Abstract
Complicated skin and soft tissue infections (cSSTIs) are among the most rapidly increasing reasons for hospitalization. To describe inpatients with regard to patient characteristics, cSSTI origin, appropriateness of initial antibiotics, and outcomes, we performed a retrospective cohort study in patients hospitalized for cSSTI. To identify independent predictors of outcomes, we performed multivariate analyses. Of 1,096 eligible patients, 48.7% had health care-associated (HCA) cSSTI and 51.3% had community-acquired (CA) cSSTI. After adjustment for baseline variables, hospital length of stay (LOS) was longer for HCA than for CA cSSTI (difference, 2.1 days; 95% confidence interval [CI], 0.8 to 3.5; P < 0.05). Other covariates associated with a longer LOS were need for dialysis (regression coefficient ± standard error, 4.5 ± 1.1) and diabetic wound diagnosis (2.6 ± 1.0) (all P < 0.05). In the subset with culture-positive cSSTI within 24 h of admission, the most common pathogen was Staphylococcus aureus (298/449 [66.4%]), of which 74.8% (223/298) were methicillin-resistant S. aureus (MRSA). Eighty-three patients (18.5%) received inappropriate initial antibiotics. After adjustment for other variables, the following were associated with inappropriate initial therapy: direct admission to hospital (not via emergency department), cSSTI caused by MRSA or mixed pathogens, and cSSTI caused by pathogens other than S. aureus or streptococci (all P < 0.05). We did not find an association between inappropriate therapy and outcomes, except in the subset with ulcers (adjusted odds ratio, 11.8; 95% CI, 1.3 to 111.1; P = 0.03). More studies are needed to examine the impact of HCA cSSTI and inappropriate initial therapy on outcomes.
INTRODUCTION
Complicated skin and soft tissue infections (cSSTIs) typically involve deep soft tissue and occur in patients with underlying disease, often requiring intravenous antibiotic therapy, surgical intervention, or both. cSSTIs are among the most rapidly increasing reasons for hospitalization (7, 15) and are associated with substantial health care costs (11, 13, 16).
The recent rise in antibiotic resistance among causative pathogens (23), particularly methicillin-resistant Staphylococcus aureus (MRSA), has complicated management of cSSTIs. These pathogens, previously confined to health care settings, are increasingly common in the community (5, 9) as some care traditionally provided in hospitals is now provided in ambulatory settings. This shift has blurred the distinction between community-acquired and nosocomial infections, prompting the introduction of a new category of health care-associated infections to describe infections in community-based patients who have had contact with the health care system and thus may have been exposed to potentially resistant pathogens.
The concept of health care-associated infection has been studied extensively in patients with pneumonia and bacteremia; the epidemiology, microbiology, and outcomes of health care-associated infections coincide more closely with those of nosocomial infections. Furthermore, studies in these types of infections have shown that inappropriate initial antibiotic therapy is associated with worse outcomes (1, 8, 12, 17, 18, 22). It is unclear whether these concepts hold true for cSSTI because of the paucity of specific studies.
We conducted a retrospective cohort study with chart review of hospitalized patients with an admission diagnosis of cSSTI. Our primary objectives were to (i) describe patients with cSSTI with regard to origin of infection (health care associated or community acquired), (ii) describe patients with regard to appropriateness of initial antibiotic therapy in the subset of patients with culture-positive cSSTI, and (iii) determine whether origin of infection and/or appropriateness of empirical therapy is a predictor of hospital length of stay (LOS) and readmission or death within 30 days of discharge.
(The preliminary findings of this study were presented as posters at the 19th Annual Scientific Meeting of the Society for Healthcare Epidemiology of America [SHEA], San Diego, CA, 19 to 22 March 2009 [14], and the 47th Annual Meeting of the Infectious Diseases Society of America, Philadelphia, PA, 29 October to 1 November 2009 [27].)
MATERIALS AND METHODS
This retrospective cohort study was conducted at a 900-bed urban teaching hospital of a large health care system in the midwestern region of the United States. We enrolled adult patients (age, ≥18 years) who had a primary or secondary admission diagnosis of cSSTI, had been hospitalized for at least 24 h between 24 December 2005 and 17 October 2008, and had received at least one antibiotic by intravenous administration during hospitalization. Eligible patients were randomly selected from a master list of electronic medical record numbers. Only first admissions were included. Infection was defined as the presence of a predominant organism(s) plus signs and symptoms of infection, with the organism(s) identified from operative cultures done at the time of debridement or incision and drainage of abscess. To be included in the analysis of appropriateness of therapy, patients also had to have had a culture-positive wound specimen obtained within 24 h after presentation to the emergency room or hospital, whichever occurred first. In addition, patients whose culture-positive wound specimen was obtained within 48 h after presentation to the emergency room or hospital, whichever occurred first, were considered separately in a sensitivity analysis. Patients were excluded if any of the following were present: transfer to or from another hospital, comorbid infection other than cSSTI, admission for scheduled amputation, cSSTI acquired during current hospitalization, or diagnosis of burn, gangrene, animal or human bite, or osteomyelitis. The protocol was approved by the Institutional Review Board. Informed consent was waived. Data were collected in compliance with the Health Insurance Portability and Accountability Act (HIPAA).
