Abstract
Bloodstream infections are a leading cause of admissions to hospital intensive care units and carry a high mortality rate. Clinical outcome can be greatly improved by early effective antibiotic therapy; therefore, broad-spectrum antimicrobial therapy is often initiated when there is a clinical suspicion of bloodstream infection. Unfortunately, this method may not always be effective when dealing with inherently resistant organisms and can also result in iatrogenic infection and the development of resistant isolates. Rapid identification of the infecting organism may aid in choosing appropriate antimicrobial therapy, thereby reducing these potential adverse events. We compared the matrix-assisted laser desorption ionization (MALDI) Biotyper system with Sepsityper specimen processing (Bruker Daltonics, Billerica, MA) to routine methods for the identification of microorganisms from 164 positive blood cultures. The MALDI Biotyper/Sepsityper identified 85.5% of bacterial isolates directly from positive monomicrobial blood cultures with 97.6% concordance to genus and 94.1% concordance to species with routine identification methods. Gram-negative isolates were more likely to produce acceptable confidence scores (97.8%) than Gram-positive isolates (80.0%); however, genus and species concordance with routine identification methods were similar in both groups. Reanalysis of collected spectra using modified blood culture-specific parameters resulted in an improved overall identification rate for Gram-positive bacteria (89.0%). Median times to identification using the MALDI Biotyper/Sepsityper were 23 to 83 h faster than routine methods for Gram-positive isolates and 34 to 51 h faster for Gram-negative isolates.
INTRODUCTION
Sepsis is a serious condition often stemming from bacterial infection of the bloodstream. Bacterial sepsis accounts for up to 11% of intensive care unit (ICU) admissions, and in-hospital mortality rates range from 25 to 80% (1, 15). Early diagnosis and treatment of bacterial sepsis is critical to a favorable patient outcome. Kumar et al. report a 7.6% mean decrease in survival for every hour effective antibiotic therapy is delayed following the onset of sepsis-related hypotension (11). Rapid identification of the etiology of bacterial sepsis has the potential to positively impact patient care when coupled with an active antibiogram and predictable antibiotic resistance profiles.
Currently, microbiologic diagnosis of bacteremia relies on subculture of positive blood culture bottles to solid medium and 18 to 24 h of incubation prior to identification using biochemical tests or automated preformed enzyme assays. In total, the process can require 18 to 48 h, or more, from initial blood culture positivity to a definitive bacterial identification. Molecular and fluorescent in situ hybridization (FISH) assays enable the identification of pathogens directly from positive blood culture bottles, thus eliminating the need for subculture (3, 10, 20). Unfortunately, these assays are capable of detecting a limited number of microorganisms and are expensive compared to routine biochemical methods. FISH assays are more cost-effective, but setup can be cumbersome and labor-intensive.
Matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) has been routinely used in clinical laboratories in Europe since 2009 and has recently been adopted in the United States (12). Several reports have demonstrated excellent performance of MALDI-TOF MS for the identification of bacteria and yeast from solid media (2, 5–7, 18, 23–26, 29). In all studies, MALDI-TOF MS demonstrated equivalence or superiority to current methods for identification of microorganisms with the added benefits of reduced turnaround time and decreased cost. These characteristics make the application of MALDI-TOF MS to identification of microorganisms directly from positive blood cultures an attractive option.
Recent studies using MALDI-TOF MS for identification of microorganisms directly from positive blood cultures have demonstrated identification rates of 80% to 90% with 59% to 95% concordance with routine identification methods (4, 13, 16, 28). Variability in MALDI-TOF MS performance may be related to the various methods used for isolation of organisms from blood cultures. Reported methods include an array of differential centrifugation, washing, gel-based separation, and protein extraction steps to isolate bacteria and remove substances that can interfere with MALDI-TOF analysis (4, 8, 12, 16, 21, 28). These procedures can be tedious and lack standardization.
In this study, we evaluate the commercially available Sepsityper kit (Bruker Daltonics, Billerica, MS) coupled with the MALDI Biotyper system (Bruker) for isolation and identification of microorganisms directly from positive blood cultures. Specifically, we aim to compare the performance of the MALDI Biotyper/Sepsityper system to routine identification methods used for positive blood cultures. Additionally, we evaluate a modified blood culture-specific parameter for analysis of collected spectra and establish the potential time savings using MALDI-TOF MS compared to routine identification methods.
MATERIALS AND METHODS
Collection of blood cultures.
Positive blood cultures (n = 164) from patients at Froedtert Hospital (Milwaukee, WI) and Dynacare Laboratories (Milwaukee, WI) were prospectively collected between January and June 2011. Inclusion criteria included a positive Gram stain to confirm the presence of microorganisms prior to MALDI-TOF and routine analysis. Cultures were held at room temperature following removal from the blood culture instrument and were analyzed within 8 h of culture positivity. Only the initial positive culture from each patient was enrolled to avoid duplicate analysis of the same septic episode; however, positive aerobic and anaerobic cultures from the same set of cultures were accepted when available.
