correlated with the estimates of λ for all surface materials. Hence, λ was set equal to 0.079 (slightly larger than 50 CFU/645.16 cm2) for all surface materials, because the measured FNR was 0 for tests of ≥50 CFU/645.16 cm2.
Fig 3.
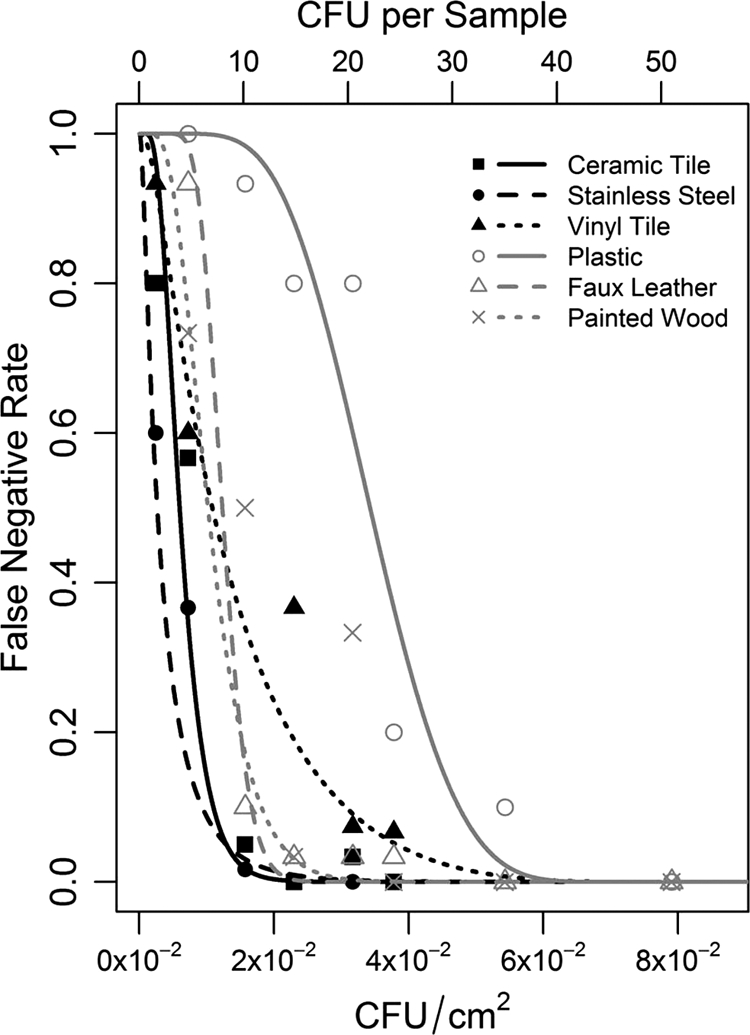
Average false-negative rate data and fitted equations as a function of B. atrophaeus concentration (from positive controls) for each of six surface materials.
The FNR-concentration equations listed in Table 3 and shown in Fig. 3 are subject to considerable uncertainty, because the data used to fit the equations are subject to considerable uncertainty. The relatively high uncertainty in the test data and, hence, in the fitted curves, leads to differences between the curves and certain data points in some cases. The FNR curves are consistent with RE data, showing a trend of smoother surfaces resulting in higher REs and lower FNRs. Stainless steel generally had the lowest FNRs, and plastic had the highest FNRs. Figure 3 curves below FNR = 0.4 have false-negative profiles that trend with the surface roughness (stainless steel < ceramic < faux leather < painted wood < vinyl < plastic light covers). Table 2 summarizes the mean FNR (across all concentrations) for each surface material. The lowest mean FNR (0.12) occurs for the smoothest surface (stainless steel) and the largest mean FNR (0.48) occurs for the roughest surface (plastic panel). The linear trend between FNR and roughness index is not as strong as for RE, because FNR depends on concentration as well as surface material. Hence, the mean FNR value for each surface material is affected by the nature of FNR dependence on concentration for each surface material.
Limit of detection.
Table 4 lists the estimates of LOD90 and LOD95 for the sponge wipe method with each of the six surface materials. The LOD95 values for the six surface materials were calculated using the FNR-concentration equations of the form in equation 5 with coefficients listed in Table 3. Also shown in Table 4 is the 95% confidence interval on each LOD95 value.