Predefined patient characteristics, cSSTI therapy (antibiotics and procedures), and outcomes were abstracted from chart review and electronic medical records and entered into a study-specific database. Outcomes included hospital LOS, mortality during hospitalization, and, among those discharged alive, readmission or death within 30 days of discharge.
The origin of infection was designated health care associated or community acquired on the basis of patient history. Infections were designated health care associated if patients had been hospitalized within 180 days, were transferred from a nursing home, rehabilitation hospital, or other long-term nursing care facility, had received antibiotics in the past 30 days, were receiving dialysis, or were immunosuppressed at time of admission. Immunosuppression was defined as corticosteroid therapy, seropositivity for human immunodeficiency virus, solid organ or bone marrow transplantation, treatment with radiotherapy or chemotherapy for malignancy within 6 months, or primary or secondary immunodeficiency. Infections not meeting health care-associated criteria were designated community acquired. cSSTI classifications comprised diabetic wound infection (International Classification of Diseases [ICD-9] code for diabetes [250.xx] and any SSTI code), severe cellulitis, surgical wound infection, surgical device-associated or prosthesis-associated infection, necrotizing fasciitis, major or deep abscess, decubitus ulcer, or nondiabetic wound. Initial antibiotic therapy was defined as appropriate if it was given within 24 h of admission and was active against all identified pathogens on the basis of in vitro susceptibility testing or spectrum coverage (a proxy measure for susceptibility when susceptibility results were unavailable) based on the Sanford Guide (10). Polymicrobial infection was defined as infection with more than one causative pathogen; mixed infection was defined as infection with both Gram-positive and Gram-negative pathogens.
Statistical analysis.
Descriptive statistics included relative frequencies for categorical variables and means (and standard deviations) or medians (and interquartile ranges) for continuous variables. Bivariate associations were tested by χ2 or Fisher's exact test for two categorical variables, Student's t or Wilcoxon rank sum test for one binary and one continuous variable, and Pearson or Spearman correlation coefficients for two continuous variables, depending on whether assumptions were met; variables with associations resulting in P values of <0.20 were included in initial multivariate models. Multiple linear regression analysis was performed using the log of LOS as the dependent variable. Duan's smearing technique (4) was used to “retransform” log-estimated differences to derive estimates of differences in days. The procedure was performed using both the group-specific smearing factor and the common smearing factor; upon review of the standard deviations, one method was chosen. Multiple logistic regression analysis was conducted for dichotomous outcome variables (e.g., readmission), and adjusted odds ratios and 95% confidence intervals (CI) were derived for categorical independent variables. Multivariate procedures were performed using a monitored iterative backwards elimination approach in which the variable with the largest P value was eliminated at each step, provided it was not a main effect of an interaction. Variables were retained in final multivariate models if P was <0.05 or if they were the primary exposure variables (i.e., health care-associated versus community-acquired cSSTI and appropriate versus inappropriate antibiotic therapy). In the analysis to determine whether appropriateness of initial antibiotic therapy was predictive of outcome, bivariate and multivariate analyses were performed using data from the subset of patients who had a culture taken within 24 h of admission; a sensitivity analysis was performed in the larger subset of patients who had a culture taken within 48 h. All tests were two-tailed.
RESULTS
Health care-associated versus community-acquired cSSTI analyses (in all patients).
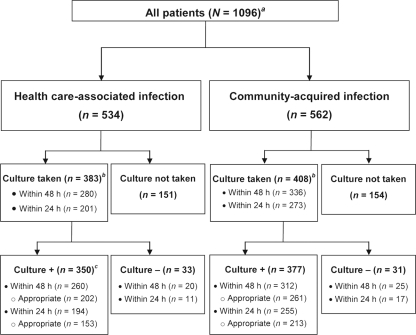
We abstracted chart data from a total of 1,096 eligible patients with cSSTIs, including 534 (48.7%) with health care-associated infections and 562 (51.3%) with community-acquired infections (Fig. 1). Compared with patients with community-acquired cSSTIs, patients with health care-associated cSSTIs were older, were generally more likely to have comorbidities such as cancer, cardiovascular disease, and chronic kidney disease, and were more likely to have Medicare insurance. Patients with community-acquired infections were more likely to be African American, to have no insurance, to have abused intravenous drugs, and to have been admitted through the emergency department (Table 1). The most common types of cSSTIs were cellulitis and abscess, both of which were more likely to be community acquired; surgical wound infection and ulcer were more likely to be health care associated.
Fig 1.
Flow diagram of patients with cSSTIs. a, This includes patients who met the inclusion/exclusion criteria. Originally, we included randomly selected patients hospitalized in 2007. To increase the sample size, we later added patients who had positive cultures from December 2005 to October 2008. b, Culture obtained at any time during hospitalization. c, Four patients had missing pathogen information and were excluded from the analysis within 48 h; one had missing pathogen information and was excluded from the analysis within 24 h. “Appropriate” refers to appropriate initial antibiotic therapy.
Table 1.