Sepsityper processing.
A 1.0-ml sample was collected from Bactec Plus Aerobic/F and Bactec Lytic/10 Anaerobic/F blood culture bottles (Becton Dickinson, Sparks, MD) following indication of a positive culture. Each specimen was processed using the Sepsityper kit (Bruker) prior to analysis using the Bruker Microflex LT MALDI-TOF MS and Biotyper 3.0 software. Briefly, 1.0 ml of residual blood culture from positive blood culture bottles was transferred to a 1.5-ml centrifuge tube (provided). A 200-μl aliquot of lysis buffer (provided) was added to the blood specimen, and the mixture was vortexed for 15 s prior to centrifugation (16,000 × g, 1 min). Following centrifugation, the supernatant was removed and the bacterial pellet was resuspended in 1.0 ml of wash buffer (provided), vortexed, and centrifuged as before. The supernatant was discarded, and the pellet was resuspended in 70% ethanol, vortexed, and centrifuged at 16,000 × g for 2 min. The ethanol was discarded, and the pellet was allowed to dry completely. When dry, 50 μl of 70% formic acid (Sigma-Aldrich, St. Louis, MO) and 50 μl of acetonitrile (Sigma-Aldrich) were added, and the pellet was resuspended. The suspension was centrifuged a final time (16,000 × g, 1 min), and 1 μl of the resulting supernatant was analyzed using MALDI-TOF MS.
MALDI-TOF analysis.
Duplicate 1-μl aliquots of extracted protein supernatant were transferred to individual spots on a 96-spot polished stainless steel target plate (Bruker) and allowed to dry. Each spot was overlaid with 1 μl of alpha-cyano-4-hydroxy cinamic acid (HCCA) matrix and allowed to dry. When dry, the target plate was inserted into the Bruker Microflex LT MALDI-TOF MS system for analysis. A composite profile of proteins with m/z of 3,000 to 15,000 was generated based on a minimum of 240 measurements (laser shots) for each specimen. The composite profile was analyzed using Biotyper 3.0 software (Bruker), which queried a reference bank of 3,995 spectra and returned the top 10 identification matches along with confidence scores ranging from 0.0 to 3.0. Scores of 2.0 and higher were considered high-confidence (secure species) identification, scores of 1.7 to 1.99 were considered intermediate-confidence (genus only) identification, and scores <1.7 were considered unacceptable identification. A second analysis of the collected spectra was conducted using blood culture-specific parameters that excluded mass peaks with m/z ratios <4,000. The identification with the highest confidence score for each specimen was compared to the final identification released by the clinical laboratory using routine identification methods appropriate for each isolate.
Routine identification methods.
Positive blood cultures were analyzed according to standard methods used by Dynacare Laboratories. Blood culture bottles were subcultured to the appropriate media and incubated aerobically or anaerobically until sufficient growth was present to proceed with testing (usually 18 to 24 h). Specific identification methods differ by organism but include the Phoenix automated microbiology system (BD), Vitek 2 (bioMérieux, Marcy l'Etoile, France), RapID NF (Remel, Lenexa, KS), and various latex agglutination and biochemical spot tests. The time to identification using routine methods was defined as the time from report of a positive Gram stain to the entry of a final result in the laboratory information management system. Sequencing of one isolate was carried out using the ABI PRISM 310 Genetic Analyzer and BigDye Terminator version 1.1 cycle sequencing kit with 16S 5′ (5′ TGG AGA GTT TGA TCC TGG CT) and 3′ (5′ TAC CGC GGC TGC TGG CAC) primers.
Statistical analysis.
Statistical significance was calculated using one-tailed Student's t test analysis of matched groups. Standard deviation was calculated using Microsoft Excel.
RESULTS
A total of 164 positive blood cultures representing 134 unique bacteremic episodes were prospectively collected. Of 150 monomicrobial cultures, 100 (67%) contained Gram-positive organisms, 45 (30%) contained Gram-negative organisms, and 5 (3%) contained yeast. The remaining 14 (9%) cultures were polymicrobial. Of the 145 monomicrobial bacterial cultures, 124 (85.5%) produced acceptable identification scores of ≥1.7 using the MALDI Biotyper/Sepsityper identification system. This included 94 (64.8%) cultures with high confidence scores of ≥2.0 and 30 (20.7%) cultures with intermediate confidence scores of 1.70 to 1.99. Gram-negative bacteria were more likely to produce high confidence scores (91.1%) than Gram-positive bacteria (53.0%). Overall concordance with routine identification methods was 97.6% to genus and 94.1% to species for isolates with confidence scores of ≥1.7.
Gram-positive bacteria.
The distribution of confidence scores within the 100 Gram-positive cultures was 20% unacceptable (<1.7), 27% intermediate (1.7 to 1.99), and 53% high (2.0 to 3.0) (Table 1). Among isolates with high confidence scores, there was 98.1% (52/53) concordance to genus and 93.3% (42/45) concordance to species with routine identification methods. Concordance was similar among isolates with intermediate confidence scores (96.3% to genus, 92.0% to species). Of the 32 cultures containing Staphylococcus epidermidis, only 5 produced high confidence scores of >2.0, while 16 produced intermediate and 11 produced unacceptable confidence scores (Table 1). Despite the low scores, all of these isolates were confirmed as S. epidermidis using Vitek 2.
