Abstract
Natural antibiotics are thought to function in the defense, fitness, competitiveness, biocontrol activity, communication, and gene regulation of microorganisms. However, the scale and quantitative aspects of antibiotic production in natural settings are poorly understood. We addressed these fundamental questions by assessing the geographic distribution of indigenous phenazine-producing (Phz+) Pseudomonas spp. and the accumulation of the broad-spectrum antibiotic phenazine-1-carboxylic acid (PCA) in the rhizosphere of wheat grown in the low-precipitation zone (<350 mm) of the Columbia Plateau and in adjacent, higher-precipitation areas. Plants were collected from 61 commercial wheat fields located within an area of about 22,000 km2. Phz+ Pseudomonas spp. were detected in all sampled fields, with mean population sizes ranging from log 3.2 to log 7.1 g−1 (fresh weight) of roots. Linear regression analysis demonstrated a significant inverse relationship between annual precipitation and the proportion of plants colonized by Phz+ Pseudomonas spp. (r2 = 0.36, P = 0.0001). PCA was detected at up to nanomolar concentrations in the rhizosphere of plants from 26 of 29 fields that were selected for antibiotic quantitation. There was a direct relationship between the amount of PCA extracted from the rhizosphere and the population density of Phz+ pseudomonads (r2 = 0.46, P = 0.0006). This is the first demonstration of accumulation of significant quantities of a natural antibiotic across a terrestrial ecosystem. Our results strongly suggest that natural antibiotics can transiently accumulate in the plant rhizosphere in amounts sufficient not only for inter- and intraspecies signaling but also for the direct inhibition of sensitive organisms.
INTRODUCTION
Antibiotics are bioactive microbial metabolites that at low concentrations inhibit the growth or metabolic activities of other organisms (42). Natural antibiotics have been a subject of intense research for the past 70 years and, together with their semisynthetic derivatives, form the foundation of modern antimicrobial therapy (45). However, the role of natural antibiotics has been debated for decades. Antibiotic-producing species are common in microbial communities throughout nature, and traditionally, it has been thought that natural antibiotics contribute to microbial defense, fitness, interference, and competitiveness (1, 5, 7, 10, 11, 19, 20, 24, 38, 52). It is generally accepted that in terrestrial ecosystems, natural antibiotics accumulate in inhibitory concentrations primarily in nutrient-rich environments, such as those encountered by antibiotic producers during colonization of the plant rhizosphere or animal hosts. The antibiotic-mediated inhibition of plant pathogens by rhizosphere-inhabiting biocontrol microorganisms is well documented in numerous studies and summarized in several comprehensive review papers (11, 12, 34, 50). Perhaps the best-known example is the suppression of take-all disease in wheat by 2,4-diacetylphloroglucinol, produced by Pseudomonas fluorescens in the rhizosphere (50, 51). Among examples of antibiosis involved in the protection of eukaryotic hosts and their symbionts from pathogens are interactions between attine ants and Pseudonocardia species that produce antibiotics that protect ant fungal gardens from the mycoparasite Escovopsis (7) and the association of the southern pine beetle Dendroctonus frontalis with the fungus Entomocorticium sp. A, which is protected from the pathogen Ophiostoma minus by a mycangimycin-producing actinomycete (38). Other examples include Janthinobacterium lividum, which colonizes the skin of the red-backed salamander and produces indole-3-carboxaldehyde and violacein, metabolites which protect the amphibian from the chytrid pathogen Batrachochytrium dendrobatidis (5), and actinobacteria that produce a suite of antibiotics that protect beewolf digger wasp larvae and cocoons against entomopathogens (19).
In recent years, it has been argued that antibiotics rarely accumulate in natural habitats in inhibitory concentrations and therefore function less as weapons of defense and more as signaling compounds important in bacterial physiology, communication, and gene regulation (8–10, 22). In actuality, antibiotics in natural settings are likely to function both in antagonism and as signaling compounds. However, there is a glaring lack of data on the amounts and spatial and temporal patterns of antibiotic production in the field, which severely hampers our understanding of the role of these compounds in nature. Natural antibiotics are notoriously hard to detect and quantify in situ due to microbial degradation, chemical decomposition, and/or binding to soil and organic matter (3, 41). As a consequence, all studies published to date have examined the role and production patterns of antibiotics under controlled conditions and/or with arbitrarily chosen strains introduced, often at high densities, into soil or onto plants or animals (1, 2, 4, 15–17, 21, 23, 29, 33, 39, 44).
Recently, we reported that the rhizospheres of cereal crops grown in areas around Lind, WA, and Ritzville, WA, supported large populations (105 to 106 CFU g−1 of roots) of indigenous phenazine-producing (Phz+) bacteria (25). Phenazines are a class of well-studied natural antibiotics that are produced by diverse plant-associated bacteria, exhibit unique redox properties and broad-spectrum antibiotic activity, and play a wide variety of roles in nature (24). The majority of Phz+ bacteria isolated from plants near Lind and Ritzville belong to the P. fluorescens species complex and produce the broad-spectrum antibiotic phenazine-1-carboxylic acid (PCA). This finding represents the first example of soils from the United States enriched in indigenous Phz+ pseudomonads.
The farming communities of Lind and Ritzville, WA, are located in the heart of the low-precipitation zone (<350 mm) of the Columbia Plateau, which stretches from north central Washington into Oregon. This region, which has been farmed for over 125 years, encompasses 1.6 million cropland hectares and is the largest contiguous production region in the western United States (36). The low-precipitation zone is a basalt plateau, with canyons and coulees cut by flooding during prehistoric times and soils that are derived from windblown sediments called loess. Winter precipitation is efficiently stored in the soil profile, underpinning the alternate winter wheat-summer fallow rotation that has been the dominant cropping system since the onset of farming in this region (36). The abundance of indigenous PCA-producing pseudomonads and homogeneous climate conditions, soil types, and cropping and agronomic management practices make wheat fields in the low-precipitation zone of the Columbia Plateau an ideal environmental system for investigating the large-scale distribution of indigenous beneficial rhizobacteria, the frequency and amounts of natural antibiotics, and the factors affecting the population dynamics and activity of antibiotic-producing microbial communities.
To address these questions, we surveyed 86 sites in 61 commercial wheat fields scattered over an area of approximately 22,000 km2 in the low-precipitation zone and adjacent, higher-precipitation areas in central and eastern Washington, northeastern Oregon, and northern Idaho. Results of this 2-year study (2008 to 2009) revealed that wheat from all sampled fields was colonized by Phz+ Pseudomonas spp., but these bacteria were particularly abundant in the rhizospheres of plants grown in areas with an annual precipitation of less than 350 mm. The presence of Phz+ pseudomonads resulted in the accumulation of significant (nanomolar) quantities of PCA in the plant rhizosphere, and the amounts recovered correlated positively (r2 = 0.46, P = 0.0006) with mean population sizes of Phz+ pseudomonads. To our knowledge, this is the first example of production of significant quantities of a natural antibiotic by indigenous microorganisms across a large-scale terrestrial ecosystem. Our results also strongly suggest that natural antibiotics can accumulate in the plant rhizosphere in amounts sufficient not only for inter- and intraspecies signaling but also for the direct inhibition of sensitive organisms.
MATERIALS AND METHODS
Collection of samples.