Table 4.
Estimates of the LOD90 and LOD95 values for sponge wipe method and six surface materialsa
| Surface material | LOD90, CFU/cm2 | LOD95, CFU/cm2 (95% CI) |
|---|---|---|
| Stainless steel | 0.015 | 0.013 (0.010, 0.015) |
| Ceramic tile | 0.015 | 0.013 (0.007, 0.015) |
| Vinyl tile | 0.031 | 0.038 (0.029, 0.047) |
| Faux leather | 0.015 | 0.018 (0.010, 0.022) |
| Painted wood | 0.023 | 0.021 (0.018, 0.024) |
| Plastic | 0.039 | 0.051 (0.049, 0.054) |
LOD90 is the lowest concentration tested that yielded 90% samples with >1 CFU per sample. LOD95 is the concentration at which the contamination would be correctly detected 95% of the time, calculated as the concentration corresponding to the 5th percentile of the FNR versus concentration equation for each surface material (Table 3). The 95% confidence intervals (CI) for the LOD95 estimates are shown.
Stainless steel and ceramic tile have the smallest LOD90 and LOD95 values, while the plastic lighting panel and vinyl tile have the largest LOD90 and LOD95 values. These results are consistent with stainless steel and ceramic tile having the lowest and the plastic lighting panel and vinyl tile having the largest values of surface roughness index. Figure 3 curves cross midway. However, at low FNRs (e.g., below 0.4), the effects of surface materials on FNR, LOD90, and LOD95 occur in the same order (stainless steel < ceramic < faux leather < painted wood < vinyl < plastic light cover).
DISCUSSION
Recovery efficiency.
Comparing our study's RE results to those from previous sampling studies is problematic because of many differences. The studies used a variety of sampling methods and materials (collection, extraction, analytical), surface materials, spore sizes and characteristics, and surface concentrations, and they were subject to different environmental conditions, each of which may affect REs (10, 40). Using stainless steel as a test surface, the RE values from our study (which ranged from 36 to 63%, with an average of 52%) were larger than in other surface sampling studies. Presumably our study's higher REs resulted from using the improved sponge wipe method and possibly differences in test methods (e.g., spore strain, deposition method, or environmental controls). In our study, REs were not influenced by the low spore concentrations tested (3 × 10−3 to 1.86 CFU/cm2). Rose et al. (39) conducted a national validation study with stainless steel coupons using the same sampling and analytical methods as used in our study. Their study resulted in an overall average RE of 29.7 (ranging from 24.4 to 34.6%) when testing liquid-deposited B. anthracis Sterne concentrations of 0.013, 0.277, and 17.127 CFU/cm2. As with our study, Rose et al. (39) saw no effect of spore concentration on RE. Our higher RE may be due in part to using a surrogate and the lack of a mixed microbial culture.
Studies that used aerosol depositions have the difficult task of collecting samples with loosely attached spores, particularly under conditions of low relative humidity. Spores can potentially be reaerosolized during sampling. Edmonds (13) found that recovery of liquid-deposited spores differs significantly from recovery of dry aerosol-deposited spores. He reported 89% RE from liquid-deposited spores and 61% from aerosol-deposited spores when using macrofoam swabs and testing recovery from glass coupons. Estill et al. (15), using gauze wipes to sample aerosol-deposited spores on stainless steel, reported REs ranging from 18% (deposition concentration of 2.7 CFU/cm2) to 31% (deposition concentration of 0.08 CFU/cm2). Brown et al. (2), using polyester-rayon gauze wipes to sample aerosol-deposited spores on stainless steel, reported a mean RE of 35%.
Because swabs made from macrofoam have material similarity to cellulose sponge wipes, whereas cotton swabs or gauze wipes are considerably different from sponge wipes, RE information from macrofoam swabs is of interest. The study by Hodges et al. (22) using macrofoam swab samples and B. anthracis Sterne spores on stainless steel coupons resulted in REs ranging from 32 to 49% (spore concentrations from 0.1 to 2.9 × 103 CFU/cm2). In the Edmonds et al. (14) study using macrofoam swabs, the RE increased as the concentration of liquid-deposited spores increased (103 to 106 CFU/cm2). The Edmonds et al. (14) results are counter to the results of our study, but the spore concentrations used by Edmonds were far greater and the test methods differed. High spore concentrations can result in layers of spores rather than a monolayer of spores. This layering can result in greater recovery of the top layers of spores because they are not subject to van der Waals, Coulombic, and other surface forces.