Characteristics of all patientsa
| Variable | No. (%) of patients, unless otherwise stated |
P valueb | ||
|---|---|---|---|---|
| HCAI (n = 534) | CAI (n = 562) | Total (n = 1,096) | ||
| Demographics | ||||
| Median age (IQR) (yr) | 56 (47, 73) | 52 (41, 63) | 54 (44, 67) | <0.0001 |
| Male gender | 268 (50.2) | 298 (53.0) | 566 (51.6) | 0.35 |
| Race: Caucasian | 186 (34.8) | 165 (29.4) | 351 (32.0) | 0.05 |
| Race: African American | 327 (61.2) | 377 (67.1) | 704 (64.2) | 0.0436 |
| Race: Asian or Middle Eastern | 11 (2.1) | 7 (1.3) | 18 (1.6) | 0.29 |
| Race: Hispanic | 7 (1.3) | 13 (2.3) | 20 (1.8) | 0.22 |
| Ethnicity: Hispanic | 10 (1.9) | 14 (2.5) | 24 (2.2) | 0.48 |
| Insurance | ||||
| Medicaid | 42 (7.9) | 39 (6.9) | 81 (7.4) | 0.56 |
| Medicare | 228 (42.7) | 142 (25.3) | 370 (33.8) | <0.0001 |
| Private/commercial | 225 (42.1) | 275 (48.9) | 500 (45.6) | 0.0239 |
| None | 38 (7.1) | 104 (18.5) | 142 (13.0) | <0.0001 |
| Admission source | ||||
| Prior admission to same hospital at ≤180 days | 347 (65.0) | 0 (0.0) | 347 (31.7) | <0.0001 |
| Transfer from home | 431 (80.7) | 551 (98.0) | 982 (89.6) | <0.0001 |
| Transfer from nursing home, rehab, or hospice | 93 (17.4) | 0 (0.0) | 93 (8.5) | <0.0001 |
| Transfer from other environment | 8 (1.5) | 11 (2.0) | 19 (1.7) | 0.56 |
| ER prior to admission | 427 (80.1) | 497 (88.8) | 924 (84.5) | <0.0001 |
| Comorbidities | ||||
| Intravenous drug abuse | 56 (10.5) | 97 (17.3) | 153 (14.0) | 0.0013 |
| Diabetes | 189 (35.5) | 196 (34.9) | 385 (35.2) | 0.84 |
| Peripheral vascular disease | 59 (11.1) | 50 (8.9) | 109 (10.0) | 0.23 |
| Hypertension | 321 (60.2) | 292 (52.0) | 613 (56.0) | 0.0059 |
| Cancer | 84 (15.8) | 25 (4.5) | 109 (10.0) | <0.0001 |
| Chronic obstructive pulmonary disease | 40 (7.5) | 16 (2.9) | 56 (5.1) | 0.0005 |
| Congestive heart failure | 82 (15.4) | 45 (8.0) | 127 (11.6) | 0.0001 |
| Coronary artery disease | 72 (13.5) | 37 (6.6) | 109 (10.0) | 0.0001 |
| Chronic kidney disease | 83 (15.6) | 33 (5.9) | 116 (10.6) | <0.0001 |
| Anemia | 98 (18.4) | 42 (7.5) | 140 (12.8) | <0.0001 |
| Need for dialysis | 58 (10.9) | 0 (0.0) | 58 (5.3) | <0.0001 |
| Intravenous antibiotic within 30 daysc | 102 (19.3) | 0 (0.0) | 102 (9.4) | <0.0001 |
| Immunosuppression | 128 (24.0) | 0 (0.0) | 128 (11.7) | <0.0001 |
| SSTI subtype | ||||
| Cellulitis | 284 (53.2) | 338 (60.1) | 622 (56.8) | 0.0201 |
| Surgical wound | 96 (18.0) | 2 (0.4) | 98 (8.9) | <0.0001 |
| Necrotizing fasciitis | 9 (1.7) | 13 (2.3) | 22 (2.0) | 0.46 |
| Abscess | 137 (25.7) | 249 (44.1) | 385 (35.1) | <0.0001 |
| Diabetic wound | 30 (5.6) | 42 (7.5) | 72 (6.6) | 0.22 |
| Nondiabetic wound | 23 (4.3) | 16 (2.9) | 39 (3.6) | 0.19 |
| Ulcer | 82 (15.4) | 31 (5.5) | 113 (10.3) | <0.0001 |
| Other | 23 (4.3) | 25 (4.5) | 48 (4.4) | 0.91 |
| Time of first culture relative to ER/hospital admission | ||||
| ≤24 hd | 190 (35.6) | 256 (45.6) | 446 (40.7) | 0.0001 |
| ≤48 hd | 260 (48.7) | 311 (55.3) | 571 (52.1) | 0.0052 |
| >48 hd | 90 (16.9) | 66 (11.7) | 156 (14.2) | 0.0304 |
| Did not have positive culture taken | 184 (34.5) | 185 (32.9) | 369 (33.7) | 0.59 |
CAI, community-acquired infection; HCAI, health care-associated infection; ER, emergency room.
P values for HCAI versus CAI.
The most common antibiotics were vancomycin (n = 35), cefazolin (n = 21), and clindamycin (n = 17).
Time of culture in subset of patients with positive cultures.
