Abstract
Quantitative HIV RNA viral load (QVL) assays (Roche Diagnostics) were sensitive and specific when used to diagnose HIV infection in (i) HIV-exposed infants (sensitivity of 100% [63.1 to 100%] and specificity of 100% [97.9 to 100%]) and (ii) suspected acute HIV infection patients with a negative/indeterminate Western blot (sensitivity of 97.6% [91.6 to 99.7%] and specificity of 100% [96.1 to 100%]). No false-positive QVL results were identified.
TEXT
Third-generation enzyme immunoassays (EIAs) for human immunodeficiency virus (HIV) screening have an estimated window of 3 to 4 weeks for the detection of antibodies to HIV after acute infection (9). However, these EIAs are not helpful in the diagnosis of congenital/perinatal HIV infections since transplacental maternal antibodies may persist for 18 months in infants (4). These assays are also limited as diagnostic tests for patients with acute HIV infection (AHI) who have not yet developed confirmatory HIV antibodies detected by Western blotting (WB) (8).
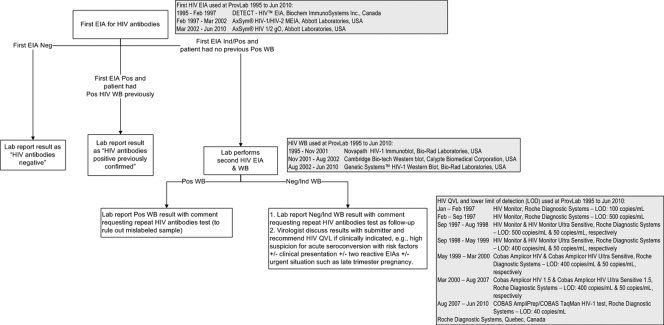
Data from the Alberta Provincial Laboratory for Public Health (ProvLab) were extracted and analyzed to examine the performance of quantitative HIV RNA viral load (QVL) assays licensed for the clinical monitoring of HIV-infected patients as diagnostic tools in two populations: (i) HIV-exposed infants and (ii) patients with suspected AHI not confirmed by a WB (Fig. 1). Diagnostic laboratories that opted to use qualitative nucleic acid assays (currently only licensed for the screening of blood, organ, and tissue donors) to diagnose HIV infections in these two populations needed to maintain two platforms and inventories of nucleic acid assays to support both HIV diagnosis and disease monitoring.
Fig 1.
HIV antibody testing algorithm and quantitative HIV RNA viral load assays used at the Provincial Laboratory for Public Health, Alberta, Canada, from 1995 to 2010. EIA, enzyme immunoassay; WB, Western blot; QVL, quantitative HIV RNA viral load; Lab, Provincial Laboratory for Public Health, Alberta (ProvLab); Neg, negative; Ind, indeterminate; Pos, positive.
The inclusion criteria for HIV-exposed infants were that the infants were born to HIV-infected mothers between 1995 and 30 May 2009 in central and northern Alberta and that they were tested with QVL assays prior to 18 months of age. Confirmed congenital/perinatal HIV-infected cases were defined as infants with multiple (≥2) detectable QVL using the Public Health Agency of Canada (PHAC) case definition for HIV (14). Infants with undetectable QVL and negative follow-up HIV antibody test results were defined as “confirmed not HIV infected” based on the Centers for Disease Control and Prevention 2008 HIV case definition (2). The second study population of suspected AHI cases included individuals ≥2 years old with initial positive/indeterminate EIA screens and negative/indeterminate WBs between April 1996 and 30 April 2010 who had QVL assays performed within 150 days of their negative/indeterminate WBs (Fig. 1). Suspected AHI cases who had detectable QVL or a follow-up positive WB were defined as “confirmed HIV infected” (2), and patients who had no detectable QVL and failed to demonstrate progression on serial HIV antibody tests (at least 7 days apart) were defined as not HIV infected. Statistical comparisons were made using Mann-Whitney tests for medians and chi-square tests for proportions.