False negative rate and level of detection.
The FNR performance of sampling methods has not been investigated in previous studies documented in the literature (30). Hence, there are no previous results to compare with our FNR results. In contrast, values for LOD (defined and estimated in various ways) have been reported in previous studies. Rose et al. (39) reported a LOD90 of 0.031 CFU/cm2 using the sponge wipe method on stainless steel. Our study found a slightly lower LOD90 value for stainless steel (0.015 CFU/cm2). In a study that used aerosol-deposited spores (102 to 105 CFU/cm2) on stainless steel and polyester-rayon gauze, Brown et al. (2) calculated a LOD of 3.6 CFU/cm2 (using 90 CFU for a 25-cm2 sample area). However, Brown et al. assume that the number of CFU required for detection is independent of the sample surface area. The efficiency of sampling and processing procedures will vary with the surface material and texture, the soil load, the size of the sample area, the bacterial species, and many other factors. Hence, any LOD is a reflection of the environmental conditions in which the test was conducted.
The FNR of a sampling and analysis method (such as the sponge wipe method addressed in this article) can potentially have a major impact on health risk decisions. For concentrations below the level at which false negatives begin to occur but above levels determined to have a health risk, a negative sample result cannot be completely trusted to indicate the absence of contamination or health risk. One solution that compensates for an FNR of >0 is to collect more samples, which increases the probability of detecting the contamination. The FNR-concentration equations developed in this study can be used to estimate the FNR of the sponge wipe method for various concentrations and surface materials. This FNR estimate can in turn be used in responding to a future B. anthracis contamination event. For example, estimates of the FNR are required as inputs to formulas for calculating the number of samples required (with a given statistical sampling approach) to obtain the desired confidence in characterization and clearance decisions. Also, after samples are collected and analyzed following a contamination event, FNR values are needed as inputs to formulas for calculating the statistical confidence in characterization and clearance decisions.
Effect of surface materials.
The environmental surfaces selected for this study are hard, nonporous surfaces, which varied in surface roughness. The surface characteristics evaluated (including hydrophobicity, statics, contact angle, and porosity; unpublished data) did not show a relationship with RE, FNR, or LOD. However, surface chemistry effects were previously shown to influence REs of spores from sample media (10). In this study, the sampling and analysis methods were constant so that the effects of surface materials could be evaluated. A roughly linear relationship was seen between mean RE (ranging from 9.8 to 48.9%) and surface roughness, with the highest REs from the smoothest surfaces.
Probst et al. (32, 33) reported that REs for B. atrophaeus spores from different surfaces showed a variation from 5.9 to 62.0%, depending on the roughness of the surface analyzed. However, roughness values were not provided. Estill et al. (15) tested stainless steel and carpet and found higher RE on stainless steel for all spore concentrations tested. These findings, combined with the similar findings of our study, may have implications for field sampling. Specifically, personnel could sample locations with smooth surfaces and take a quick measurement of the roughness index in order to roughly estimate RE, FNR, and LOD, which could then be used to guide the sampling plan development.
Effects of sample media and surrogate microorganism.
Our study used premoistened 3M cellulose sponge wipes, with the depths of folded sponge wipe samples varying from 6 to 18 mm. This may have introduced variation in RE because the difference in sponge volume could lead to a difference in buffer distribution throughout the sponge, which would cause the outside of the sponge to be drier, particularly in a low-humidity environment.
The extraction and recovery characteristics of a different Bacillus species, native spore materials, or additives may lead to results different from those reported in this study. Spore inoculum used in this study did not include any surface treatment, such as silicon dioxide coating. Also, the Bacillus species investigated in this study, B. atrophaeus, does not possess an exosporium like B. anthracis. Probst et al. (32) reported that the REs for B. atrophaeus spores removed from stainless steel coupons were greater than those found with B. anthracis spores. This result suggests that different physiochemical adhesive properties, such as hydrophobicity or molecular composition of spore sheaths, may affect the release of spores from surfaces (37).