Table 1.
Performance of MALDI-TOF/Sepsityper for identification of Gram-positive bacteria from positive blood culture bottles
| Organism (identified by routine methods) | No. of isolates | MALDI scorel |
Organism identified by MALDI (if discrepant) | ||
|---|---|---|---|---|---|
| <1.7 | 1.7–1.99 | >2.0 | |||
| Actinomyces spp. | 2 | 2a | |||
| Bacillus spp. | 2 | 1a | 1a | ||
| Corynebacterium spp. | 2 | 1 (1)a | 1a | Burkholderia saccharolyticusb | |
| Enterococcus faecalis | 12 | 1 | 11 | ||
| Enterococcus faecium | 6 | 2 | 4 | ||
| Facklamia hominis | 1 | 1 | |||
| Gemella spp. | 1 | 1a | |||
| Lactobacillus spp. | 3 | 3a | |||
| Micrococcus leuteus | 2 | 1a | 1 | ||
| Propionibacterium acnes | 3 | 2 (1) | 1 | Lactobacillus satsumensisc | |
| Staphylococcus aureus | 10 | 10 | |||
| Staphylococcus capitisd | 2 | 1 | 1 | ||
| Staphylococcus lugdunensis | 1 | 1 | |||
| Staphylococcus epidermidise | 32 | 11 | 16 | 5 | |
| Staphylococcus hominisf | 4 | 1 | 3 | ||
| Staphylococcus simulansg | 1 | 1 | |||
| Streptococcus pyogenes | 4 | 1 | 3 | ||
| Streptococcus agalactiae | 1 | 1 | |||
| Streptococcus pneumoniae | 1 | 1 | |||
| Streptococcus oralis | 3 | 2 (2) | 1 (1) | Streptococcus pneumoniaeh (3 isolates) | |
| Streptococcus viridans group | 5 | 2 (1) | 3 (3) | Facklamia languida,iLactobacillus curvatus,jStreptococcus pneumoniaek (2 isolates) | |
| Streptococcus milleri group | 2 | 2a | |||
| Total no. of isolates | 100 | 20 | 27 | 53 | |
| % genus agreement | 90.0 | 96.3 | 98.1 | ||
| % species agreement | 82.4 | 92.0 | 93.3 | ||
Identified to the genus level only by using routine methods, not included in species agreement statistics.
MALDI identification score was 1.37 (unacceptable), and Gram stain supported routine identification methods.
MALDI identification score was 1.51 (unacceptable), and catalase test supported routine identification methods.
Both isolates initially identified only as CoNS using routine methods, and species identification was confirmed using Vitek2.
Twenty of 32 isolates initially identified only as CoNS using routine methods, and species identification was confirmed using Vitek2.
Three of four isolates initially identified only as CoNS using routine methods, and species identification was confirmed using Vitek2.
Isolate initially identified only as CoNS using routine methods, and species identification was confirmed using Vitek2.
MALDI identification scores were 1.67, 1.88, and 1.91. All 3 isolates were optochin resistant, supporting routine identification.
MALDI identification score was 1.79. Isolate was identified as F. languida by 16S rRNA gene sequencing, confirming MALDI identification.
MALDI identification score was 2.05. Gram stain and vancomycin disk diffusion test support MALDI identification of L. curvatus.
MALDI identification scores were 2.22 and 2.28. Both isolates were optochin resistant, supporting routine identification.
Numbers in parentheses indicate the number of discrepant results in a given group.
There were five discrepant identification results among isolates with acceptable confidence scores, all of which were identified as Streptococcus oralis or viridans group streptococci using routine methods. Three of these were identified as Streptococcus pneumoniae using the MALDI Biotyper. Each of the isolates was resistant to optochin, supporting the biochemical identification. The remaining two discrepant results were identified as Facklamia languida and Lactobacillus curvatus by the MALDI Biotyper. 16S rRNA sequence analysis confirmed the MALDI Biotyper identification of F. languida (NCBI reference sequence NR_026487.1; E value, 0.0; max identity, 99%) (data not shown). The final discrepant isolate was identified as Lactococcus lactis by Vitek 2; however, observation of Gram stain (short, Gram-positive rods), negative catalase test, and resistance to vancomycin are most consistent with Lactobacillus spp. and support the MALDI Biotyper identification.
Gram-negative bacteria.
Forty-one of 45 (91%) Gram-negative cultures generated high confidence scores, and 3 (7%) generated intermediate confidence scores (Table 2). Only 1 culture failed to produce an acceptable identification. There was 97.6% (40/41) concordance to genus and 95.1% (39/41) concordance to species with routine identification methods among isolates with high (>2.0) confidence scores. All 3 isolates identified with intermediate confidence scores (1.7 to 1.99) were 100% concordant to genus and species with routine methods.