In 2008 and 2009, we collected 86 samples of winter wheat (and, to a lesser extent, spring wheat and barley) from 61 commercial fields located throughout nine Washington and five Oregon counties situated partially or completely within the low-rainfall zone (36) and six counties in adjacent, higher-rainfall zones, including some fields in Idaho (Fig. 1). In 2008, some fields were sampled up to four times, and in 2009, each field was sampled only one time. In both years, the sampling was carried out between March and June and sampled plants corresponded to Feekes growth stages 5 (leaf sheaths strongly erect) to 10.5 (anthesis). At each location, a spot about 50 m into the field from the road was selected and tagged using a global positioning system (GPS) (Table 1). Next, clumps of plants with adhering soil were dug to a depth of 20 to 25 cm every few meters along three transects running in different directions from the reference point. Plants from each transect were kept in separate plastic bags and treated as replicates. All plant samples were transported to the laboratory and stored at 4°C for no more than 24 h before processing.
Fig 1.
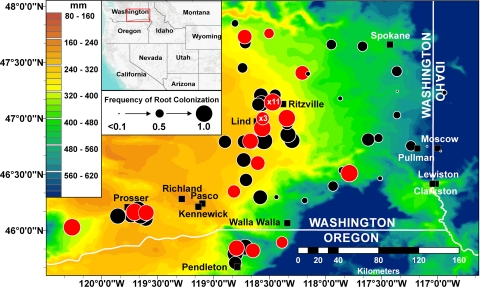
Distribution of Phz+ pseudomonads in wheat fields from eastern Washington, northeastern Oregon, and western Idaho. Map of the surveyed area is overlaid with mean annual precipitation values for the years 1971 through 2000 (see inset for scale). The locations of sampling sites are indicated by circles whose sizes are proportional to the frequency of rhizospheres colonized by Phz+ Pseudomonas spp., as determined for 8 to 16 individual plants (see inset for scale). Red circles indicate sites from which samples were extracted for PCA. Black rectangles indicate local cities and towns. Precipitation values and global positioning system coordinates of sampled fields were managed with the ArcGIS 9.3 software. (Map created with ArcGIS and ArcMap [ESRI].)
Table 1.
Populations of Phz+ Pseudomonas spp. and amounts of PCA recovered from rhizospheres of cereals collected in 2008 to 2009 in the low-precipitation zone of the Columbia Plateau
| Cropa | Geographic coordinates | Sampling date (mo/day/yr) | Phz+Pseudomonas spp. |
Amount of PCA detected (ng g−1 of roots [fresh wt] ± SD)d | |
|---|---|---|---|---|---|
| Population densityb (log CFU g−1 of roots [fresh wt] ± SD) | Frequencyc | ||||
| WW | 47°8′34′′N, 118°28′22′′W | 03/18/08 | 5.7 ± 1.0 | 1.0 | ND |
| WW | 47°0′1′′N, 118°33′45′′W | 03/18/08 | 5.2 ± 0.9 | 0.8 | 16.9e |
| cSW | 47°8′34′′N, 118°28′22′′W | 04/24/08 | 6.3 ± 1.1 | 0.9 | 1,000.0e |
| SB | 47°8′34′′N, 118°28′22′′W | 04/24/08 | 6.7 ± 0.9 | 1.0 | 1,600.0e |
| SW | 47°8′34′′N, 118°28′22′′W | 04/24/08 | 6.6 ± 0.9 | 0.9 | 866.7e |
| WW | 47°8′34′′N, 118°28′22′′W | 04/24/08 | 6.1 ± 0.9 | 1.0 | 151.6 ± 43.2 |
| WW | 47°8′34′′N, 118°28′22′′W | 04/24/08 | 6.8 ± 1.8 | 1.0 | 316.7 ± 215.4 |
| WW | 47°0′1′′N, 118°33′45′′W | 04/24/08 | 5.2 ± 0.8 | 0.9 | 34.9 ± 1.9 |
| WW | 46°49′27′′N, 117°31′41′′W | 04/24/08 | 4.1 ± 0.8 | 0.5 | – |
| WW | 46°48′24′′N, 118°18′16′′W | 04/24/08 | 6.0 ± 0.8 | 0.9 | – |
| WW | 46°57′2′′N, 118°20′36′′W | 04/24/08 | 6.4 ± 1.1 | 0.6 | – |
| WW | 46°52′5′′N, 118°32′47′′W | 04/24/08 | 3.2 ± 0.3 | 0.5 | – |
| cSW | 47°8′34′′N, 118°28′22′′W | 05/16/08 | 7.1 ± 1.0 | 0.9 | 925.8 ± 884.3 |
| SB | 47°8′34′′N, 118°28′22′′W | 05/16/08 | 6.8 ± 0.9 | 0.8 | 159.2 ± 113.8 |
| SW | 47°8′34′′N, 118°28′22′′W | 05/16/08 | 7.2 ± 1.3 | 0.9 | 466.7 ± 370.7 |
| WW | 47°8′34′′N, 118°28′22′′W | 05/16/08 | 7.3 ± 0.9 | 0.9 | 440.7 ± 393.0 |
| WW | 47°0′1′′N, 118°33′45′′W | 05/16/08 | 5.3 ± 1.0 | 0.6 | 29.2 ± 2.4 |
| WW | 46°47′52′′N, 118°33′3′′W | 05/16/08 | 5.3 ± 1.0 | 1.0 | – |
| WW | 46°47′40′′N, 118°39′42′′W | 05/16/08 | 6.3 ± 0.6 | 0.8 | – |
| WW | 46°47′29′′N, 118°53′56′′W | 05/16/08 | 5.5 ± 1.1 | 0.8 | – |
| WW | 46°55′13′′N, 118°34′1′′W | 05/16/08 | 5.5 ± 1.2 | 0.8 | – |
| WW | 47°8′34′′N, 118°28′22′′W | 05/16/08 | 6.3 ± 0.9 | 1.0 | ND |
| cSW | 47°8′34′′N, 118°28′22′′W | 06/09/08 | 6.1 ± 1.1 | 0.9 | – |
| SB | 47°8′34′′N, 118°28′22′′W | 06/09/08 | 6.4 ± 0.9 | 0.9 | – |
| SW | 47°8′34′′N, 118°28′22′′W | 06/09/08 | 6.4 ± 1.3 | 1.0 | – |
| WW | 47°8′34′′N, 118°28′22′′W | 06/09/08 | 5.5 ± 0.7 | 1.0 | – |
| WW | 47°8′34′′N, 118°28′22′′W | 06/09/08 | 7.0 ± 0.5 | 1.0 | – |