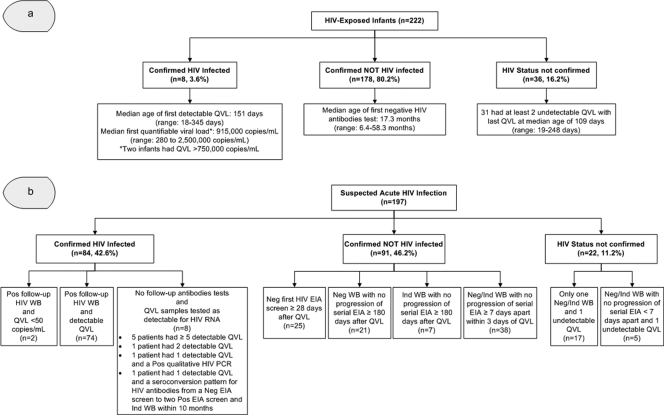
Among 222 HIV-exposed infants, 8 (3.6%) were confirmed as being HIV infected with multiple (≥5) detectable QVL (Fig. 2a). Among these 8 infected infants, the QVL was initially undetectable for only one infant at 2 days of age, and this infant subsequently had detectable QVL at 27 days and in follow-up tests. Of the other 7 infants, one also had the first detectable QVL by 1 month of age (18 days), two by 3 months (63 and 73 days), and four by 11 months of age, depending on the timing of the first QVL. The remaining 214 infants had undetectable QVL; 192 (89.7%) had ≥2 QVL tests (median, 3 tests; range, 1 to 7 tests) performed, with most specimens (609/698, 87.2%) submitted at an age of <6 months. A total of 178 infants had negative HIV serology at a median age of 17.3 months (range, 6.4 to 58.3 months), confirming that they were not HIV infected. For the remaining 36 infants with no follow-up serology, 31 (86.1%) had at least two undetectable QVL. Among all infants with confirmed HIV status, QVL assays had 100% sensitivity and 100% specificity (Table 1).
Fig 2.
Quantitative viral load results and serological follow-up for the two study populations in Central and Northern Alberta, HIV-exposed infants (a) and suspected acute HIV infections (b). (a) HIV-Exposed Infants, infants born to HIV-infected mothers between 1995 and 30 May 2009 in Central and Northern Alberta who were tested with QVL assays prior to 18 months of age; Confirmed HIV Infected, infants (<18 months of age) with ≥2 detectable QVL; Confirmed NOT HIV Infected, infants with all QVL samples tested as undetectable and a negative HIV antibody test on follow-up; HIV Status Not Confirmed, infants with all QVL samples tested as undetectable who were lost to follow-up with no follow-up HIV antibody test. (b) Suspected Acute HIV Infection, patients ≥2 years old with initial positive/indeterminate EIA screens and negative/indeterminate WBs between April 1996 and 30 April 2010 who had at least one detectable QVL within 150 days of their negative/indeterminate WB; Confirmed HIV Infected, patients with detectable QVL and/or a follow-up positive WB; Confirmed NOT HIV Infected, patients with all QVL samples tested as undetectable who failed to demonstrate progression/seroconversion on serial HIV antibody tests that were at least 7 days apart; HIV Status Not Confirmed, patients with all QVL samples tested as undetectable who had limited or no follow-up HIV antibody test. QVL, quantitative HIV RNA viral load; EIA, enzyme immunoassay; WB, Western blot; Pos, positive; Neg, negative; Ind, indeterminate.
Table 1.
Sensitivity and specificity for QVL assay results among HIV-exposed infants and cases of suspected acute HIV infection who had multiple detectable QVL and/or follow-up serological tests to confirm their statusa
| Population | No. positive by QVL assay/no. positive by serial serology and/or repeat detectable QVL | Sensitivity (%) (95% CI) | No. negative by QVL/no. negative by serial serology | Specificity (%) (95% CI) |
|---|---|---|---|---|
| HIV-exposed infants (n = 186) | 8/8 | 100 (63.1–100) | 178/178 | 100 (97.9–100) |
| Suspected acute HIV infection cases (n = 175) | 82/84b | 97.6 (91.6–99.7) | 91/91 | 100 (96.1–100) |
CI, confidence interval.
One of the two patients with serologically confirmed HIV infection had an initially undetectable QVL but a low viral load on a follow-up QVL (see text).
With respect to our second study population of suspected AHI cases, 197 had QVL assays performed within 150 days of their negative/indeterminate WB, representing 9.1% of the 2,157 patients with negative/indeterminate WBs during the study period. A total of 84 patients were confirmed to be HIV infected (Fig. 2b). Two of these 84 patients tested as undetectable on their first QVL assay. The first QVL from one of these two patients was undetectable using the Cobas Amplicor HIV 1.5 assay (Roche Diagnostic Systems, Quebec, Canada), which had a limit of detection (LOD) of 400 copies/ml; the same patient then had detectable QVL 72 and 218 days later at 850 copies/ml and 680 copies/ml, respectively, using the Cobas Amplicor HIV UltraSensitive 1.5 assay (LOD, 50 copies/ml). The other suspected AHI patient, who was confirmed as HIV infected based on a follow-up positive WB and detection of HIV antibodies by radioimmunoprecipitation, continued to have undetectable QVL for the next 5 years using both the Cobas Amplicor HIV UltraSensitive 1.5 and the Cobas AmpliPrep/Cobas TaqMan HIV-1 assays (Roche Diagnostic Systems) (LOD, 40 copies/ml). The remaining 82 suspected AHI cases with confirmed HIV infection all had detectable QVL: 25 had first QVL that were greater than the quantifiable upper limit of the assays (7 at >10,000, 17 at >750,000, and 1 at >10,000,000 copies/ml), and the remaining 57 had a median first QVL of 82,000 copies/ml (range, 430 to 23,000,000 copies/ml). For the 113 suspected AHI cases with undetectable QVL, 91 (80.5%) had follow-up serological tests to confirm that they were not HIV infected (Fig. 2b). The QVL assays had 97.6% sensitivity and 100% specificity for diagnosing AHI (Table 1).