Table 2.
Performance of MALDI-TOF/Sepsityper for identification of Gram-negative bacteria from positive blood culture bottles
| Organism (identified by routine methods) | No. of isolates | MALDI scorec |
Organism identified by MALDI (if discrepant) | ||
|---|---|---|---|---|---|
| <1.7 | 1.7–1.99 | >2.0 | |||
| Bacteroides fragilis | 1 | 1 | |||
| Enterobacter cloacae | 3 | 3 (1) | Enterobacter asburiaea | ||
| Escherichia coli | 22 | 22 | |||
| Haemophilus influenzae | 1 | 1 | |||
| Haemophilus parainfluenzae | 1 | 1 | |||
| Klebsiella oxytoca | 3 | 3 (1) | Enterobacter cloacaeb | ||
| Klebsiella pneumoniae | 6 | 6 | |||
| Neisseria gonorrhoeae | 1 | 1 (1) | MALDI failed to generate ID | ||
| Proteus mirabilis | 1 | 1 | |||
| Pseudomonas aeruginosa | 4 | 4 | |||
| Serratia marcescens | 1 | 1 | |||
| Stenotrophomonas maltophilia | 1 | 1 | |||
| Total no. of isolates | 45 | 3 | 41 | ||
| % genus agreement | 0 | 100 | 97.6 | ||
| % species agreement | 0 | 100 | 95.1 | ||
MALDI identification score was 2.25. Vitek2, Voges-Proskauer, and motility tests support routine identification.
MALDI identification score was 2.19. Vitek2 and spot indole tests support the MALDI identification.
Numbers in parentheses indicate the number of discrepant results in a given group.
Two cultures generated discrepant identification results, both of which were isolates producing high confidence scores. The first was identified as Enterobacter cloacae using routine methods and as Enterobacter asburiae using the MALDI Biotyper. Both the BD Phoenix and Vitek 2 systems identified the isolate as E. cloacae or E. cloacae complex. The isolate was motile and produced a positive Voges-Proskauer test, both of which are consistent with E. cloacae and refute the MALDI identification. The second discrepant isolate was identified as Klebsiella oxytoca using the BD Phoenix system and was identified as E. cloacae using the MALDI Biotyper. Vitek 2 analysis was in agreement with the MALDI identification of E. cloacae, and a spot indole test was negative (>90% of K. oxytoca isolates are indole positive [17]). Both results support the MALDI Biotyper identification.
The single culture which failed to produce an acceptable identification (score of <1.7) was identified as Neisseria gonorrhoeae using routine methods. MALDI analysis of a colony following subculture returned an identification of N. gonorrhoeae with a high confidence score of 2.32. This suggests that the poor identification obtained directly from the blood culture was a consequence of suboptimal isolation or preparation of the bacteria from the blood culture.
Yeast.
None of the 5 monomicrobial cultures containing yeast generated acceptable confidence scores using the standard spectral analysis parameters.
Polymicrobial cultures.
The MALDI Biotyper correctly identified one of the organisms present in 9 of 14 (64.3%) polymicrobial cultures with a high confidence score (Table 3).
Table 3.
Performance of MALDI-TOF/Sepsityper for identification of microorganisms from polymicrobial blood culture bottles
| Organism(s) (identified by routine methods) | MALDI identification | Scoreb | Gram staina |
|---|---|---|---|
| E. coli, CoNS | E. coli | 2.43 | GNR, GPCCL |
| S. pyogenes, CoNS, viridans group Streptococcus | S. pyogenes | 2.19 | GPCCH |
| Candida albicans, Candida glabrata, CoNS | Unable to identify | NA | YST |
| Enterococcus faecalis, CoNS | E. faecalis | 2.35 | GPCCH |
| Klebsiella pneumoniae, Flavobacterium indologenes, Candida tropicalis | K. pneumoniae | 2.36 | GNR |
| C. glabrata, Lactobacillus spp. | Aromatoleum spp. | 1.29 | YST, GPCCH |
| E. coli, Citrobacter amalonaticus | E. coli | 2.36 | GNR |
| Eubacterium lentum, Peptostreptococcus asaccharolyticus | Mixed Pseudomonas spp. | <1.4 | GNR, GPC |
| Citrobacter freundii, Streptococcus salivarius | C. freundii | 2.47 | GNR, GPCCH |
| Bacillus spp. CoNS | Unable to identify | NA | GNR, GPCCH |
| Klebsiella oxytoca, Enterococcus faecium, E. coli | E. coli | 2.41 | GNR |
| S. aureus, Streptococcus agalactiae | S. agalactiae | 2.43 | GPCCH |
| Acinetobacter spp., CoNS 1, CoNS 2 | S. hominis | 2.14 | GNR,GPCCL |
| B. fragilis, Bacteriodes vulgatus, Clostridium clostridioforme | Unable to identify | NA | GNR |
Primary Gram stain result. GNR, Gram-negative rod; GPCCL, Gram-positive cocci in clusters; GPCCH, Gram-positive cocci in chains; YST, yeast.