| WW | 47°7′57′′N, 118°35′7′′W | 06/09/08 | 5.9 ± 1.1 | 1.0 | – |
| cSW | 47°8′34′′N, 118°28′22′′W | 06/30/08 | 6.3 ± 0.8 | 0.9 | – |
| SB | 47°8′34′′N, 118°28′22′′W | 06/30/08 | 6.1 ± 1.1 | 0.9 | – |
| SW | 47°8′34′′N, 118°28′22′′W | 06/30/08 | 6.0 ± 1.0 | 0.8 | – |
| WW | 47°8′34′′N, 118°28′22′′W | 06/30/08 | 6.1 ± 1.1 | 1.0 | – |
| cSW | 47°8′34′′N, 118°28′22′′W | 06/30/08 | 6.6 ± 0.9 | 1.0 | – |
| WW | 47°50′45′′N, 118°48′30′′W | 06/30/08 | 5.1 ± 1.0 | 0.4 | – |
| WW | 47°36′47′′N, 118°43′13′′W | 06/30/08 | 4.2 ± 0.2 | 0.4 | – |
| WW | 46°27′51′′N, 117°30′50′′W | 03/17/09 | 3.2 ± 0.3 | 0.3 | – |
| WW | 46°30′33′′N, 117°47′18′′W | 03/17/09 | 5.9 ± 1.1 | 1.0 | 14.0e |
| WW | 46°32′7′′N, 117°51′13′′W | 03/17/09 | 5.2 ± 0.8 | 0.4 | – |
| WW | 46°23′41′′N, 117°56′1′′W | 03/17/09 | 5.5 ± 0.7 | 0.6 | – |
| WW | 46°15′59′′N, 118°11′15′′W | 03/17/09 | 6.2 ± 2.1 | 0.3 | – |
| WW | 46°17′48′′N, 118°26′23′′W | 03/17/09 | 5.6 ± 1.2 | 0.3 | – |
| WW | 46°17′44′′N, 118°35′3′′W | 03/17/09 | 4.6 ± 1.5 | 0.8 | – |
| WW | 46°20′52′′N, 118°49′7′′W | 03/17/09 | 6.0 ± 1.4 | 0.8 | 16.8 ± 0.5 |
| WW | 46°27′1′′N, 118°42′40′′W | 03/17/09 | 4.1 ± 1.1 | 0.8 | – |
| WW | 46°36′3′′N, 118°36′21′′W | 03/17/09 | 5.1 ± 1.3 | 0.8 | ND |
| WW | 46°9′49′′N, 119°42′23′′W | 03/24/09 | 6.4 ± 0.9 | 1.0 | 34.6 ± 11.2 |
| WW | 46°7′58′′N, 119°42′21′′W | 03/24/09 | 6.5 ± 1.7 | 0.8 | – |
| WW | 46°7′37′′N, 119°44′20′′W | 03/24/09 | 6.1 ± 0.8 | 0.8 | – |
| WW | 46°7′38′′N, 119°51′21′′W | 03/24/09 | 5.6 ± 1.3 | 0.9 | – |
| WW | 46°1′36′′N, 120°15′53′′W | 03/24/09 | 7.0 ± 1.3 | 1.0 | 557.6 ± 84.5 |
| WW | 46°6′8′′N, 119°36′20′′W | 03/24/09 | 5.5 ± 0.8 | 0.8 | – |
| WW | 46°9′27′′N, 119°36′7′′W | 03/24/09 | 6.9 ± 0.9 | 0.9 | 85.7 ± 35.3 |
| WW | 46°11′19′′N, 119°40′27′′W | 03/24/09 | 6.2 ± 1.1 | 0.9 | – |
| WW | 46°48′39′′N, 117°37′12′′W | 04/21/09 | 5.5 ± 1.8 | 0.8 | – |
| WW | 46°48′8′′N, 118°18′3′′W | 04/21/09 | 6.3 ± 1.2 | 0.9 | – |
| WW | 46°51′15′′N, 118°20′10′′W | 04/21/09 | 5.2 ± 1.3 | 0.9 | – |
| WW | 47°0′4′′N, 118°20′48′′W | 04/21/09 | 5.7 ± 1.6 | 1.0 | 29.2 ± 14.5 |
| WW | 47°7′26′′N, 118°36′4′′W | 04/21/09 | 5.7 ± 2.0 | 0.8 | – |
| WW | 46°54′45′′N, 118°33′58′′W | 04/21/09 | 6.2 ± 1.3 | 1.0 | DNQ |
| WW | 46°47′41′′N, 118°33′28′′W | 04/21/09 | 6.0 ± 1.5 | 0.9 | – |
| WW | 46°47′46′′N, 118°40′19′′W | 04/21/09 | 6.4 ± 0.9 | 0.9 | DNQ |
| WW | 46°47′35′′N, 118°43′48′′W | 04/21/09 | 5.9 ± 1.4 | 0.8 | – |
| WW | 46°45′14′′N, 117°14′20′′W | 04/21/09 | 4.8 ± 1.0 | 0.6 | – |
| WW | 47°1′49′′N, 117°21′47′′W | 05/04/09 | 6.0 ± 1.3 | 0.4 | – |
| WW | 47°25′12′′N, 117°21′52′′W | 05/04/09 | 4.5 ± 1.2 | 0.6 | – |
| WW | 47°38′34′′N, 117°40′15′′W | 05/04/09 | 5.6 ± 0.9 | 0.6 | – |
| WW | 47°39′28′′N, 117°56′54′′W | 05/04/09 | 4.0 ± 0.9 | 0.6 | – |
| WW | 47°45′29′′N, 118°30′19′′W | 05/04/09 | 6.3 ± 0.8 | 0.6 | DNQ |
| WW | 47°43′43′′N, 118°43′23′′W | 05/04/09 | 6.9 ± 1.1 | 0.8 | 21.1e |
| WW | 47°27′0′′N, 118°44′30′′W | 05/04/09 | 4.7 ± 1.4 | 0.6 | – |
| WW | 47°24′20′′N, 118°12′29′′W | 05/04/09 | 6.3 ± 1.0 | 0.8 | 94.2e |
| WW | 47°10′39′′N, 117°52′12′′W | 05/04/09 | 4.1 ± 0.6 | 0.3 | – |
| WW | 47°23′50′′N, 118°9′26′′W | 05/04/09 | 4.6 ± 1.3 | 0.3 | – |
| SW | 47°13′43′′N, 116°56′19′′W | 06/07/09 | 5.5 ± 0.3 | 0.3 | – |
| WW | 46°43′2′′N, 116°58′50′′W | 06/07/09 | 4.1 | 0.1 | – |
| WW | 45°53′33′′N, 118°23′24′′W | 06/09/09 | 5.1 ± 2.1 | 0.7 | DNQ |
| WW | 45°49′13′′N, 118°38′57′′W | 06/09/09 | 6.0 ± 0.6 | 0.8 | 16.3e |
| WW | 45°51′0′′N, 118°43′7′′W | 06/09/09 | 5.2 ± 1.1 | 0.9 | – |
| WW | 45°50′31′′N, 118°47′47′′W | 06/09/09 | 5.1 ± 0.9 | 0.9 | DNQ |
| WW | 45°47′29′′N, 118°48′28′′W | 06/09/09 | 5.6 ± 0.8 | 0.9 | – |
| WW | 45°42′38′′N, 118°48′29′′W | 06/09/09 | 4.6 ± 0.9 | 0.8 | – |
| WW | 46°7′52′′N, 119°36′37′′W | 06/09/09 | 4.1 ± 0.9 | 0.7 | – |
| WW | 46°7′40′′N, 119°39′9′′W | 06/09/09 | 4.9 ± 0.7 | 0.9 | – |
| WW | 46°11′19′′N, 119°40′27′′W | 06/09/09 | 5.5 ± 0.8 | 0.92 | – |
| WW | 46°37′40′′N, 118°43′54′′W | 06/09/09 | 4.2 ± 1.7 | 0.8 | – |
WW, winter wheat; cSW, continuous spring wheat; SB, spring barley; SW, spring wheat.
As determined by the PCR-based endpoint dilution assay.
Frequency of individual rhizospheres colonized by Phz+ pseudomonads.
Detected by HPLC–Q-TOF–MS/MS. ND, none detected; –, sample was not extracted; DNQ, detectable but not quantifiable (corresponds to 2.3 to 13.3 ng PCA g−1 roots [fresh weight]).