In bivariate analyses, health care-associated infection was associated with significantly worse outcomes than community-acquired infection (Table 2). The unadjusted mean hospital LOS was 1.7 days longer for health care-associated infection than for community-acquired infection. The rates of hospital mortality and of readmission or death within 30 days after hospital discharge were more than twice as high for health care-associated infection. After adjustment for the variables in Table 1, hospital LOS was longer for health care-associated infection than for community-acquired infection (difference, 2.11 days; 95% CI, 0.75 to 3.48; P < 0.05; Table 2) (common smearing estimate = 1.52, yielding a difference of 2.24 days). Other covariates significantly (P < 0.05) associated with a longer LOS were need for dialysis (regression coefficient ± standard error [SE], 4.52 ± 1.10) and diagnosis of diabetic wound (2.56 ± 0.98) (data not shown). Patients with both health care-associated infection and anemia were more likely to have a longer LOS (regression coefficient of interaction term ± SE, 3.90 ± 1.57; P < 0.05). Patients with both community-acquired infection and either ulcer (−8.47 ± 1.78) or necrotizing fasciitis (−22.81 ± 3.47) were also more likely to have a longer LOS, whereas patients with community-acquired infection and abscess were more likely to have a shorter LOS (−2.80 ± 1.05) (all P < 0.05). Covariates that were independently associated with hospital readmission or death within 30 days after hospital discharge were older age (odds ratio [95% CI], 1.02 [1.01 to 1.02]), previous hospitalization (2.39 [1.49 to 3.85]), cSSTI diagnosis other than abscess (0.63 [0.42 to 0.93]), and cSSTI diagnosis of ulcers (2.51 [1.61 to 3.91]) (all P < 0.05).
Table 2.
Unadjusted and multivariate analyses of outcomes stratified by health care-associated infection (HCAI) and community-acquired infection (CAI) (n = 1,096)
| Outcome | Unadjusted analysis |
Multivariate analysisa | ||
|---|---|---|---|---|
| HCAI (n = 534) | CAI (n = 562) | P value | ||
| Hospital length of stay (days) | 2.11 (0.75-3.48)b | |||
| Median (IQR) | 5 (1, 73) | 4 (1, 103) | <0.0001c | |
| Mean ± SD | 8.11 ± 8.46 | 6.39 ± 9.71 | <0.0001d | |
| No. (%) of patients with in-hospital mortality | 15 (2.8) | 6 (1.1) | <0.05e | 1.58 (0.58–4.29)f |
| No. (%) of patients with readmission/death within 30 days | 136 (25.5) | 67 (11.9) | <0.05e | 1.08 (0.66–1.76)f |
Adjusted for variables in Table 1.
Regression mean difference (95% CI); P < 0.05; smearing retransformed mean = 2.24 (0.87–3.61).
Wilcoxon rank sum test.
t test on log-transformed length of stay.
Chi-square test.
Adjusted odds ratio (95% CI).
In the subset of 449 patients who had positive cultures obtained within 24 h, 43.2% had a health care-associated cSSTI and 56.8% had a community-acquired cSSTI. The most common pathogen was S. aureus (66.4%), of which 74.8% were MRSA (Table 3), followed by Streptococcus species (26.1%). Other pathogens were cultured in less than 7% of patients.
Table 3.
Distribution of the most common pathogens among patients with a positive culture obtained at <24 h from time of admission or emergency room visit
| Pathogen(s) | No. (%) of patientsa |
P value | ||
|---|---|---|---|---|
| HCAI (n = 194) | CAI (n = 255) | Total (n = 449) | ||
| S. aureus | 131 (67.5) | 167 (65.5) | 298 (66.4) | 0.65 |
| Methicillin resistant | 99 (75.6) | 124 (74.3) | 223 (74.8) | 0.71 |
| Methicillin susceptible | 31 (23.7) | 43 (25.7) | 74 (24.8) | |
| Missing sensitivity data | 1 (0.8) | 0 (0.0) | 1 (0.3) | |
| Streptococcus species | 44 (22.7) | 73 (28.6) | 117 (26.1) | 0.16 |
| Enterococcus species | 9 (4.6) | 6 (2.4) | 15 (3.3) | 0.18 |
| Proteus species | 15 (7.7) | 15 (5.9) | 30 (6.7) | 0.44 |
| Other Enterobacteriaceae | 14 (7.2) | 13 (5.1) | 27 (6.0) | 0.35 |
| Pseudomonas aeruginosa | 11 (5.7) | 7 (2.7) | 18 (4.0) | 0.12 |
| Other Gram-negative bacteria | 4 (2.1) | 8 (3.1) | 12 (2.7) | 0.57 |
| Polymicrobial infection | 35 (18.0) | 34 (13.3) | 69 (15.4) | 0.17 |
| Type of pathogen | 0.35 | |||
| ≥1 Gram-positive pathogen (and no Gram-negative pathogens) | 160 (82.5) | 216 (84.7) | 376 (83.7) | |
| ≥1 Gram-negative pathogen (and no Gram-positive pathogens) | 24 (12.4) | 21 (8.2) | 45 (10.0) | |
| Mixed (both Gram-positive and -negative pathogens) | 5 (2.6) | 12 (4.7) | 17 (3.8) | |
| Other | 5 (2.6) | 6 (2.4) | 11 (2.4) | |
CAI, community-acquired infection; HCAI, health care-associated infection.
Appropriate versus inappropriate initial antibiotic treatment analyses (in patients with positive cultures).