NA, not applicable.
Analysis of spectra using blood-culture-specific parameters.
Reanalysis of all collected spectra using modified blood-culture-specific parameters resulted in equal or higher raw scores for 67% (97/145) of monomicrobial bacterial cultures. This included 72% (72/100) of Gram-positive cultures and 56% (25/45) of Gram-negative cultures. The mean increase in confidence score was statistically significant (0.12 ± 0.10, P = 1.74 × 10−7) for Gram-positive but not for Gram-negative (0.01 ± 0.07, P = 0.19) cultures. Among Gram-positive cultures, this resulted in 20 cultures moving into a higher confidence category (Table 4). Notably, this included 11 cultures containing S. epidermidis. Classification of Gram-negative cultures remained largely unchanged, with only 2 cultures moving into a higher confidence category. One of 5 yeast cultures generated an acceptable confidence score using the modified parameters. Reanalysis resulted in 3 cultures moving into lower confidence categories (Actinomyces turicensis, F. languida, Bacteriodes fragilis). Importantly, there was no change in identification of isolates when blood culture specific parameters were applied.
Table 4.
Cultures with change in confidence category using blood culture-specific parameters
| Organism | No. of isolatesa |
||||
|---|---|---|---|---|---|
| Increased confidence |
Decreased confidence |
||||
| Unacceptable to intermediate | Unacceptable to high | Intermediate to high | High to intermediate | Intermediate to unacceptable | |
| Gram-positive | |||||
| A. turicensis | 1 | ||||
| E. faecalis | 1 | ||||
| E. faecium | 1 | 1 | |||
| F. languida | 1 | ||||
| Micrococcus leuteus | 1 | ||||
| P. acnes | 1 | ||||
| Staphylococcus capitis | 1 | ||||
| S. epidermidis | 6 | 5 | |||
| S. gordonii | 1 | ||||
| S. pneumoniae | 1 | ||||
| S. pyogenes | 1 | ||||
| Gram-negative | |||||
| B. fragilis | 1 | ||||
| H. influenzae | 1 | ||||
| S. maltophilia | 1 | ||||
| Yeast | |||||
| C. glabrata | 1 | ||||
| Total | 10 | 1 | 12 | 1 | 1 |
Scores: unacceptable, <1.70; intermediate, 1.70 to 1.99; high, >2.0.
Time to identification.
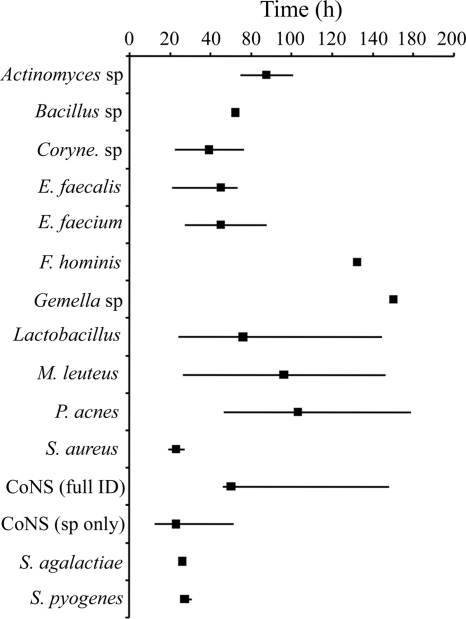
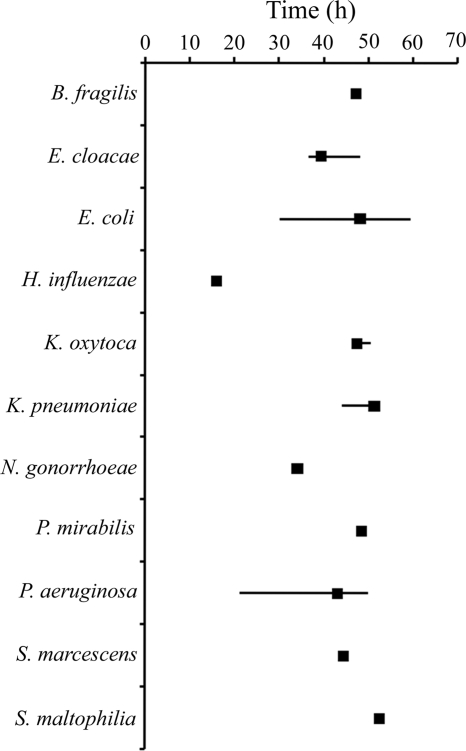
The time to identification of Gram-positive and -negative bacteria from initial blood culture positivity was highly variable using routine identification methods. Staphylococcus aureus (19.25 h to 27.72 h; median, 23.50 h) and Streptococcus pyogenes (26.93 h to 31.07 h; median, 27.75 h) identification times were relatively short, requiring only simple biochemical or agglutination tests. In contrast, difficult-to-identify organisms or those with slow growth rates, such as Facklamia hominis (112.48 h) and Propionibacterium acnes (46.55 h to 139.12 h; median, 83.20 h), required greatly extended times (Fig. 1). Identification times of Gram-negative bacteria were more consistent (16.08 h to 59.65 h), because these organisms were identified primarily using automated identification systems following 18 to 24 h of incubation (Fig. 2).