Replicates were pooled for PCA extraction.
Quantification of the population size and frequency of root colonization by indigenous, root-associated Phz+ Pseudomonas spp.
Indigenous Phz+ Pseudomonas spp. in the rhizosphere were enumerated by a modification of the terminal dilution endpoint assay (27). Briefly, four plants from each replicate bag were selected for processing and gently shaken to remove loosely adhering soil. The roots with rhizosphere soil from each plant were excised at the crown and placed in a 50-ml Falcon tube containing 10 to 30 ml (depending on the amount of roots) of sterile water, and bacteria were dislodged by vortexing (1 min) and sonication (1 min) in an ultrasonic cleaner. Root washes were then serially diluted in water in wells of a 96-well microtiter plate (200 μl per well), and then dilutions from each well were used to inoculate another microtiter plate filled (200 μl per well) with one-third-strength King's medium B (18) amended with cycloheximide, chloramphenicol, and ampicillin (100, 15, and 40 μg ml−1, respectively), a medium which is semiselective for pseudomonads (27). Cycloheximide was used to inhibit soilborne fungi. Chloramphenicol and ampicillin were added to reduce the growth of competing soil bacteria because Phz+ Pseudomonas spp. are naturally resistant to both antibiotics. After the plates were incubated at room temperature in the dark for 72 h, the optical density at 600 nm (OD600) was recorded in each well. All wells with an OD600 of ≥0.1 were considered positive for bacterial growth, and the cells in these wells were screened for the presence of the phenazine biosynthesis gene phzF by PCR with primers Ps_up1 and Ps_low1 (25). The population size of Phz+ Pseudomonas spp. in the rhizosphere of each plant was calculated based on the final dilution testing positive for phzF. When a replicate of a sample had plants without Phz+ pseudomonads, the mean population size for the replicate was calculated using only colonized plants. Population sizes of total culturable aerobic bacteria were determined by the terminal dilution endpoint assay using 1/10-strength Tryptic soy broth (BD Biosciences, Franklin Lakes, NJ) amended with cycloheximide (100 μg ml−1) (28). All population data were converted to log CFU per gram (fresh weight) of roots. The frequency of rhizospheres of plants colonized by Phz+ pseudomonads was calculated by dividing the number of Phz+ plants by the total number of plants sampled. In our experience, assessments of the overall abundance of any specific group of microorganism, like Phz+ Pseudomonas spp., in the field requires two complementary measures, population density and plant colonization frequency. It is well established in the literature that populations of a specific microorganism are not uniformly distributed along a root, among roots of the same plant, or among root systems of plants in the same field (48).
Detection of phenazine-1-carboxylic acid in the rhizosphere of field-grown wheat and barley.
Roots of plants in each replicate of each sample that were not used to determine populations of Phz+ pseudomonads were excised from the shoots and stored in plastic bags at −80°C. Fifteen grams of frozen roots with adhering rhizosphere soil was cut into pieces of about 5 mm and shaken for 2 h in 500-ml flasks filled with 30 ml of 80% acetone acidified to pH 2.0 with 10% trifluoroacetic acid. Each 15-g sample was spiked with 2 μg of phenazine (Sigma, St. Louis, MO) as an internal standard in order to determine the extraction efficiency of PCA. The mixture was then filtered, the solvent-phase samples were centrifuged (12,500 × g) and evaporated, and the remaining aqueous fraction was adjusted to pH 2.0 and extracted twice with 10 ml of ethyl acetate. The organic-phase samples were collected and evaporated to dryness, and the dried samples were reconstituted in 1 ml of 98% acetonitrile-2% acetic acid and clarified by passage through a 0.22-μm filter (4).
PCA was detected and quantified with a Waters 2695 liquid chromatograph equipped with a 996 photodiode array and coupled to a detector-quadrupole orthogonal time-of-flight (Q-TOF) Q-Tof 2 mass spectrometer with an IonSabre atmospheric chemical ionization probe (all from Waters Corp., Milford, MA). Samples were separated on a Symmetry C18 column (particle size, 5 μm; 3.0 by 150 mm; Waters Corp.) using a flow rate of 450 μl min−1 and a 2-min initiation with 10% acetonitrile-2% acetic acid followed by a 20-min linear gradient to 100% acetonitrile in 2% acetic acid. Spectral scanning by photodiode array was from 180 to 470 nm, with monitoring for PCA at 248 nm, its spectral maximum in this solvent system. Q-Tof 2 profiles were monitored at m/z 225.0664, the exact mass for PCA under the atmospheric pressure chemical ionization (APCI) positive ion mode. Optimized conditions for detection and quantification of PCA included a corona current of 2.7 μA, cone voltage of 25 V, collision energy of 12, source temperature of 120°C, and probe temperature of 650°C for exact (time-of-flight) mass measurements. For tandem mass spectrometry (MS-MS) measurements, the cone voltage was 35 V and the collision energy was 25. Data were analyzed by using MassLynx, OpenLynx, and QuantLynx software (Waters Corp.). PCA was quantified by comparing values to those of a six-point calibration curve (0.2 to 10 μg), with samples prepared and extracted as described above from suspensions (15 g) of roots of wheat plus rhizosphere soil grown in the greenhouse in a Quincy virgin soil (Shano sandy loam) that was pasteurized to ensure that it was free of Phz+ isolates. The determination coefficient (r2) of the calibration equations ranged from 0.9942 to 0.9997. The detection limit for PCA in the rhizosphere samples was 35 ng per 15-g rhizosphere sample.
Biocontrol assays.
To assess their role in the rhizosphere of dryland cereals, four strains of Phz+ pseudomonads were tested as wheat seed treatments for their ability to suppress Rhizoctonia root rot caused by Rhizoctonia solani AG-8. Pseudomonas sp. strains R2-7-07, R4-34-07, and R11-23-07 were from Ritzville, WA (25), and P. fluorescens strain 2-79 was isolated in 1979 from Lind, WA (43). R. solani AG-8 isolate C1 (49) was routinely cultured on potato dextrose agar (PDA), consisting of potato dextrose broth (PDB; 24 g) (BD, Sparks, MD), agar (20 g) (Sigma Chemical Co., St. Louis, MO), and water (1 liter). To prepare an oat kernel inoculum, 250 ml of oat grain and 250 ml of water were mixed in a 1-liter flask and autoclaved on two consecutive days. Each flask was inoculated with R. solani AG-8 isolate C1 grown on PDA for 7 days. Half of a plate was cut into small pieces and added to a flask. After 4 weeks at room temperature, the colonized oat grain was removed from the flasks, dried for 72 h under a sterile airflow, and then stored at 4°C. Colonized oat kernels were fragmented in a Waring blender and sieved into particles of 250 μm to 1 mm. Inocula were enumerated from a suspension of 100 mg of homogenized material in 5 ml of water and from a 10-fold dilution of the suspension on Rhizoctonia-selective medium (RSM) plates containing 100 μg ml−1 chloramphenicol, a broad-spectrum antibacterial agent, and 1 μg ml−1 active ingredient (a.i.) benomyl (Benlate, DuPont, Wilmington, DE), a fungicide with activity against ascomycetes. The inoculum titer was calculated after incubation at room temperature in darkness for 3 days (30). For biocontrol assays, R. solani AG-8 isolate C1 was added to sieved and pasteurized Palouse silt loam soil from the Spillman Agronomy Farm, Pullman, WA, to reach 100 propagules per gram (ppg) of soil. Sixty grams of pasteurized Spillman soil infested with R. solani AG-8 isolate C1 at 100 ppg of soil was placed inside a tapered plastic tube (Cone-tainer; 15 cm long, 2.5 cm in diameter) (Stuewe and Sons, Inc., Corvallis, OR). Cone-tainers were held in plastic racks (100 cones per rack). Each Cone-tainer was watered (10 ml), one wheat seed (Triticum aestivum cv. Penawawa) was sown and covered with a 1-cm layer of noninoculated soil, and the rack with the tubes was covered with a sheet of plastic until the plants emerged.