A total of 449 patients were evaluable for appropriateness of initial antibiotic therapy on the basis of culture results obtained within 24 h, including 366 who received appropriate therapy (81.5%) and 83 who did not (18.5%). The proportions of patients who received inappropriate therapy were similar between the health care-associated and community-acquired groups (Fig. 1). After conducting bivariate analyses for those variables in Table 1, we found the following differences between patients who received appropriate and inappropriate therapies (data not shown). Patients who received appropriate antibiotic therapy were younger (median [interquartile range {IQR}] for appropriate versus inappropriate therapy, 51 years [40, 58] versus 54 years [44, 65]; P < 0.05), more likely to have been admitted to the emergency department immediately before hospitalization (321 [87.7%] versus 59 [71.1%]; P = 0.0003), and more likely to have a history of intravenous drug abuse (78 [21.3%] versus 8 [9.6%]; P = 0.01). They were more likely to have a cSSTI diagnosis of abscess (195 [53.3%] versus 33 [39.8%]; P = 0.03) and less likely to have ulcer as a diagnosis (24 [6.6%] versus 11 [13.3%]; P = 0.04).
There were differences in the distribution of pathogens between patients who received appropriate therapy and those who did not (data not shown). Compared with patients who received appropriate therapy, patients who received inappropriate therapy had a higher prevalence of cultures that were positive for MRSA as a percentage of patients with S. aureus (172/243 [71.1%] versus 51/55 [92.7%]; P = 0.0005), enterococci as a percentage of all patients (9/366 [2.5%] versus 6/55 [7.2%]; P = 0.03), or Pseudomonas aeruginosa as a percentage of all patients (8/366 [2.2%] versus 10/83 [12.0%]; P = 0.0003). They had a lower prevalence of cultures that were positive for streptococci (107/366 [29.2%] versus 10/83 [12.0%]; P = 0.0009). There were also differences in the types of pathogens (P = 0.002), such as Gram-positive (317/366 [86.6%] versus 59/83 [71.1%]) and mixed (12/366 [3.3%] versus 5/83 [6.0%]) pathogens.
The following variables remained in the final multivariate model and were associated with inappropriate initial therapy in patients for whom culture was performed within 24 h: direct admission to the hospital (not via the emergency department), cSSTI due to MRSA or mixed Gram-positive and -negative pathogens, and cSSTI due to pathogens other than S. aureus or Streptococcus species (all P < 0.05) (data not shown).
Variables for which there were differences between appropriately and inappropriately treated patients as well as those significantly associated with outcomes for the entire sample were included in initial multivariate models to assess the significance of the exposure variable with regard to LOS and readmission or death within 30 days of discharge. Among the 449 patients, there were no statistically significant differences in outcomes between patients who received appropriate initial antibiotics and those who did not in both bivariate and multivariate) analyses (Table 4), except for one subset. Patients with ulcers who received inappropriate antibiotics were nearly 12 times more likely to be readmitted or to die within 30 days than patients with ulcers who received appropriate antibiotics (adjusted odds ratio, 11.76 [95% CI, 1.30 to 111.11]; P = 0.03).
Table 4.
Unadjusted and multivariate analyses of outcomes stratified by appropriateness of empirical antibiotic therapy in patients who had a positive culture within 24 h (n = 449)
| Outcome | Unadjusted analysis |
Multivariate analysisa | ||
|---|---|---|---|---|
| Appropriate (n = 366) | Inappropriate (n = 83) | P value | ||
| Hospital length of stay (days) | ||||
| All patients | 1.00 (−1.15-3.15)b | |||
| Median (IQR) | 4 (2, 7) | 4 (3, 6) | 0.26c | |
| Mean ± SD | 5.6 ± 6.2 | 6.1 ± 7.9 | 0.31c | |
| Subset of patients discharged alive | 0.99 (−1.16-3.14)b | |||
| Median (IQR) | 4 (2, 6) | 4 (3, 6) | 0.20c | |
| Mean ± SD | 5.6 ± 6.1 | 6.1 ± 7.9 | 0.25d | |
| No. (%) of patients with in-hospital mortality | 5 (1.4) | 0 | 0.59e | —f |
| No. (%) of patients with readmission/death within 30 days | ||||
| All patients | 48 (13.1) | 8 (9.6) | 0.46e | With ulcers, 11.76 (1.30–111.11)g,h; without ulcers, 1.05 (0.43–2.60)g |
| Subset of patients discharged alive | 43 (11.9) | 8 (9.6) | 0.70e | 1.48 (0.63–3.48)g |
Adjusted for variables in Table 1.
Regression mean difference in days (95% CI).
Wilcoxon rank sum test.
t test on log-transformed length of stay.
Chi-square test.
—, not done because of small sample size.
Adjusted odds ratio (95% CI).
P = 0.03.
Similar bivariate and multivariate results were found in the subset of 570 evaluable patients who had positive cultures within 48 h (data not shown).
DISCUSSION
In our retrospective cohort study of 1,096 patients hospitalized for the treatment of cSSTI, the origin of infection was nearly evenly distributed between the health care and community settings. We are aware of only two previous retrospective cohort studies that considered origin of infection in patients hospitalized for cSSTI. Health care-associated infection occurred in 27.2% of 12,506 patients with skin, soft tissue, and bone and joint infections in a multi-institutional study by Lipsky et al. (16) and 73.6% of 717 patients with cSSTI in a single-center study by Zilberberg et al. (29). This variability may be attributable to differences in study populations and criteria for defining health care-associated infection, such as the time frame to define previous hospitalization and previous antibiotic use. A standardized definition for health care-associated cSSTI would be helpful for future studies.