Environmental contaminant concentrations and health risk.
Price et al. (31) and Hong et al. (24) discussed approaches for linking environmental concentrations of B. anthracis spores on surfaces in buildings with human health risk. For example, for a retrospective inhalation risk of 10−3, Hong et al. calculate an estimated environmental concentration range of 0.36 to 3.4 × 102 spores/m2 (3.6 × 10−5 to 0.034 CFU/cm2) for an 8-h exposure. Hong et al. state that the ability to reliably sample is a prerequisite for the implementation of environmental standards and that sampling in this very low concentration range would require large sampling areas. Our study found an LOD for stainless steel of 0.015 CFU/cm2 using the validated sponge wipe method; LODs for other surface materials tested ranged from 0.015 to 0.039 CFU/cm2. These findings suggest that the performance improvements obtained with the validated sponge wipe method may be approaching what is required to detect spores at lower surface concentrations. However, sampling techniques for large areas is an important topic for future efforts to increase detection capabilities.
ACKNOWLEDGMENTS
The Sandia National Laboratories (SNL) and Pacific Northwest National Laboratory (PNNL) work was funded by the Chemical and Biological Research and Development Branch of the Chemical and Biological Division in the Science and Technology Directorate of the Department of Homeland Security. SNL is a multiprogram national laboratory operated by Sandia Corporation, a Lockheed Martin company, for the US Department of Energy's National Nuclear Security Administration under contract DE-AC04-AL8500. PNNL is a multiprogram national laboratory operated for the US Department of Energy by Battelle under contract DE-AC05-76RL01830.
Footnotes
Published ahead of print 2 December 2011
REFERENCES
- 1. Almeida JL, Harper B, Cole KD. 2008. Bacillus anthracis spore suspensions: determination of stability and comparison of enumeration techniques. J. Appl. Microbiol. 104:1442–1448 [DOI] [PubMed] [Google Scholar]
- 2. Brown GS, et al. 2007. Evaluation of a wipe surface sample method for collection of Bacillus spores from nonporous surfaces. Appl. Environ. Microbiol. 73:706–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brown GS, et al. 2007. Evaluation of rayon swab surface sample collection method for Bacillus spores from nonporous surfaces. J. Appl. Microbiol. 103:1074–1080 [DOI] [PubMed] [Google Scholar]
- 4. Brown GS, et al. 2007. Evaluation of vacuum filter sock surface sample collection method for Bacillus spores from porous and non-porous surfaces. J. Environ. Monitor. 9:666–671 [DOI] [PubMed] [Google Scholar]
- 5. Buttner MP, Cruz-Perez P, Stetzenbach LD. 2001. Enhanced detection of surface-associated bacteria in indoor environments by quantitative PCR. Appl. Environ. Microbiol. 67:2564–2570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buttner MP, et al. 2004. Determination of the efficacy of two building decontamination strategies by surface sampling with culture and quantitative PCR analysis. Appl. Environ. Microbiol. 70:4740–4747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buttner MP, et al. 2004. Evaluation of the biological sampling kit (BiSKit) for large-area surface sampling. Appl. Environ. Microbiol. 70:7040–7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Centers for Disease Control and Prevention 6 December 2006, posting date. Facts about the Laboratory Response Network. http://www.bt.cdc.gov/lrn/pdf/lrnfactsheet.pdf
- 9. Centers for Disease Control and Prevention and National Institute for Occupational Safety and Health 7 September 2010, posting date. Surface sampling for Bacillus anthracis spores from smooth, non-porous surfaces. Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, Atlanta, GA: http://www.cdc.gov/niosh/topics/emres/surface-sampling-bacillus-anthracis.html [Google Scholar]
- 10. Da Silva SM, Filliben JJ, Morrow JB. 2011. Parameters affecting spore recovery from wipes used in biological surface sampling. Appl. Environ. Microbiol. 77:2374–2380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davison AC, Hinkley D. 2006. Bootstrap methods and their application, 8th ed. Cambridge University Press, New York, NY [Google Scholar]