Seeds were treated with strain 2-79, R2-7-07, R4-34-07, or R11-23-07 (106 CFU per seed) using methylcellulose, as previously described (43). Controls were treated with only methylcellulose. Each tube served as a replicate (20 per treatment). Tubes were arranged in a completely randomized design and incubated in a growth room (15°C, 12-h photoperiod). Tubes received 10 ml of water twice a week and one-third-strength Hoagland's nutrient solution (macroelements only) once a week. After 2 weeks, plants were removed from the tubes and shoot height and root and seedling weights were measured. The results from two separate experiments were pooled.
Data analysis.
GPS data were managed using ArcGIS 9.3.1 software (ESRI, Redlands, CA). Precipitation data were from the PRISM Climate Group, Oregon State University, Corvallis, OR (http://www.prismclimate.org). Statistical analyses were performed with STATISTIX 8.0 software (Analytical Software, St. Paul, MN). In the biocontrol experiments, differences in shoot length, seedling weight, and root weight among treatments were determined by standard analysis of variance or the Kruskal-Wallis test (P ≤ 0.05), and mean comparisons among treatments were performed by using Fisher's protected least-significant-difference test (P ≤ 0.05) or the Kruskal-Wallis test for all pairwise comparisons (P ≤ 0.05). Regression analysis was used to determine the relationship between precipitation and plant colonization frequencies of Phz+ Pseudomonas spp. and the relationship between log PCA ng g−1 (fresh weight) of roots and population densities of Phz+ Pseudomonas spp.
RESULTS
Populations and colonization frequency of indigenous Phz+ Pseudomonas spp. in the rhizosphere of dryland cereal crops.
Phz+ Pseudomonas spp. were detected in all 86 samples collected from 61 fields in 2008 and 2009. Population sizes ranged from log 5.1 to log 7.3 and from log 3.2 to log 7.0 CFU per gram (fresh weight) of roots in 2008 and 2009, respectively. However, across both years, populations were high, with 82.5% of sampled plants supporting densities of Phz+ pseudomonads greater than 105 CFU per gram (fresh weight) of roots. In 2008, densities in fields sampled multiple times between March and June remained fairly consistent. For example, in spring barley (47°8′34′′N, 118°20′22′′W) sampled on 24 April, 16 May, 9 June, and 30 June 2008, population densities of Phz+ pseudomonads were log 6.7, log 6.8, log 6.4, and log 6.1, respectively (Table 1). In both 2008 and 2009, population densities of total culturable aerobic bacteria varied from 107 to 109 CFU g−1 of roots (fresh weight) (data not shown).
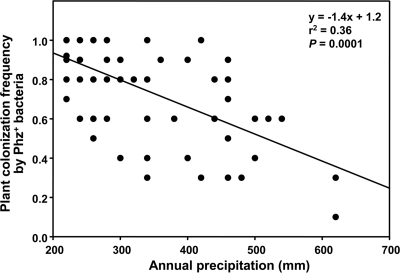
The frequency of colonization of individual plants by Phz+ pseudomonads was also consistently high. At 71% of the sites sampled, Phz+ pseudomonads were present in more than 80% of plant rhizospheres. The highest population densities and colonization frequencies were on plants from fields located in the most arid areas (Fig. 1). Populations and frequencies decreased in samples taken from fields to the east of the low-precipitation zone. Linear regression analysis confirmed this observation and demonstrated a significant (r2 = 0.36, P = 0.0001) inverse relationship between the colonization frequency of individual plants by Phz+ Pseudomonas spp. and the mean annual precipitation (averaged for sampled sites for a 30-year period from 1971 to 2000) (Fig. 2). There was no significant correlation between population densities and annual precipitation or between the colonization frequency and sampling date or the plant growth phase (data not shown).
Fig 2.
Relationship between frequencies of plant colonization by indigenous Phz+ rhizobacteria and annual precipitation in sampled locations. In each sampled location, the mean colonization frequency was calculated based on screening 8 to 16 individual wheat rhizospheres for the presence of Phz+ rhizobacteria with the PCR-based dilution endpoint assay. The mean annual precipitation values are based on precipitation records for the years 1971 through 2000.
Accumulation of phenazine-1-carboxylic acid in the rhizosphere of cereal crops.
For measurements of PCA in the rhizosphere, we used roots from fields that had yielded consistently high populations of Phz+ rhizobacteria (Fig. 1). We recovered PCA from plant roots using a protocol that has been developed, optimized, and successfully employed in our laboratory for extraction and high-performance liquid chromatography (HPLC)-based detection of antibiotics from the rhizosphere of greenhouse-grown wheat (33, 44). In this study, however, the detection and quantification of PCA was carried out using the HPLC–Q-Tof 2 mass spectrometer. To compensate for variability in the PCA recovery due to differences in soil properties at various locations and in time of sampling, all samples were spiked prior to extraction with a known amount of an internal standard consisting of synthetic unsubstituted phenazine, which has the same extraction efficiency from roots as PCA and is easily distinguished from PCA by HPLC or mass spectrometry. Extraction efficiency, determined by comparing the ion peak area of 2 μg of phenazine extracted from rhizosphere samples with that of the same amount analyzed without extraction, ranged from 59% to 72%. The intrarun precision, expressed as a coefficient of variation (CV), was less than 13% for 40 ng on the column, and the interday CV for the calibration curves was less than 5%.
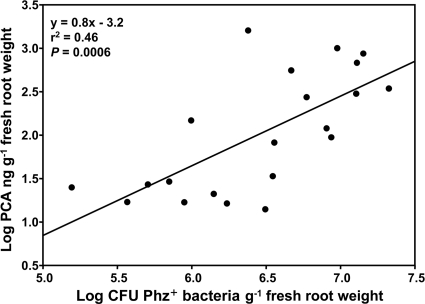
The optimized extraction protocol was applied to root samples that had been collected during 2008 and 2009 and that included winter wheat, spring wheat, and spring barley (Table 1). Of 29 processed root samples, PCA was quantifiable in 21, with amounts of the recovered antibiotic varying approximately 114-fold, from 14 to 1,600 ng g−1 of roots (fresh weight) (Table 1). Of the remaining eight samples, PCA was detectable in five, but the amounts were below the quantification limit of our assay (13.3 ng g−1 [fresh weight] of roots), and in three samples, the amounts of antibiotic were below the detection limit of our assay (2.3 ng g−1 [fresh weight] of roots). Linear regression analysis demonstrated a significant positive relationship (r2 = 0.46, P = 0.0006) between the numbers of CFU of Phz+ isolates and the concentration of PCA on the roots (Fig. 3). The largest amounts of PCA were recovered from spring wheat and barley that had been collected in late April to mid-May.
Fig 3.