As in previous studies, our patient characteristics differed between cohorts with health care-associated and community-acquired infections. Considering that patients with health care-associated infections were older, it is not surprising that they were more likely to have age-related comorbidities and Medicare insurance than patients with community-acquired infections. On the other hand, patients with community-acquired infections had characteristics generally representative of an underserved population; they were more likely to be African American, abuse intravenous drugs, have no health insurance, and seek treatment in the emergency room.
In the subset of patients with positive cultures within 24 h of admission, the distribution of pathogens was similar to that reported by Zilberberg et al. (29). One striking difference between prior studies and ours was the prevalence rates of MRSA for health care-associated and community-acquired cSSTIs, with 51.0% versus 48.6% in our study, 19.1% versus 13.9% in the study by Lipsky et al. (16), and 32.1% versus 43.7% in the study by Zilberberg et al. (29). Although the exact reasons for these differences are unclear, they may be attributed to increasing rates of MRSA, particularly in this metropolitan region, with our study capturing the most recent experience.
Hospital LOS was approximately 2 days longer for health care-associated cSSTI than for community-acquired cSSTI in our study (8.1 versus 6.4 days; P < 0.001) and, albeit with different patient populations, the one by Lipsky et al. (8.0 versus 6.2 days; P < 0.001) (16). Zilberberg et al. (29) reported an absolute difference of approximately 4 days (9.4 versus 5.5 days; P < 0.001). Hospital mortality was higher in patients with health care-associated infection in our study and other studies (16, 29). Readmission/death within 30 days followed the same trend in our study. Collectively, these findings suggest that health care-associated cSSTI leads to worse outcomes than community-acquired cSSTI, but both types appear to be associated with adverse outcomes.
Initial antibiotic therapy was inappropriate in 18.5% of our patients whose cultures were taken within 24 h of admission and were positive. These rates are slightly lower than the 22%-to-23% range in other analyses of patients hospitalized with cSSTI, including two retrospective cohort studies (6, 29) and a microcosting study (25). Interestingly, the rate of inappropriate initial antibiotic therapy dropped from 43% in 2004 to 3% in 2008 in patients with MRSA cSSTI who presented to emergency departments in a multicenter study; overall rates for patients with cSSTI due to any pathogen were not reported (24). Another difference is that only 16% of patients were hospitalized in that study (24), whereas all were hospitalized in our study and the other studies (6, 25, 29).
In our study, the following factors were predictive of inappropriate initial antibiotic therapy: admission without presentation to the emergency room and cSSTI caused by difficult-to-treat pathogens (i.e., MRSA, both Gram-positive and Gram-negative pathogens, or pathogens other than S. aureus or streptococci). Antimicrobial resistance is increasingly common in these difficult-to-treat organisms (3). Edelsberg et al. (6) reported that the following variables were independently associated with treatment failure: initiation of antibiotics in the intensive care unit, older age, higher Charlson comorbidity index, postoperative wound infection, cSSTI other than cellulitis, and receipt of certain antibiotic therapies or vasoactive medications. Although these variables differ from those in our study, they are also indicative of a more severe infection that is usually difficult to treat.
We did not detect statistically significant evidence of worse outcomes in patients who received inappropriate initial antibiotic therapy in multivariate analyses, except in the subset of patients with ulcers. These patients have comorbidities and often have infections due to antimicrobial-resistant bacteria that recur and are difficult to treat. Few studies, however, have examined the impact of appropriate antibiotic therapy on outcomes in patients with cSSTI or evaluated initial therapy and outcomes in infected ulcers (2). Available results are contradictory, possibly because of differences in study methods, definitions, and sample sizes. Zilberberg et al. (28) reported that inappropriate therapy was independently associated with an excess hospital LOS (attributable LOS, 1.8 days; 95% CI, 1.4 to 2.3; P < 0.001), but not excess mortality, in 527 patients with health care-associated cSSTI. When patients were stratified by the presence of bacteremia, however, LOS differences were observed only in those with bacteremia. Ruhe et al. (21) reported higher cure rates among patients treated with active antibiotic therapy (i.e., at least one agent to which the organism showed in vitro susceptibility) in a study of 492 adults with community-acquired MRSA cSSTI (95% versus 87%; P = 0.001); inactive therapy was the only independent predictor of treatment failure (adjusted odds ratio, 2.8; 95% CI, 1.3 to 6.2; P = 0.01). In contrast, Moran et al. (19) reported that antibiotic activity against MRSA was not associated with outcomes in the subset of 248 patients with MRSA cSSTI and follow-up information, but this number was deemed insufficient to assess this relationship. These outcomes were not reported when this study was repeated (24). Similarly, clinical cure rates were equivalent regardless of antibiotic activity in two other studies of patients with staphylococcal cSSTI (20, 26). Others used failure of initial antibiotic therapy as a surrogate for inappropriate therapy. Edelsberg et al. (6) reported that this surrogate was predictive of increased mortality (odds ratio, 2.9; 95% CI, 2.3 to 3.6), excess antibiotic days (5.7 days), excess hospital LOS (5.4 days), and excess inpatient charges ($5,285) (all P < 0.01) in multivariate analyses of 47,291 patients. Tarricone et al. (25) reported that failure of initial antibiotic therapy added 7 days to hospital LOS and €2,850 to hospital cost in a microcosting study of 307 patients.