- 12. Dorsa WJ, Siragusa GR, Cutter CN, Berry ED, Koohmaraie M. 1997. Efficacy of using a sponge sampling method to recover low levels of Escherichia coli O157:H7, Salmonella typhimurium, and aerobic bacteria from beef carcass surface tissue. Food Microbiol. 14:63–69 [Google Scholar]
- 13. Edmonds JM. 2009. Efficient methods for large-area surface sampling of sites contaminated with pathogenic microorganisms and other hazardous agents: current state, needs, and perspectives, Appl. Microbiol. Biotechnol. 84:811–816 [DOI] [PubMed] [Google Scholar]
- 14. Edmonds JM, et al. 2009. Surface sampling of spores in dry-deposition aerosols. Appl. Environ. Microbiol. 75:39–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Estill CF, et al. 2009. Recovery efficiency and limit of detection of aerosolized Bacillus anthracis Sterne from environmental surface samples. Appl. Environ. Microbiol. 75:4297–4306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Frawley DA, et al. 2008. Recovery efficiencies of anthrax spores and ricin from nonporous or nonabsorbent and porous or absorbent surfaces by a variety of sampling methods. J. Forensic Sci. 53:1102–1107 [DOI] [PubMed] [Google Scholar]
- 17. Government Accountability Office (GAO) 2005. Anthrax detection: agencies need to validate sampling activities in order to increase confidence in negative results (report to the Chairman, Subcommittee on National Security, Emerging Threats, and International Relations, House Committee on Government Reform, House of Representatives), GAO-05-251. US Government Accountability Office, Washington, DC [Google Scholar]
- 18. Government Accountability Office (GAO) 2005. Anthrax detection: agencies need to validate sampling activities in order to increase confidence in negative results (testimony before the Chairman, Subcommittee on National Security, Emerging Threats, and International Relations, House Committee on Government Reform, House of Representatives), GAO-05-493T. US Government Accountability Office, Washington, DC [Google Scholar]
- 19. Government Accountability Office (GAO) 2003. Bioterrorism-Public Health Response to Anthrax Incidents of 2001. (Report to the Honorable Bill Frist, Majority Leader, U.S. Senate), GAO-04-152, GAO-05-251. US Government Accountability Office, Washington, DC [Google Scholar]
- 20. Hahn GJ, Shapiro SS. 1968. Statistical models in engineering. John Wiley and Sons, New York, NY [Google Scholar]
- 21. Hitchins AD, Mishra-Szymanski A. 1999. Qualitative and quantitative microbiology guidelines for methods validation. J. AOAC Int. 82(2):402–416 [Google Scholar]
- 22. Hodges LR, Rose LJ, Peterson A, Noble-Wang J, Arduino MJ. 2006. Evaluation of a macrofoam swab protocol for the recovery of Bacillus anthracis spores from a steel surface. Appl. Environ. Microbiol. 72:4429–4430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hodges LR, Rose LJ, O'Connell H, Arduino MJ. 2010. National validation study of a swab protocol for the recovery of Bacillus anthracis spores from surfaces. J. Microbiol. Methods 81:141–146 [DOI] [PubMed] [Google Scholar]
- 24. Hong T, Gurian PL, Dudley Ward NF. 2010. Setting risk-informed environmental standards for Bacillus anthracis spores. Risk Anal. 30:1602–1622 [DOI] [PubMed] [Google Scholar]
- 25. Lewandowski R, Kozlowska K, Szpakowska M, Stepinska M, Trafny EA. 2010. Use of a foam spatula for sampling surfaces after bioaerosol deposition. Appl. Environ. Microbiol. 76:688–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mathwave 2011. Johnson SB distribution, Mathwave Technologies, Dnepropetrovsk, Ukraine. http://www.mathwave.com/help/easyfit/html/analyses/distributions/johnson_sb.html Accessed 10 November 2011
- 27. Miller RG. 1981. Simultaneous statistical inference, 2nd ed. Springer Verlag, New York, NY [Google Scholar]
- 28. Nellen J, Rettberg P, Horneck G, Streit WR. 2006. Planetary protection—approaching uncultivable microorganisms. Adv. Space Res. 38:1266–1270 [Google Scholar]