Relationship between the accumulation of PCA in field-grown cereals and populations of indigenous Phz+ rhizobacteria. Amounts of PCA were determined by extracting the antibiotic from 15-gram samples of wheat roots and performing a quantitation of PCA by HPLC–Q-TOF–MS/MS. In each location from which samples were extracted for PCA, the populations of Phz+ rhizobacteria were determined by analyzing 8 to 16 individual wheat rhizospheres using the PCR-based endpoint dilution assay with phzF-specific primers.
Biocontrol of Rhizoctonia root rot by Phz+ Pseudomonas spp.
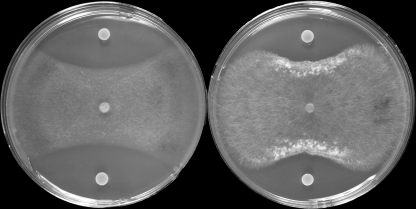
All four Pseudomonas treatments provided significant (P = 0.05) suppression of Rhizoctonia root rot compared to that of the nontreated control based upon measurements of shoot length, seedling weight, or root weight (Table 2). All of these growth parameters are reduced in wheat by infection with Rhizoctonia. Strain R2-7-07 was the most effective of the four strains tested. The tested Phz+ strains were also strongly inhibitory to R. solani AG-8 under in vitro conditions (Fig. 4).
Table 2.
Biological control of Rhizoctonia root rot by Phz+ pseudomonads
| Control or strain used for treatmenta | Shoot lengthb (cm) | Seedling wtb (mg) | Root wtb (mg) |
|---|---|---|---|
| Control | 16.1 ± 0.3 c | 306 ± 16 b* | 86 ± 5 c |
| 2-79 | 17.1 ± 0.3 b | 357 ± 40 ab* | 96 ± 4 bc |
| R2-7-07 | 18.0 ± 0.4 a | 390 ± 21 a* | 115 ± 6 a |
| R4-34-07 | 17.9 ± 0.3 ab | 349 ± 18 ab* | 104 ± 5 ab |
| R11-23-07 | 17.4 ± 0.3 ab | 361 ± 17 ab* | 113 ± 5 a |
Pasteurized Spillman soil was infested with R. solani AG-8 isolate C1 at 100 ppg soil, and seeds of spring wheat (Triticum aestivum cv. Penawawa) were treated with bacteria at ∼106 CFU per seed. Seeds in the control treatment received 1% methylcellulose only.
Combined results of two independent experiments are shown. Data are the averages of 40 replications ± standard error (SE). Numbers in the same column followed by different letters are significantly different according to Fisher's protected least-significant-difference test (P = 0.05), and numbers in the same column followed by asterisks are significantly different according to the Kruskal-Wallis test for all pairwise comparisons (P ≤ 0.05).
Fig 4.
Antagonistic activity of Pseudomonas sp. R2-7-07 against plant pathogens G. graminis var. tritici ARS-A1 (left) and R. solani AG-8 isolate C1 (right). Agar plugs with mycelium (5-mm diameter) were taken from the edges of actively growing cultures of R. solani AG-8 C1 and G. graminis var. tritici ARS-A1 and transferred to the center of one-quarter-strength PDA agar plates. The plates were incubated at room temperature in the dark for 24 h, and then 5-μl aliquots of bacterial culture (OD600 of 0.1) were spotted 1.5 cm from the edge of the plate. The coinoculated plates were kept in the dark at room temperature and examined when the fungus reached the edge of the negative-control plate.
DISCUSSION
Despite decades of study, fundamental questions have remained unanswered about the quantity and frequency of antibiotic production by indigenous microorganisms in natural habitats. We addressed these questions by determining the geographic distribution of Phz+ Pseudomonas spp. and the concomitant accumulation of the broad-spectrum antibiotic PCA in the rhizosphere of wheat and barley grown commercially on the Columbia Plateau of the Pacific Northwest. This arid region, situated in the rain shadow of the Cascade Mountains, has cool, moist winters, warm, dry summers, and annual precipitation of less than 350 mm, two-thirds of which falls between November and May. A winter wheat-summer fallow rotation is used throughout the region because precipitation accumulating in the soil profile is sufficient to support only one crop every 2 years. Growers typically plant seeds as deep as 20 cm in late August or September with deep-furrow drills to find moisture adequate for seed germination.
Our results revealed that indigenous Phz+ Pseudomonas spp. are highly abundant (generally >105 CFU g−1 of roots [fresh weight]) and thrive on roots of cereal crops grown under these arid conditions (Fig. 1 and 3). In fact, our data suggest that Phz+ Pseudomonas spp. are uniquely adapted to the rhizosphere under conditions of water stress and that soil moisture represents a major abiotic factor that drives the development of the indigenous phenazine-producing microbial community. This notion is supported by the inverse correlation (r2 = 0.36, P = 0.0001) between the proportion of plants colonized by Phz+ Pseudomonas spp. and local annual precipitation values (Fig. 2). Especially notable is that wheat collected in the Horse Heaven Hills area was heavily colonized by phenazine producers (mean colonization frequency of 0.87; mean Phz+ population level of log 5.9 CFU g−1 of roots [fresh weight]) and had readily quantifiable levels of PCA (34.6 to 557.6 ng g−1 of roots [fresh weight]). The Horse Heaven Hills (46°7′59′′N, 119°52′4′′W) are situated in Benton County of Washington State and encompass 121,400 cultivated dryland hectares that receive less annual precipitation (∼165 mm) than any other nonirrigated, wheat-producing region in the United States. Our findings are in contrast to the idea (14) that pseudomonads require water potentials near field capacity and percolating water for rapid growth and antibiotic production in the rhizosphere.
Our study is also the first to demonstrate the capacity of an indigenous microbial community to produce significant quantities of antibiotic across a large terrestrial ecosystem. The amounts of PCA we recovered from the rhizosphere of field-grown cereals were greater than or equal to those previously reported for experimental systems that utilized introduced rhizobacteria and/or controlled plant growth conditions (41). We isolated phenazines by solvent extraction of pooled root samples, and thus, our measured amounts of PCA probably underestimated actual concentrations in and around microcolonies of Phz+ pseudomonads. Given that bacteria inhabit only 12 to 15% of the rhizosphere volume (47) and that Phz+ Pseudomonas spp. generally comprise only 1 to 10% of the culturable rhizosphere community (data not shown), we estimate that localized PCA concentrations could easily reach amounts of 100 nmol nanomoles. The synthesis of phenazines in Pseudomonas spp. is regulated by quorum sensing (24), and thus, it was not surprising that the amount of PCA recovered from the rhizosphere was positively correlated with the population density of Phz+ bacteria colonizing the roots (Fig. 3).
What is the biological significance of PCA accumulating in the rhizosphere of dryland wheat? Phenazines are known to function as molecular signals controlling morphology and biofilm formation (24) and as electron shuttles facilitating the mobilization of Fe and Mn in alkaline soils (13, 32, 46). The concentrations of PCA we detected in the rhizosphere of field-grown wheat are sufficient to carry out these functions. The 100 nM amounts of PCA are also sufficient to inhibit other microorganisms, as they approach the MICs for some Gram-positive bacteria and fungi (40). We suggest that PCA in the rhizosphere contributes to the longevity and sustainability of wheat production in the low-precipitation zone by serving as the first line of microbial defense of roots against one or more fungal pathogens.
