Abstract
The disease spectrum associated with human bocavirus-1 infection remains to be fully defined. We report a case of bocavirus-1-associated bronchiolitis, leading to severe respiratory failure and extracorporeal membrane oxygenation in a 4-year-old child, and suggest blood testing for human bocavirus-1 in children with severe respiratory tract infection.
CASE REPORT
The patient was a 4-year-old girl, prematurely born in gestational week 29. She had an ordinary upbringing with mild infection-associated wheezing and was hospitalized once at 1 year of age for wheezing. She had no continuous medication but was prescribed albuterol inhalation as needed. The disease episode started rather suddenly with mild dyspnea. Albuterol inhalation gave no amelioration, and this prompted two outpatient visits to the emergency ward of the local hospital on the first 2 days of the disease. Fever (38.2°C) was noted, and she was given albuterol and budesonide inhalations and oral (p.o.) penicillin V with moderate effect. During the second night of the disease, her respiration deteriorated with heavy dyspnea and wheezing, and she returned to the hospital for a third time. Because of severe airway constriction she was now referred to a pediatrics clinic in a secondary hospital. A chest X-ray showed subcutaneous emphysema, pneumomediastinum, and left-sided pneumothorax, which are known complications of severe wheezing in children (5), as well as bilateral minor infiltrates. She was given intravenous (i.v.) cefotaxime and erythromycin. Tracheal intubation was performed and bilateral chest tubes were inserted without an improvement in oxygen saturation. Ventilating the patient was problematic, requiring a peak inspiratory pressure up to 55 cm H2O to maintain tidal volumes of 9 ml/kg. At this point the patient had a partial CO2 pressure (pCO2) of 11.0 kPa (82 mm Hg), and the extracorporeal membrane oxygenation (ECMO) unit at Karolinska University Hospital was contacted. The patient was cannulated for venovenous (VV)-ECMO (15F double-lumen Origen cannulae) locally and transferred to Karolinska in Stockholm. The patient was stabilized, but the ECMO treatment was complicated by cannula perforation of the right atrium and an acute thoracotomy had to be performed. Her respiration improved gradually over the following days. She could be removed from ECMO support after 2 days and extubated after 5 days. The girl made a full recovery and was healthy on follow-up.
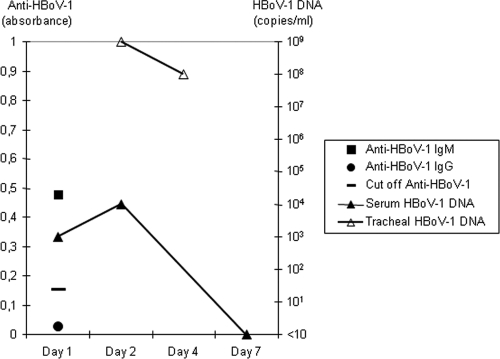
At the start of ECMO treatment, plasma C-reactive protein was 37 mg/liter and the white blood cell count was 8.0 × 109/liter. Bacterial and fungal cultures from tracheal aspirate, urine, and blood collected on the first and second day of ECMO treatment were negative, including cultures for Legionella pneumophila (tracheal aspirate) and Bordetella pertussis (nasopharyngeal swab). However, the samples were obtained after initiation of antibiotic therapy. An immunofluorescence test for respiratory syncytial virus (RSV) on a nasopharyngeal sample made at the secondary hospital was negative. A tracheal aspirate sample, drawn on the first day of ECMO treatment, was tested for atypical bacterial agents and 15 respiratory viruses by hydrolysis probe-based real-time PCR (11). It was negative for Mycoplasma pneumoniae, Chlamydophila pneumoniae, Legionella pneumophila, adenovirus, coronaviruses (229E, HKU1, NL63, and OC43), enteroviruses, influenza A and B, metapneumovirus, parainfluenza viruses 1 to 3, rhinoviruses, and RSV. The only finding in this sample was human bocavirus-1 (HBoV-1) DNA, present at high copy numbers, and also detected in a repeat tracheal aspirate collected 2 days later (Fig. 1). Importantly, HBoV-1 DNA was also detected in two serum samples drawn during the course of the disease but not in a serum sample drawn immediately after recovery. The specificity of the real-time PCR result for HBoV-1 was confirmed by a second, conventional PCR assay followed by sequencing of the product (1). A serum sample drawn just before the initiation of ECMO treatment was tested for IgG and IgM antibodies against HBoV-1, with an assay based on HBoV-1 virus like particles (10). IgM but not IgG antibodies against HBoV-1 were detected (Fig. 1). Anti-HBoV-1 was not analyzed during or after ECMO treatment because of the heavy transfusion exposure associated with the procedure, leading to passively transferred antibodies that remain for several months.
Fig 1.
HBoV-1 diagnostic findings in tracheal aspirate and serum. Day 1 is the starting day of ECMO treatment. Anti-HBoV-1 was not analyzed during or after ECMO treatment because of the heavy transfusion exposure associated with the procedure.