Fig 1.
Times to identification for Gram-positive bacteria using routine methods. The range of times to full identification of each isolate (solid lines) and median time to identification (solid squares) from initial blood culture positivity ranged from 19.25 h for S. aureus to 139.12 for P. acnes. All identifications were complete within 20 min by using the MALDI Biotyper/Sepsityper.
Fig 2.
Times to identification for Gram-negative bacteria using routine methods. The range of times to full identification of each isolate (solid lines) and median time to identification (solid squares) from initial blood culture positivity ranged from 16.08 h for Haemophilus influenzae to 59.65 h for Escherichia coli. All identifications were complete within 20 min by using the MALDI Biotyper/Sepsityper.
Identification of organisms from positive blood cultures using the MALDI Biotyper and Sepsityper processing kit required approximately 20 min and was independent of species. Batch processing added approximately 1 min per blood culture analyzed.
DISCUSSION
This study examined monomicrobial blood cultures containing Gram-positive and Gram-negative bacteria, as well as yeast and polymicrobial cultures. Proportions of each culture type are similar to those reported in a large national study and are also representative of all blood cultures routinely analyzed at this institution (15). Using standard spectral analysis parameters, MALDI Biotyper/Sepsityper identified the bacterium present in 85.5% of monomicrobial cultures with an acceptable confidence score (>1.7). A larger proportion of Gram-negative cultures generated acceptable scores (97.8%) than Gram-positive bacteria (80.0%). Similarly, a larger proportion of Gram-negative cultures generated high confidence scores (91.1%) than Gram-positive cultures (53.0%). This was primarily attributable to the large proportion of S. epidermidis cultures which generated intermediate (1.70 to 1.99) or unacceptable (<1.7) scores. Using standard parameters, 11 S. epidermidis isolates generated unacceptable confidence scores, and 5 remained in the unacceptable category following reanalysis using blood culture specific parameters. Despite the low scores, all coagulase-negative staphylococcus (CoNS) isolates with scores of >1.7 (n = 29 using standard parameters, n = 35 using blood culture specific parameters) were correctly identified to the species level by MALDI, as confirmed by Vitek 2. The cause of low or unacceptable scores associated with S. epidermidis is not readily apparent but has been reported previously for S. epidermidis isolates obtained from blood culture and solid media (18, 28). All five blood cultures containing yeast produced unacceptable confidence scores. This may be a result of the Sepsityper processing protocol not being optimized for recovery of yeast. A recent study investigated the isolation and identification of yeast from blood cultures using a modified Sepsityper processing protocol and demonstrated excellent results (32). The MALDI Biotyper was unable to identify multiple organisms in polymicrobial cultures; however, when a high confidence score identification was obtained, the identified organism was present.
When modified parameters excluding peaks with m/z ratios below 4,000 were used to analyze the collected spectra, the proportion of Gram-positive cultures giving acceptable confidence scores increased from 80% to 89%. Raw confidence scores increased for 68% (102/150) of monomicrobial cultures. The increase was statistically significant for Gram-positive cultures and resulted in an overall increase in secure species identifications from 64.8% to 73.1% (Gram negative, 91.1% to 95.6%; Gram positive, 53.0% to 63.0%). This modification appeared to be especially helpful in generating higher confidence scores for cultures containing S. epidermidis. The modified parameter did not change the identification of any isolate. Because MALDI-TOF identification is based on matching spectral peaks in a sample to a best-match reference library, additional peaks due to contaminants will lower confidence scores. Ferroni et al. report additional spectral peaks with low m/z ratios when analyzing the same strain from blood culture compared to solid media (9). Excluding mass spectrum peaks with m/z ratios from 3,000 to 4,000, which were included in the original algorithm, eliminated peaks likely corresponding to residual blood component debris and as a result increased overall confidence scores.
Regardless of which parameters were used for spectral analysis, the MALDI Biotyper/Sepsityper identifications were highly concordant with routine methods. Vales of concordance with genus (∼98%) and species (∼94%) were similar among both Gram-positive and Gram-negative bacteria, producing acceptable confidence scores. Similar concordance to species was observed in isolates with high (>2.0) and intermediate (1.7 to 1.99) confidence scores, indicating that the manufacturer-recommended cutoffs for species versus genus may be overly conservative and that lower confidence score thresholds could be considered.
Characterization of discordant results supported the MALDI Biotyper identification in 3 of 7 cultures that generated acceptable confidence scores (>1.7). Three of the four incorrect identifications by MALDI were viridans group streptococci misidentified as S. pneumoniae. The inability of the MALDI Biotyper to distinguish between S. pneumoniae and viridans group streptococci (specifically the mitis group) is due to the high similarity in protein profiles generated by these strains. This has been previously reported and is acknowledged by the manufacturer in the product insert (16, 18, 28). The remaining incorrect MALDI identification was an E. cloacae isolate identified as E. asburiae by the MALDI Biotyper. E. asburiae is closely related to E. cloacae and resides within the E. cloacae complex. Species within this complex are difficult to distinguish using biochemical tests and are often misidentified using automated systems (19, 31).