Phenazines have been implicated in the biocontrol of soilborne plant diseases by introduced fluorescent pseudomonads, and Thomashow et al. used a molecular genetic approach (43) and analytical techniques (44) to demonstrate that PCA produced in the wheat rhizosphere under greenhouse conditions was responsible for the inhibition of Gaeumannomyces graminis var. tritici, the take-all pathogen of wheat. In the Châteaurenard region of Dijon, France, indigenous phenazine-producing bacteria working in concert with nonpathogenic Fusarium oxysporum form the basis of natural disease suppression in these Fusarium wilt-suppressive soils (26, 43). In the inland Pacific Northwest, root and crown rots caused by Fusarium culmorum, Fusarium pseudograminearum, Rhizoctonia solani AG-8, and Rhizoctonia oryzae are common soilborne diseases of dryland wheat for which commercial varieties lack resistance (6, 31). Recently, Schillinger and Paulitz (37) reported a spontaneous decline in the severity of Rhizoctonia root rot in wheat grown near Ritzville that is similar to declines mentioned in reports in Australia (35, 52). PCA-producing isolates from the Ritzville and Lind areas are inhibitory to Rhizoctonia spp. in vitro (Fig. 4), and application of Phz+ Pseudomonas spp. as seed treatments to wheat resulted in significant suppression of Rhizoctonia root rot (Table 2).
To summarize, we discovered a large-scale and continuously distributed antibiotic-producing microbial community associated with the rhizosphere of commercially grown cereal crops. This microbial community has the capacity to produce large (up to 1.6 micrograms per gram of roots [fresh weight]) quantities of PCA in the rhizosphere of cereals. The broad activity of phenazine metabolites makes the widespread occurrence of PCA in agricultural soils of the low-precipitation zone of the Columbia Plateau highly biologically significant. Our findings also raise numerous unanswered questions, including those pertaining to the identities of the biotic and abiotic factors influencing PCA accumulation in the plant rhizosphere, the dynamics of antibiotic turnover in soil, and the possibility of uptake/accumulation of phenazines by plant tissues.
ACKNOWLEDGMENTS
We are grateful to Karen Adams, Renee Espinosa, and Karen Hansen for excellent technical assistance. We also thank Marc Evans of the Department of Statistics at Washington State University for assistance with the analysis of our data.
Footnotes
Published ahead of print 2 December 2011
REFERENCES
- 1. Becker MH, Brucker RM, Schwantes CR, Harris RN, Minbiole KPC. 2009. The bacterially produced metabolite violacein is associated with survival of amphibians infected with a lethal fungus. Appl. Environ. Microbiol. 75:6635–6638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bergsma-Vlami M, Prins ME, Raaijmakers JM. 2005. Influence of plant species on population dynamics, genotypic diversity and antibiotic production in the rhizosphere by indigenous Pseudomonas spp. FEMS Microbiol. Ecol. 52:59–69 [DOI] [PubMed] [Google Scholar]
- 3. Bonsall RF, Thomashow LS, Mavrodi DV, Weller DM. 2007. Extraction and detection of antibiotics in the rhizosphere metabolome. Curr. Trends Mass Spectrom. (Suppl. Spectrosc.) 11:14–19 [Google Scholar]
- 4. Bonsall RF, Weller DM, Thomashow LS. 1997. Quantification of 2,4-diacetylphloroglucinol produced by fluorescent Pseudomonas spp. in vitro and in the rhizosphere of wheat. Appl. Environ. Microbiol. 63:951–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brucker RM, et al. 2008. Amphibian chemical defense: antifungal metabolites of the microsymbiont Janthinobacterium lividum on the salamander Plethodon cinereus. J. Chem. Ecol. 34:1422–1429 [DOI] [PubMed] [Google Scholar]
- 6. Cook RJ. 1986. Wheat management systems in the Pacific Northwest. Plant Dis. 70:894–898 [Google Scholar]
- 7. Currie CR, Poulsen M, Mendenhall J, Boomsma JJ, Billen J. 2006. Coevolved crypts and exocrine glands support mutualistic bacteria in fungus-growing ants. Science 311:81–83 [DOI] [PubMed] [Google Scholar]
- 8. Davies J. 2006. Are antibiotics naturally antibiotics? J. Ind. Microbiol. Biotechnol. 33:496–499 [DOI] [PubMed] [Google Scholar]
- 9. Davies J, Spiegelman GB, Yim G. 2006. The world of subinhibitory antibiotic concentrations. Curr. Opin. Microbiol. 9:445–453 [DOI] [PubMed] [Google Scholar]
- 10. Fajardo A, Martinez JL. 2008. Antibiotics as signals that trigger specific bacterial responses. Curr. Opin. Microbiol. 11:161–167 [DOI] [PubMed] [Google Scholar]
- 11. Haas D, Defago G. 2005. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat. Rev. Microbiol. 3:307–319 [DOI] [PubMed] [Google Scholar]
- 12. Haas D, Keel C. 2003. Regulation of antibiotic production in root-colonizing Pseudomonas spp. and relevance for biological control of plant disease. Annu. Rev. Phytopathol. 41:117–153 [DOI] [PubMed] [Google Scholar]
- 13. Hernandez ME, Kappler A, Newman DK. 2004. Phenazines and other redox-active antibiotics promote microbial mineral reduction. Appl. Environ. Microbiol. 70:921–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Howie WJ, Cook RJ, Weller DM. 1987. Effects of soil matric potential and cell motility on wheat root colonization by fluorescent pseudomonads suppressive to take-all. Phytopathology 77:286–292 [Google Scholar]
- 15. Keel C, et al. 1992. Suppression of root diseases by Pseudomonas fluorescens CHA0-importance of the bacterial secondary metabolite 2,4-diacetylphloroglucinol. Mol. Plant Microbe Interact. 5:4–13 [Google Scholar]
- 16. Kempf HJ, Bauer PH, Schroth MN. 1993. Herbicolin A-associated with crown and roots of wheat after seed treatment with Erwinia herbicola B247. Phytopathology 83:213–216 [Google Scholar]
- 17. Kempf HJ, Sinterhauf S, Muller M, Pachlatko P. 1994. Production of two antibiotics by a biocontrol bacterium in the spermosphere of barley and in the rhizosphere of cotton, p 114–116 In Ryder MH, Stephens PM, Bowen GD. (ed), Improving plant productivity with rhizosphere bacteria. CSIRO Division of Soils, Adelaide, South Australia, Australia [Google Scholar]
- 18. King EO, Ward MK, Raney DE. 1954. Two simple media for the demonstration of pyocyanin and fluorescein. J. Lab. Clin. Med. 44:301–307 [PubMed] [Google Scholar]
- 19. Kroiss J, et al. 2010. Symbiotic Streptomycetes provide antibiotic combination prophylaxis for wasp offspring. Nat. Chem. Biol. 6:261–263 [DOI] [PubMed] [Google Scholar]
- 20. Little AE, Robinson CJ, Peterson SB, Raffa KF, Handelsman J. 2008. Rules of engagement: interspecies interactions that regulate microbial communities. Annu. Rev. Microbiol. 62:375–401 [DOI] [PubMed] [Google Scholar]