Suspected AHI patients confirmed to be HIV infected and those who were not HIV infected were similar in age, with median ages of 35.0 (range, 16.1 to 62.2 years) and 30.8 (range, 15.3 to 80.3 years) years, respectively (P = 0.09), but were more likely to be male (61.7% versus 39.3%, respectively; P = 0.004), to have reactive results by both EIAs (91.5% versus 18.0%, respectively; P < 0.001), and to have an indeterminate rather than a negative WB (75.9% versus 60.4%, respectively; P = 0.03). The median signal/cutoff for the first EIA for the AHI cases, 8.5 (range, 1.0 to 31.6), was higher than that for the uninfected patients, 1.5 (range, 0.74 to 15.88), P < 0.001. (The sample with the median signal/cutoff of 0.74 had a reactive EIA screen at a different laboratory.) Risk factors were available for 70 (83.3%) of the 84 confirmed AHI cases from the Provincial Notifiable Disease Database: 35 (50%) were injection drug users, 12 (17%) were heterosexuals with a high-risk sex partner, and 10 (14%) were men who have sex with men.
While our study supports the position endorsed by the American Academy of Pediatrics for the use of QVL as a diagnostic tool for HIV in infants <18 months old (15), our results should be interpreted with caution due to the small number of infected infants (n = 8) in our sample and because the sample collection time points were not standardized among the exposed infants. Although we cannot make firm recommendations on optimal time points and frequency of QVL measurements, the PHAC recommendation of testing exposed infants at multiple time points is supported by the observation of an undetectable QVL on day 2 of life and a detectable QVL on day 27 in one of our infants (14). Of note, reported sensitivities of QVL assays range from 25 to 50% in the first few days of life to 100% by 6 to 12 weeks of age (15). Furthermore, while some published studies suggest that the use of maternal and infant antiretroviral drugs does not decrease the sensitivity of QVL assays performed during the first 6 months of age, others suggest that the use of zidovudine may result in lower infant viral loads (10, 16).
With respect to suspected AHI cases, we had two interesting confirmed infected cases with low viral set points and undetectable QVL during acute HIV infection. So while QVL may be an important and viable diagnostic tool, these cases suggest that a complete serological follow-up is still important for case ascertainment. Our observations suggest that the QVL assay licensed for patient monitoring has utility as a supplementary diagnostic tool for the diagnosis of HIV infection in the two population groups studied.
Unlike other studies (3, 5, 13), we found no false-positive QVL in either of our study populations. Brambilla et al. (1) postulated that many false-positive QVL may be due to poor specimen handling. The excellent specificity (100%) of the QVL assays in our laboratory likely reflects the staff's technical expertise and the quality assurance measures in place at the ProvLab. The specificity was likely also improved by the ProvLab requirement of using only dedicated plasma samples for QVL assays, which meant that follow-up samples were collected from all suspected acute seroconverters. Potential disadvantages of this approach are a delay in diagnosis and incomplete compliance with follow-up. A recent study reported good performance of QVL assays using serum aliquots of the original samples submitted for HIV antibody tests (12). Other limitations of our approach are that we did not perform QVL assays on all patients with negative/indeterminate WBs and that our risk factor screening to select suspected seroconverters could also have enhanced the positive predictive value of the QVL. Furthermore, QVL assays have variable sensitivity for non-B HIV clades (7, 17), and commercial QVL assays cannot diagnose HIV-2 infections, which are rare but present in Canada (6, 11).
In summary, our data show good performance characteristics of QVL assays in the diagnosis of HIV infection in HIV-exposed infants and in cases of suspected acute HIV infection before the development of confirmatory antibodies. Further studies with larger sample sizes, incorporation of various HIV clades, and use of nondedicated serum samples will provide further validation data for the diagnostic use of these assays.
ACKNOWLEDGMENTS
We would like to thank the virology and molecular diagnostic staff at the Provincial Laboratory for Public Health, Edmonton, Alberta, Canada, for their help with testing and John Kim and the staff at the National Microbiology Laboratory for providing reference HIV tests for some of the patients.