A major advantage of MALDI-TOF-based identification is a departure from reliance on physical, biochemical, and metabolic characteristics in favor of protein profile analysis. This allows better discrimination of species that have similar biochemical/metabolic profiles, such as CoNS. Accurate identification of CoNS can be helpful in differentiating a true infection from contamination. Identification based on the protein profile may also be advantageous when working with biochemically inert bacteria or organisms isolated from patients undergoing antibiotic therapy. Pretreated isolates often produce poor or inconsistent biochemical/metabolic profiles, making identification by routine methods difficult. Identification using MALDI-TOF relies primarily on ribosomal protein profiles which are of high abundance and are unaffected by many classes of antibiotics (22). Finally, automated identification systems require correct interpretation of Gram stain results for selection of an appropriate identification panel. Misinterpretation of the Gram stain can lead to a failed identification, which further extends turnaround time.
Empiric broad-spectrum antibiotic therapy is initiated when there is clinical suspicion of sepsis. While effective, broad-spectrum therapy is associated with adverse events, including Clostridium difficile disease, and potentially contributes to the selection of more resistant microorganisms (14, 27, 30). Earlier deescalation of broad-spectrum therapy may reduce these risks. Therefore, rapid identification of the etiologic agents of bacteremia and septicemia is an important component to management of the infection and can affect clinical outcome. The MALDI Biotyper/Sepsityper can be used to directly analyze positive blood cultures in real time and provide definitive species identification within 20 min. In the current study, the use of MALDI Biotyper/Sepsityper reduced the time to positive identification of isolates by as much as 130 h (Gemella spp.) and minimally by approximately 24 h (S. aureus, Streptococcus group A and group B). Combined with predictable antibiotic resistance profiles and effective real-time antibiograms, these reduced times to bacterial identification could aid in guidance of antibiotic therapy in patients with bacteremia.
ACKNOWLEDGMENTS
We thank Bruker Daltonics for material support in the form of Sepsityper kits and Raymond Podzorski, ProHealth Laboratories (Waukesha, WI), for ribosomal nucleic acid sequencing.
Footnotes
Published ahead of print 7 December 2011
REFERENCES
- 1.Angus DC, Wax RS. 2001. Epidemiology of sepsis: an update. Crit. Care Med. 29:S109–S116 [DOI] [PubMed] [Google Scholar]
- 2.Bessede E, et al. 2011. Matrix-assisted laser-desorption/ionization biotyper: experience in the routine of a university hospital. Clin. Microbiol. Infect. 17:533–538 [DOI] [PubMed] [Google Scholar]
- 3.Cattoir V, et al. 2010. Rapid detection of Pseudomonas aeruginosa from positive blood cultures by quantitative PCR. Ann. Clin. Microbiol. Antimicrob. 9:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christner M, et al. 2010. Rapid identification of bacteria from positive blood culture bottles by use of matrix-assisted laser desorption-ionization–time of flight mass spectrometry fingerprinting. J. Clin. Microbiol. 48:1584–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Degand N, et al. 2008. Matrix-assisted laser desorption ionization–time of flight mass spectrometry for identification of nonfermenting Gram-negative bacilli isolated from cystic fibrosis patients. J. Clin. Microbiol. 46:3361–3367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dhiman N, Hall L, Wohlfiel SL, Buckwalter SP, Wengenack NL. 2011. Performance and cost analysis of matrix-assisted laser desorption ionization–time of flight mass spectrometry for routine identification of yeast. J. Clin. Microbiol. 49:1614–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dubois D, et al. 2010. Identification of a variety of Staphylococcus species by matrix-assisted laser desorption ionization–time of flight mass spectrometry. J. Clin. Microbiol. 48:941–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferreira L, Sanchez-Juanes F, Munoz-Bellido JL, Gonzalez-Buitrago JM. 2010. Rapid method for direct identification of bacteria in urine and blood culture samples by matrix-assisted laser desorption ionization time-of-flight mass spectrometry: intact cell versus extraction method. Clin. Microbiol. Infect. 17:1007–1012 [DOI] [PubMed] [Google Scholar]
- 9.Ferroni A, et al. 2010. Real-time identification of bacteria and Candida species in positive blood culture broths by matrix-assisted laser desorption ionization–time of flight mass spectrometry. J. Clin. Microbiol. 48:1542–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jukes L, et al. 2010. Rapid differentiation of Staphylococcus aureus, Staphylococcus epidermidis and other coagulase-negative staphylococci and meticillin susceptibility testing directly from growth-positive blood cultures by multiplex real-time PCR. J. Med. Microbiol. 59:1456–1461 [DOI] [PubMed] [Google Scholar]