- 21. Lumsden RD, Locke JC, Adkins ST, Walter JF, Ridout CJ. 1992. Isolation and localization of the antibiotic gliotoxin produced by Gliocladium virens from alginate prill in soil and soilless media. Phytopathology 82:230–235 [Google Scholar]
- 22. Martinez JL. 2008. Antibiotics and antibiotic resistance genes in natural environments. Science 321:365–367 [DOI] [PubMed] [Google Scholar]
- 23. Maurhofer M, Keel C, Haas D, Defago G. 1995. Influence of plant species on disease suppression by Pseudomonas fluorescens strain CHA0 with enhanced antibiotic production. Plant Pathol. 44:40–50 [Google Scholar]
- 24. Mavrodi DV, Blankenfeldt W, Thomashow LS. 2006. Phenazine compounds in fluorescent Pseudomonas spp.: biosynthesis and regulation. Annu. Rev. Phytopathol. 44:417–445 [DOI] [PubMed] [Google Scholar]
- 25. Mavrodi DV, et al. 2010. Diversity and evolution of the phenazine biosynthesis pathway. Appl. Environ. Microbiol. 76:866–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mazurier S, Corberand T, Lemanceau P, Raaijmakers JM. 2009. Phenazine antibiotics produced by fluorescent pseudomonads contribute to natural soil suppressiveness to Fusarium wilt. ISME J. 3:977–991 [DOI] [PubMed] [Google Scholar]
- 27. McSpadden Gardener BB, Mavrodi DV, Thomashow LS, Weller DM. 2001. A rapid polymerase chain reaction-based assay characterizing rhizosphere populations of 2,4-diacetylphloroglucinol-producing bacteria. Phytopathology 91:44–54 [DOI] [PubMed] [Google Scholar]
- 28. McSpadden Gardener BB, Weller DM. 2001. Changes in populations of rhizosphere bacteria associated with take-all disease of wheat. Appl. Environ. Microbiol. 67:4414–4425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nielsen TH, Sorensen J. 2003. Production of cyclic lipopeptides by Pseudomonas fluorescens strains in bulk soil and in the sugar beet rhizosphere. Appl. Environ. Microbiol. 69:861–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Okubara PA, Schroeder KL, Paulitz TC. 2008. Identification and quantification of Rhizoctonia solani and R. oryzae using real-time polymerase chain reaction. Phytopathology 98:837–847 [DOI] [PubMed] [Google Scholar]
- 31. Paulitz TC, Schillinger WF, Cook RJ. 2003. Greenhouse studies of Rhizoctonia bare patch disease in soil cores from direct-seeded fields. Annu.Meet. Am. Soc. Agron. ASA, CSSA, and SSSA Abstracts, Denver, CO. [Google Scholar]
- 32. Price-Whelan A, Dietrich LE, Newman DK. 2006. Rethinking ‘secondary’ metabolism: physiological roles for phenazine antibiotics. Nat. Chem. Biol. 2:71–78 [DOI] [PubMed] [Google Scholar]
- 33. Raaijmakers JM, Bonsall RE, Weller DM. 1999. Effect of population density of Pseudomonas fluorescens on production of 2,4-diacetylphloroglucinol in the rhizosphere of wheat. Phytopathology 89:470–475 [DOI] [PubMed] [Google Scholar]
- 34. Raaijmakers JM, Vlami M, de Souza JT. 2002. Antibiotic production by bacterial biocontrol agents. Antonie Van Leeuwenhoek 81:537–547 [DOI] [PubMed] [Google Scholar]
- 35. Roget DK, Coppi JA, Herdina Gupta VVSR. 1999. Assessment of suppression to Rhizoctonia solani in a range of soils across SE Australia, p 129–130. In Magarey RC. (ed), Proceedings of the First Australasian Soilborne Disease Symposium. BSES, Brisbane, Australia [Google Scholar]
- 36. Schillinger WF, Papendick RI. 2008. Then and now: 125 years of dryland wheat farming in the Inland Pacific Northwest. Agron. J. 100:S166–S182 [Google Scholar]
- 37. Schillinger WF, Paulitz TC. 2006. Reduction of Rhizoctonia bare patch in wheat with barley rotations. Plant Dis. 90:302–306 [DOI] [PubMed] [Google Scholar]
- 38. Scott JJ, et al. 2008. Bacterial protection of beetle-fungus mutualism. Science 322:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shanahan P, O'Sullivan DJ, Simpson P, Glennon JD, O'Gara F. 1992. Isolation of 2,4-diacetylphloroglucinol from a fluorescent pseudomonad and investigation of physiological parameters influencing its production. Appl. Environ. Microbiol. 58:353–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Smirnov VV, Kiprianova EA. 1990. Bacteria of Pseudomonas genus. Naukova Dumka, Kiev, USSR [Google Scholar]
- 41. Thomashow LS, Bonsall RF, Weller DM. 1997. Antibiotic production by soil and rhizosphere microbes in situ, p 493–499 In Hurst CJ, Knudsen GR, McInerney MJ, Stetzenbach LD, Walter MV. (ed), Manual of environmental microbiology. ASM Press, Washington, DC [Google Scholar]
- 42. Thomashow LS, Weller DM. 1995. Current concepts in the use of introduced bacteria for biological disease control: mechanisms and antifungal metabolites, p 187–235 In Stacey G, Keen N. (ed), Plant-microbe interactions, vol 1. Chapman & Hall, New York, NY [Google Scholar]
- 43. Thomashow LS, Weller DM. 1988. Role of a phenazine antibiotic from Pseudomonas fluorescens in biological control of Gaeumannomyces graminis var. tritici. J. Bacteriol. 170:3499–3508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Thomashow LS, Weller DM, Bonsall RF, Pierson LS. 1990. Production of the antibiotic phenazine-1-carboxylic acid by fluorescent Pseudomonas species in the rhizosphere of wheat. Appl. Environ. Microbiol. 56:908–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. von Nussbaum F, Brands M, Hinzen B, Weigand S, Habich D. 2006. Antibacterial natural products in medicinal chemistry-exodus or revival? Angew. Chem. Int. Ed. Engl. 45:5072–5129 [DOI] [PubMed] [Google Scholar]
- 46. Wang Y, Newman DK. 2008. Redox reactions of phenazine antibiotics with ferric (hydr)oxides and molecular oxygen. Environ. Sci. Technol. 42:2380–2386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Watt M, Hugenholtz P, White R, Vinall K. 2006. Numbers and locations of native bacteria on field-grown wheat roots quantified by fluorescence in situ hybridization (FISH). Environ. Microbiol. 8:871–884 [DOI] [PubMed] [Google Scholar]
- 48. Weller DM. 1988. Biological control of soilborne plant pathogens in the rhizosphere with bacteria. Annu. Rev. Phytopathol. 26:379–407 [Google Scholar]
- 49. Weller DM, et al. 1986. Rhizoctonia root rot of small grains favored by reduced tillage in the Pacific Northwest. Plant Dis. 70:70–73 [Google Scholar]
- 50. Weller DM, et al. 2007. Role of 2,4-diacetylphloroglucinol-producing fluorescent Pseudomonas spp. in the defense of plant roots. Plant Biol. 9:4–20 [DOI] [PubMed] [Google Scholar]
- 51. Weller DM, Raaijmakers JM, Gardener BBM, Thomashow LS. 2002. Microbial populations responsible for specific soil suppressiveness to plant pathogens. Annu. Rev. Phytopathol. 40:309–348 [DOI] [PubMed] [Google Scholar]
- 52. Wiseman BM, Neate SM, Keller KO, Smith SE. 1996. Suppression of Rhizoctonia solani anastomosis group 8 in Australia and its biological nature. Soil Biol. Biochem. 28:727–732 [Google Scholar]