This study was reviewed and approved by the University of Alberta Health Research Ethics Board.
The authors declare no conflicts of interest.
The opinions expressed in this article do not necessarily reflect the views of the authors' affiliated institutions.
Footnotes
Published ahead of print 7 December 2011
REFERENCES
- 1.Brambilla D, Granger S, Jennings C, Bremer J. 2001. Rates of false-positive results on quantitative HIV RNA assays, p 112. Abstr. 8th Conf. Retrovir. Oppor. Infect. [Google Scholar]
- 2.Centers for Disease Control and Prevention 2008. Revised surveillance case definitions for HIV infection among adults, adolescents, and children aged <18 months and for HIV infection and AIDS among children aged 18 months to <13 years—United States, 2008. MMWR Recommend. Rep. 57(RR-10):1–12 [PubMed] [Google Scholar]
- 3.Daar ES, et al. 2001. Diagnosis of primary HIV-1 infection. Los Angeles County Primary HIV Infection Recruitment Network. Ann. Intern. Med. 134:25–29 [DOI] [PubMed] [Google Scholar]
- 4.Gray G, McIntyre J, Newell M-L. 2000. HIV-1 infection, p 232–257. In Newell ML, McIntyre J. (ed), Congenital and perinatal infection: prevention, diagnosis and treatment. Cambridge University Press, Cambridge, United Kingdom [Google Scholar]
- 5.Hecht FM, et al. 2002. Use of laboratory tests and clinical symptoms for identification of primary HIV infection. AIDS 16:1119–1129 [DOI] [PubMed] [Google Scholar]
- 6.Houston SC, Miedzinski LJ, Mashinter LD. 2002. Rapid progression of CD4 cell decline and subsequent response to salvage therapy in HIV-2 infection. AIDS 16:1189–1191 [DOI] [PubMed] [Google Scholar]
- 7.Kim JE, et al. 2007. Short communication: identification of a novel HIV type 1 subtype H/J recombinant in Canada with discordant HIV viral load (RNA) values in three different commercial assays. AIDS Res. Hum. Retroviruses 23:1309–1313 [DOI] [PubMed] [Google Scholar]
- 8.Kleinman SH, Lelie N, Busch MP. 2009. Infectivity of human immunodeficiency virus-1, hepatitis C virus, and hepatitis B virus and risk of transmission by transfusion. Transfusion 49:2454–2489 [DOI] [PubMed] [Google Scholar]
- 9.Lindbäck S, et al. 2000. Diagnosis of primary HIV-1 infection and duration of follow-up after HIV exposure. Karolinska Institute Primary HIV Infection Study Group. AIDS 14:2333–2339 [DOI] [PubMed] [Google Scholar]
- 10.Nesheim S, et al. 2003. Quantitative RNA testing for diagnosis of HIV-infected infants. J. Acquir. Immune Defic. Syndr. 32:192–195 [DOI] [PubMed] [Google Scholar]
- 11.Neumann PW, et al. 1989. Laboratory diagnosis of the first cases of HIV-2 infection in Canada. CMAJ 140:125–128 [PMC free article] [PubMed] [Google Scholar]
- 12.Pierce VM, Neide B, Hodinka RL. 2011. Evaluation of the Gen-Probe Aptima HIV-1 RNA qualitative assay as an alternative to Western blot analysis for confirmation of HIV infection. J. Clin. Microbiol. 49:1642–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pincus JM, et al. 2003. Acute human immunodeficiency virus infection in patients presenting to an urban urgent care center. Clin. Infect. Dis. 37:1699–1704 [DOI] [PubMed] [Google Scholar]
- 14.Public Health Agency of Canada 2009. Case definitions for communicable diseases under national surveillance. Can. Commun. Dis. Rep. 35(Suppl 2):86–87 http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/09vol35/35s2/HIV_VIH-eng.php. Accessed 10 October 2011 [Google Scholar]
- 15.Read JS. 2007. Diagnosis of HIV-1 infection in children younger than 18 months in the United States. Pediatrics 120:e1547–e1562 [DOI] [PubMed] [Google Scholar]
- 16.Swanson P, et al. 2005. Impact of human immunodeficiency virus type 1 (HIV-1) genetic diversity on performance of four commercial viral load assays: LCx HIV RNA Quantitative, AMPLICOR HIV-1 MONITOR v1.5, VERSANT HIV-1 RNA 3.0, and NucliSens HIV-1 QT. J. Clin. Microbiol. 43:3860–3868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Young NL, et al. 2000. Early diagnosis of HIV-1-infected infants in Thailand using RNA and DNA PCR assays sensitive to non-B subtypes. J. Acquir. Immune Defic. Syndr. 24:401–407 [DOI] [PubMed] [Google Scholar]