Our findings add to those from previous studies by providing more current information on microorganisms, in vitro susceptibility of S. aureus, and outcomes in cSSTI. As this is an evolving condition, information is needed to improve outcomes. Collectively, these findings may have implications for the initial management of cSSTI in patients who require hospitalization. We identified risk factors for inappropriate initial antibiotic therapy, prolonged hospitalization, and other adverse outcomes but did not find an association between inappropriate initial antibiotic therapy and outcomes. Because the benefit of providing appropriate initial antibiotic therapy remains uncertain, more studies are needed to determine optimal initial antibiotic therapy. In the meantime, cSSTI therapy should be tailored on the basis of the type and origin of infection, local resistance patterns, and risk factors. Also, given the high MRSA prevalence in patients hospitalized with cSSTI, it is prudent to provide therapy with MRSA coverage, especially when local prevalence is high.
Our study shares limitations with other retrospective studies, but we attempted to compensate for some. For example, we randomly selected patients hospitalized for cSSTI on the basis of prespecified case definitions. Generalizability and statistical power may be limited for three reasons. First, some patients did not have a culture taken within 24 h of hospital admission or did not have culture results available, which is typical in general clinical practice; however, findings were similar in the larger group who had cultures within 48 h. Second, we focused on patients hospitalized between December 2005 and October 2008, which may not be representative of the current year. Third, this study was performed in a large urban health system in the United States and therefore may not be generalizable to other hospitals and regions. Our database did not capture previous oral antibiotic use, but it included intravenous antibiotic use within 30 days; this covariate was not significant in the final multivariate analysis of appropriateness of initial antibiotic treatment. Although most of our patients received all medical care within the health system, we were not able to capture data on patients readmitted outside our study system within 30 days after discharge, which may have affected our ability to detect the effect of appropriateness of antibiotic therapy on this outcome.
In conclusion, health care-associated cSSTI should be classified as a separate category of infection because of its distinct epidemiology and worse outcomes compared with community-acquired cSSTI. It is unclear, however, whether appropriate initial therapy has an impact on outcomes because of the lack of differences between our patients who did and did not receive appropriate therapy and because of conflicting findings from previous studies. Thus, more and larger studies are needed to confirm our findings and to determine whether appropriate initial antibiotic therapy will improve outcomes for patients hospitalized with cSSTI.
ACKNOWLEDGMENTS
This study was funded by Ortho-McNeil Janssen Scientific Affairs, LLC, Raritan, NJ. L.V., M.R., and M.K. are employed by and own stock in Ortho-McNeil Janssen Scientific Affairs, LLC. M.J.Z. was a consultant for Astellas, received honoraria and speaking fees from Astellas and Cubist, and received grants from Johnson and Johnson, Cubist, and Pfizer. K.F. was a paid consultant to Ortho-McNeil Janssen for this project. N.H. and H.P. do not have any interests to declare.
We thank Katherine Miracola, Henry Ford Health System, Detroit, MI, for collecting and verifying data, and Hamilton House, Virginia Beach, VA, for assistance with manuscript preparation. Hamilton House received payment from Ortho-McNeil Janssen Scientific Affairs, LLC, for its services.
Footnotes
Published ahead of print 23 November 2011
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1. Alvarez-Lerma F. 1996. Modification of empiric antibiotic treatment in patients with pneumonia acquired in the intensive care unit. ICU-Acquired Pneumonia Study Group. Intensive Care Med. 22:387–394 [DOI] [PubMed] [Google Scholar]
- 2. Chua T, et al. 2008. Molecular epidemiology of methicillin-resistant Staphylococcus aureus bloodstream isolates in urban Detroit. J. Clin. Microbiol. 46:2345–2352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Davis SL, et al. 2007. Epidemiology and outcomes of community-associated methicillin-resistant Staphylococcus aureus infection. J. Clin. Microbiol. 45:1705–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Duan N, Manning WG, Morris CN, Newhouse JP. 1983. A comparison of alternative models for the demand for medical care. J. Bus. Econ. Stat. 1:115–126 [Google Scholar]
- 5. Eady EA, Cove JH. 2003. Staphylococcal resistance revisited: community-acquired methicillin resistant Staphylococcus aureus—an emerging problem for the management of skin and soft tissue infections. Curr. Opin. Infect. Dis. 16:103–124 [DOI] [PubMed] [Google Scholar]
- 6. Edelsberg J, et al. 2008. Clinical and economic consequences of failure of initial antibiotic therapy for hospitalized patients with complicated skin and skin-structure infections. Infect. Control Hosp. Epidemiol. 29:160–169 [DOI] [PubMed] [Google Scholar]