- 29. Piepel GF, Amidan BG, Krauter PA, Einfeld W. 2011. Experimental design for a sponge-wipe study to relate the recovery efficiency and false negative rate to the concentration of a Bacillus anthracis surrogate for six surface materials, PNNL-20060, Rev. 1. Pacific Northwest National Laboratory, Richland, WA: http://www.pnl.gov/main/publications/external/technical_reports/PNNL-20060.pdf [Google Scholar]
- 30. Piepel GF, Amidan BG, Hu R. 2011. Laboratory studies on surface sampling of Bacillus anthracis contamination: summary, gaps, and recommendations, PNNL-20910, Pacific Northwest National Laboratory, Richland, WA: http://www.pnl.gov/main/publications/external/technical_reports/PNNL-20910.pdf [DOI] [PubMed] [Google Scholar]
- 31. Price PN, Sohn MD, Lacommare KSH, McWilliams JA. 2009. Framework for evaluating anthrax risk in buildings. Environ. Sci. Technol. 43:1783–1787 [DOI] [PubMed] [Google Scholar]
- 32. Probst A, Facius R, Wirth R, Moissl-Eichinger C. 2010. Validation of a nylon-flocked-swab protocol for efficient recovery of bacterial spores from smooth and rough surfaces. Appl. Environ. Microbiol. 76:5148–5158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Probst A, Facius R, Wirth R, Wolf M, Moissl-Eichinger C. 2011. Recovery of Bacillus spore contaminants from rough surfaces: a challenge to space mission cleanliness control. Appl. Environ. Microbiol. 77:1628–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Quizon R, et al. 2007. Test and evaluation of surface sampling approaches before and after small-scale fumigation-based decontamination events, NSTD-07-0592. Applied Physics Laboratory, National Security Technology Department, John Hopkins University, Laurel, MD [Google Scholar]
- 35. Rastogi VK, Wallace L, Smith LS, Ryan SP, Martin B. 2009. Quantitative method to determine sporicidal decontamination of building surfaces by gaseous fumigants, and issues related to laboratory-scale studies. Appl. Environ. Microbiol. 75:3688–3694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rogers JV, et al. 2005. Decontamination assessment of Bacillus anthracis, Bacillus subtilis, and Geobacillus stearothermophilus spores on indoor surfaces using a hydrogen peroxide gas generator. J. Appl. Microbiol. 99:739–748 [DOI] [PubMed] [Google Scholar]
- 37. Ronner U, Husmark U, Henriksson A. 1990. Adhesion of Bacillus spores in relation to hydrophobicity. J. Appl. Bacteriol. 69:550–556 [DOI] [PubMed] [Google Scholar]
- 38. Rose L, Jensen B, Peterson A, Banerjee SN, Arduino MJ. 2004. Swab materials and Bacillus anthracis spore recovery from nonporous surfaces. Emerg. Infect. Dis. 10:1023–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rose LJ, Hodges L, O'Connell H, Noble-Wang J. 2011. National validation study of a cellulose sponge-wipe processing method for use after sampling Bacillus anthracis spores from surfaces. Appl. Environ. Microbiol. 77:8355–8359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sanderson WT, et al. 2004. Bacillus anthracis contamination and inhalational anthrax in a mail processing and distribution center. J. Appl. Microbiol. 96:1048–1056 [DOI] [PubMed] [Google Scholar]
- 41. Seber GAF, Wild CJ. 2003. Nonlinear regression, 2nd ed. John Wiley and Sons, New York, NY [Google Scholar]
- 42. Tomasino SF, et al. 2010. Use of alternative carrier materials in AOAC official method 2008.05, efficacy of liquid sporicides against spores of Bacillus subtilis on a hard, nonporous surface, quantitative three-step method. J. AOAC Int. 93:259–276 [PubMed] [Google Scholar]
- 43. US Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention and National Institutes of Health 1999. Biosafety in microbiological and biomedical laboratories, 4th ed HHS publication no. (CDC) 93-8395. CDC, Atlanta, GA [Google Scholar]
- 44. Valentine NB, et al. 2008. Evaluation of sampling tools for environmental sampling of bacterial endospores from porous and nonporous surfaces. J. Appl. Microbiol. 105:1107–1113 [DOI] [PubMed] [Google Scholar]
- 45. Valiante DJ, Schill DP, Bresnitz EA, Burr GA, Mead KR. 2003. Responding to a bioterrorist attack: environmental investigation of anthrax in New Jersey. Appl. Occup. Environ. Hyg. 18:780–785 [DOI] [PubMed] [Google Scholar]