HBoV-1 was first described in 2005 (1). Studies to date indicate that HBoV-1 is endemic worldwide and that the primary infection normally occurs in early childhood, with a seroprevalence reaching 90% in children above 3 years of age (7). The virus has mainly been detected in children with respiratory tract infection, but the causative role of HBoV-1 for respiratory tract disease has been controversial, since HBoV-1 is often detected in association with other respiratory viruses (9). Cohort studies have documented a high prevalence of HBoV-1 in respiratory tract secretions of young children and no clear association with upper respiratory tract symptoms (8, 13). On the other hand, studies of more severely ill, hospitalized children have found a statistical association between HBoV-1 and otherwise unexplained lower respiratory tract symptoms, in particular acute wheezing (2–4, 6, 10). This suggests that HBoV-1 infection, like many other viral infections, is mainly asymptomatic but occasionally severe. It is important to recognize, however, that nearly all published studies of HBoV-1 and disease have applied only PCR testing of respiratory secretions. This has turned out to be an unsuitable diagnostic approach, since HBoV-1 is often shed for a long time into the respiratory tracts of infants and young children (8, 9, 13). Recent data show that primary HBoV-1 infection can be separated from virus shedding by analysis of serum for HBoV-1 DNA and anti-HBoV-1 antibodies (2, 10). Detection of HBoV-1 DNA in blood has been more tightly linked to symptoms than other diagnostic markers (3). The usefulness of the applied serology assay has been documented in studies of children with acute wheezing. HBoV-1 IgM enzyme immunoassay (EIA) positivity correlates both with HBoV-1 viremia and with seroconversion of IgG in paired serum samples, whereas healthy subjects are generally IgM EIA negative (10). Thus, blood testing is crucial for studying HBoV-1 and disease, and new studies applying adequate diagnostic testing are urgently required. Ursic et al. (12) recently reported on a 20-month-old child who presented with acute bronchiolitis that developed into a severe course characterized by pneumothorax, pneumomediastinum, and respiratory failure with air-leak syndrome, a clinical course very similar to that of the present case. The solid diagnostic workup included electron microscopy and monitoring of HBoV-1 DNA in upper and lower respiratory secretions and plasma (12).
The present case initially presented with typical symptoms of a viral respiratory tract infection: fever and wheezing. The symptoms were then dominated by severe airway constriction, including pneumothorax, which resolved in a few days. Taken together, the clinical course, X-ray, and blood chemistry findings were compatible with a viral lower respiratory infection. While real-time PCR for 14 other respiratory viruses were negative, the course of the disease perfectly matched a primary infection by HBoV-1, supported by serology and a transient appearance of HBoV-1 DNA in serum. Of course, it cannot be excluded that the HBoV-1 infection coincided with another, unidentified infection that caused the symptoms. However, while HBoV-1 infection is frequent and shedding may be long-lasting in infants and toddlers, primary HBoV-1 infections are comparably rare among 4-year-olds, making such a coincidence unlikely. The presentation was very similar to a recently published case also linked to HBoV-1 (12). Thus, we find it likely that the observed primary HBoV-1 infection did cause the symptoms. A history of infection-associated wheezing, which was present in this case, is probably a risk factor for severe bocavirus-1 infection. This report illustrates that blood sampling is important for linking HBoV-1 to disease, and it indicates that HBoV-1 should be considered in severe respiratory tract disease in children.
ACKNOWLEDGMENTS
Potential conflicts of interest: T.A. is a coinventor on a patent application related to human bocavirus, assigned to Karolinska Institutet Innovations AB. No conflict is reported for all other authors.
Footnotes
Published ahead of print 30 November 2011
REFERENCES
- 1.Allander T, et al. 2005. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc. Natl. Acad. Sci. U. S. A. 102: 12891– 12896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allander T, et al. 2007. Human bocavirus and acute wheezing in children. Clin. Infect. Dis. 44: 904– 910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christensen A, Nordbø SA, Krokstad S, Rognlien AG, Døllner H. 2010. Human bocavirus in children: mono-detection, high viral load and viraemia are associated with respiratory tract infection. J. Clin. Virol. 49: 158– 162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fry AM, et al. 2007. Human bocavirus: a novel parvovirus epidemiologically associated with pneumonia requiring hospitalization in Thailand. J. Infect. Dis. 195: 1038– 1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Given K, Schultz A, Douglas TA, Martin AC. 2008. Air leaks in children with acute bronchiolitis. J. Paediatr. Child Health 44: 604– 606 [DOI] [PubMed] [Google Scholar]
- 6.Jacques J, et al. 2008. Human bocavirus quantitative DNA detection in French children hospitalized for acute bronchiolitis. J. Clin. Virol. 43: 142– 147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karalar L, et al. 2010. Prevalence and clinical aspects of human bocavirus infection in children. Clin. Microbiol. Infect. 16: 633– 639 [DOI] [PubMed] [Google Scholar]
- 8.Martin ET, et al. 2010. Frequent and prolonged shedding of bocavirus in young children attending daycare. J. Infect. Dis. 201: 1625– 1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schildgen O, et al. 2008. Human bocavirus: passenger or pathogen in acute respiratory tract infections? Clin. Microbiol. Rev. 21: 291– 304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Söderlund-Venermo M, et al. 2009. Clinical assessment and improved diagnosis of bocavirus-induced wheezing in children, Finland. Emerg. Infect. Dis. 15: 1423– 1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tiveljung-Lindell A, et al. 2009. Development and implementation of a molecular diagnostic platform for daily rapid detection of 15 respiratory viruses. J. Med. Virol. 81: 167– 175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ursic T, et al. 2011. Human bocavirus as the cause of a life-threatening infection. J. Clin. Microbiol. 49: 1179– 1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.von Linstow ML, Høgh M, Høgh B. 2008. Clinical and epidemiologic characteristics of human bocavirus in Danish infants: results from a prospective birth cohort study. Pediatr. Infect. Dis. J. 27: 897– 902 [DOI] [PubMed] [Google Scholar]