- 7. Edelsberg J, et al. 2009. Trends in US hospital admissions for skin and soft tissue infections. Emerg. Infect. Dis. 15:1516–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Erbay A, Idil A, Gozel MG, Mumcuoglu I, Balaban N. 2009. Impact of early appropriate antimicrobial therapy on survival in Acinetobacter baumannii bloodstream infections. Int. J. Antimicrob. Agents 34:575–579 [DOI] [PubMed] [Google Scholar]
- 9. Fridkin SK, et al. 2005. Methicillin-resistant Staphylococcus aureus disease in three communities. N. Engl. J. Med. 352:1436–1444 [DOI] [PubMed] [Google Scholar]
- 10. Gilbert DN, Moellering RC, Jr, Eliopoulos GM, Sande MA. 2007. The Sanford guide to antimicrobial therapy, 37th ed. Antimicrobial Therapy, Inc., Sperryville, VA [Google Scholar]
- 11. Hatoum HT, Akhras KS, Lin SJ. 2009. The attributable clinical and economic burden of skin and skin structure infections in hospitalized patients: a matched cohort study. Diagn. Microbiol. Infect. Dis. 64:305–310 [DOI] [PubMed] [Google Scholar]
- 12. Iregui M, Ward S, Sherman G, Fraser VJ, Kollef MH. 2002. Clinical importance of delays in the initiation of appropriate antibiotic treatment for ventilator-associated pneumonia. Chest 122:262–268 [DOI] [PubMed] [Google Scholar]
- 13. Itani KM, et al. 2009. Outcomes associated with initial versus later vancomycin use in patients with complicated skin and skin-structure infections. Pharmacoeconomics 27:421–430 [DOI] [PubMed] [Google Scholar]
- 14. Lamerato L, et al. 2009. Clinical and economic outcomes among hospitalized patients with a skin and soft tissue infection, abstr 434. Abstr. 19th Annu. Sci. Meet. Society for Healthcare Epidemiology of America, San Diego, CA, 19 to 22 March 2009 [Google Scholar]
- 15. Levit K, Wier L, Stranges L, Ryan K, Elixhauser A. 2009. HCUP facts and figures: statistics on hospital-based care in the United States, 2007. Agency for Healthcare Research and Quality, Rockville, MD: http://www.hcup-us.ahrq.gov/reports/factsandfigures/2007/pdfs/FF_report_2007.pdf [PubMed] [Google Scholar]
- 16. Lipsky BA, Weigelt JA, Gupta V, Killian A, Peng MM. 2007. Skin, soft tissue, bone, and joint infections in hospitalized patients: epidemiology and microbiological, clinical, and economic outcomes. Infect. Control Hosp. Epidemiol. 28:1290–1298 [DOI] [PubMed] [Google Scholar]
- 17. Luna CM, et al. 2006. Appropriateness and delay to initiate therapy in ventilator-associated pneumonia. Eur. Respir. J. 27:158–164 [DOI] [PubMed] [Google Scholar]
- 18. Micek ST, Kollef KE, Reichley RM, Roubinian N, Kollef MH. 2007. Health care-associated pneumonia and community-acquired pneumonia: a single-center experience. Antimicrob. Agents Chemother. 51:3568–3573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moran GJ, et al. 2006. Methicillin-resistant S. aureus infections among patients in the emergency department. N. Engl. J. Med. 355:666–674 [DOI] [PubMed] [Google Scholar]
- 20. Paydar KZ, Hansen SL, Charlebois ED, Harris HW, Young DM. 2006. Inappropriate antibiotic use in soft tissue infections. Arch. Surg. 141:850–856 [DOI] [PubMed] [Google Scholar]
- 21. Ruhe JJ, Smith N, Bradsher RW, Menon A. 2007. Community-onset methicillin-resistant Staphylococcus aureus skin and soft-tissue infections: impact of antimicrobial therapy on outcome. Clin. Infect. Dis. 44:777–784 [DOI] [PubMed] [Google Scholar]
- 22. Schwaber MJ, Carmeli Y. 2007. Mortality and delay in effective therapy associated with extended-spectrum beta-lactamase production in Enterobacteriaceae bacteraemia: a systematic review and meta-analysis. J. Antimicrob. Chemother. 60:913–920 [DOI] [PubMed] [Google Scholar]
- 23. Stevens DL, et al. 2005. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin. Infect. Dis. 41:1373–1406 [DOI] [PubMed] [Google Scholar]
- 24. Talan DA, et al. 2011. Comparison of Staphylococcus aureus from skin and soft-tissue infections in US emergency department patients, 2004 and 2008. Clin. Infect. Dis. 53:144–149 [DOI] [PubMed] [Google Scholar]
- 25. Tarricone R, et al. 2008. How complicated skin and soft tissue infections are treated in Italy: economic evaluation of inpatient intravenous antibiotic treatment in seven hospitals. J. Med. Econ. 11:265–279 [DOI] [PubMed] [Google Scholar]
- 26. Young DM, et al. 2004. An epidemic of methicillin-resistant Staphylococcus aureus soft tissue infections among medically underserved patients. Arch. Surg. 139:947–953 [DOI] [PubMed] [Google Scholar]
- 27. Zervos M, et al. 2009. Risk factors for inappropriate initial empiric therapy in hospitalized patients with skin and soft tissue infection, poster 26. Abstr. 47th Annu. Meet. Infectious Diseases Society of America, Philadelphia, PA, 29 October to 1 November 2009 [Google Scholar]
- 28. Zilberberg MD, et al. 2010. Hospitalizations with healthcare-associated complicated skin and skin structure infections: impact of inappropriate empiric therapy on outcomes. J. Hosp. Med. 5:535–540 [DOI] [PubMed] [Google Scholar]
- 29. Zilberberg MD, et al. 2009. Epidemiology and outcomes of hospitalizations with complicated skin and skin-structure infections: implications of healthcare-associated infection risk factors. Infect. Control Hosp. Epidemiol. 30:1203–1210 [DOI] [PubMed] [Google Scholar]