- 11.Kumar A, et al. 2006. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit. Care Med. 34:1589–1596 [DOI] [PubMed] [Google Scholar]
- 12.La Scola B. 2011. Intact cell MALDI-TOF mass spectrometry-based approaches for the diagnosis of bloodstream infections. Expert Rev. Mol. Diagn. 11:287–298 [DOI] [PubMed] [Google Scholar]
- 13.La Scola B, Raoult D. 2009. Direct identification of bacteria in positive blood culture bottles by matrix-assisted laser desorption ionisation time-of-flight mass spectrometry. PLoS One 4:e8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levy SB. 1998. Multidrug resistance—a sign of the times. N. Engl. J. Med. 338:1376–1378 [DOI] [PubMed] [Google Scholar]
- 15.Martin GS, Mannino DM, Eaton S, Moss M. 2003. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 348:1546–1554 [DOI] [PubMed] [Google Scholar]
- 16.Moussaoui W, et al. 2010. Matrix-assisted laser desorption ionization time-of-flight mass spectrometry identifies 90% of bacteria directly from blood culture vials. Clin. Microbiol. Infect. 16:1631–1638 [DOI] [PubMed] [Google Scholar]
- 17. Murray PR, Baron EJ, Jorgensen JH, Landry LL, Pfaller MA. 2007. Manual of clinical microbiology, 9th ed, vol 1 ASM Press, Washington, DC [Google Scholar]
- 18.Neville SA, et al. 2011. Utility of matrix-assisted laser desorption ionization–time of flight mass spectrometry following introduction for routine laboratory bacterial identification. J. Clin. Microbiol. 49:2980–2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paauw A, et al. 2008. Genomic diversity within the Enterobacter cloacae complex. PLoS One 3:e3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peters RP, et al. 2006. Faster identification of pathogens in positive blood cultures by fluorescence in situ hybridization in routine practice. J. Clin. Microbiol. 44:119–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romero-Gomez MP, Mingorance J. 2011. The effect of the blood culture bottle type in the rate of direct identification from positive cultures by matrix-assisted laser desorption/ionisation time-of-flight (MALDI-TOF) mass spectrometry. J. Infect. 62:251–253 [DOI] [PubMed] [Google Scholar]
- 22.Ryzhov V, Fenselau C. 2001. Characterization of the protein subset desorbed by MALDI from whole bacterial cells. Anal. Chem. 73:746–750 [DOI] [PubMed] [Google Scholar]
- 23.Saffert RT, et al. 2011. Comparison of Bruker Biotyper matrix-assisted laser desorption ionization-time of flight mass spectrometer to BD Phoenix automated microbiology system for identification of Gram-negative bacilli. J. Clin. Microbiol. 49:887–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saleeb PG, Drake SK, Murray PR, Zelazny AM. 2011. Identification of mycobacteria in solid-culture media by matrix-assisted laser desorption ionization–time of flight mass spectrometry. J. Clin. Microbiol. 49:1790–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seng P, et al. 2009. Ongoing revolution in bacteriology: routine identification of bacteria by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin. Infect. Dis. 49:543–551 [DOI] [PubMed] [Google Scholar]
- 26.Sogawa K, et al. 2011. Use of the MALDI BioTyper system with MALDI-TOF mass spectrometry for rapid identification of microorganisms. Anal. Bioanal Chem. 400:1905–1911 [DOI] [PubMed] [Google Scholar]
- 27.Stevens V, Dumyati G, Fine LS, Fisher SG, van Wijngaarden E. 2011. Cumulative antibiotic exposures over time and the risk of Clostridium difficile infection. Clin. Infect. Dis. 53:42–48 [DOI] [PubMed] [Google Scholar]
- 28.Stevenson LG, Drake SK, Murray PR. 2010. Rapid identification of bacteria in positive blood culture broths by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 48:444–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Veen SQ, Claas EC, Kuijper EJ. 2010. High-throughput identification of bacteria and yeast by matrix-assisted laser desorption ionization–time of flight mass spectrometry in conventional medical microbiology laboratories. J. Clin. Microbiol. 48:900–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wener KM, Schechner V, Gold HS, Wright SB, Carmeli Y. 2010. Treatment with fluoroquinolones or with beta-lactam-beta-lactamase inhibitor combinations is a risk factor for isolation of extended-spectrum-beta-lactamase-producing Klebsiella species in hospitalized patients. Antimicrob. Agents Chemother. 54:2010–2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winn W, et al. 2006. The Enterobacteriaceae, p 211–302 In Peterson N. (ed), Konemans color atlas and textbook of diagnostic microbiology. Lippincott Williams and Wilkins, Baltimore, MD: [Google Scholar]
- 32.Yan Y, et al. 2011. Improved identification of yeast species directly from positive blood culture media by combining Sepsityper specimen processing and Microflex analysis with the matrix-assisted laser desorption ionization Biotyper system. J. Clin. Microbiol. 49:2528–2532 [DOI] [PMC free article] [PubMed] [Google Scholar]