Abstract
Bacillus cereus is a rare cause of endocarditis, typically associated with intravenous drug abuse, rheumatic heart disease, prosthetic heart valves, pacemakers, or immunodeficiency. We present the first case of native valve Bacillus cereus endocarditis with no apparent risk factors. The patient had a fulminant course requiring emergent valve replacement.
CASE REPORT
A 42-year-old Thai male presented with a 3-week history of fatigue, generalized malaise, fever, chills, and dyspnea on exertion. He had no prior past medical or surgical history and no history of rheumatic heart disease. The patient admitted to intermittent use of methamphetamine (inhalation), but he denied any current or prior use of injectable drugs. According to the family, no intravenous (IV) drug using paraphernalia was found in the patient's room.
On review of systems, pertinent positive findings included fatigue, malaise, fever, chills, right and left upper quadrant pain, diarrhea for 2 weeks (nonbloody, no mucus), and left-sided toothache (no recent dental work).
Vital signs were as follows: temperature, 96.8°F; blood pressure, 92/45; heart rate, 82; respiratory rate, 20; and an oxygen saturation of 93% on room air. On physical examination, the abnormal findings were as follows: poor dentition, subconjunctival hemorrhage to the left eye (no other stigmata of infectious endocarditis were found), systolic ejection murmur (4/6) with palpable thrill, best heard at the left upper sternal border, and no skin markings to indicate IV drug use.
Pertinent laboratory findings include a urinary toxicology screen positive for methamphetamines, hemoglobin of 10.1, hematocrit of 30.3, white blood cell (WBC) count of 16.4, normal chemistry panel, and a normal urinalysis (UA).
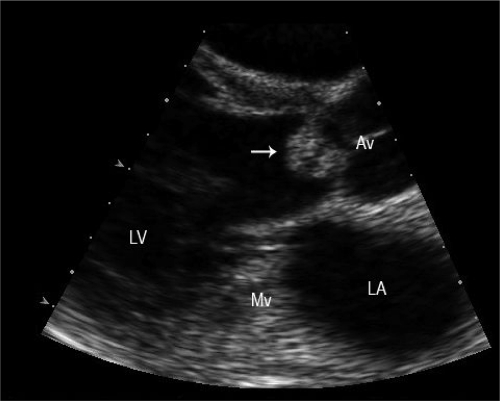
A computed tomography (CT) scan of the abdomen and pelvis was obtained on admission, which revealed a perfusion defect in both kidneys consistent with infarcts. Transthoracic echocardiography revealed an aortic valve vegetation (2 cm × 0.75 cm) (Fig. 1) prolapsing into the left ventricular outflow tract, mild mitral valve thickening with no vegetations, and an ejection fraction of 60%.
Fig 1.
Preoperative transthoracic echocardiogram showing a mobile vegetation on the aortic valve. Av, aortic valve; Mv, mitral valve; LV, left ventricle; LA, left atrium; arrow, 2.0-cm × 0.75-cm vegetation.
The patient was admitted to the hospital with a diagnosis of infectious endocarditis and septicemia. Empirical antibiotics were started with ampicillin, gentamicin, and nafcillin.
Over the ensuing 24 h, the patient's clinical condition deteriorated such that he required emergent intubation, IV pressor support, and transfer to the medical intensive care unit (ICU). Preliminary blood cultures grew Gram-positive rods, Bacillus species. Empirical antibiotics were changed to daptomycin and ampicillin. Due to the large, hypermobile aortic valve vegetation with resultant septic and cardiogenic shock, emergent aortic valve replacement was performed. After resection of a large aortic valve endocarditis vegetation on the left coronary cusp, replacement of the valve was accomplished with a 23-mm Carbomedics mechanical bileaflet top-hat valve.
Bacillus cereus was identified in two sets of blood cultures. The B. cereus group consists of closely related species: B. anthracis, B. cereus, B. thuringiensis, and B. mycoides. The members of the group are difficult to separate phenotypically and are closely related genotypically. Phenotypic testing consisted of macroscopic and microscopic morphologies in culture and Gram stain, motility, and special media. Genotypic testing consisted of nucleic acid sequencing accomplished using 16S rRNA sequencing (i.e., MicroSeq short-sequence [500 bp] analysis followed by full gene sequencing). Both approaches were used in the final bacterial identification. The finding of B. cereus prompted a change in the patient's antibiotics to daptomycin and ceftriaxone based upon the antibiogram. The organism was resistant to penicillin and tetracycline and was sensitive to cephalosporins, aminoglycosides, levofloxacin, linezolid, sulfamethoxazole-trimethoprim, and vancomycin. Pathological analysis of the tricuspid aortic valve contained acute inflammatory and fibrinous vegetations, granulation tissue, and fibrosis consistent with endocarditis. Gram stain and culture did not yield definite causative bacteria or intracellular bacteria.
Following valve replacement, the patient made an excellent clinical recovery and received ceftriaxone for 6 weeks of outpatient IV antibiotic therapy.
Bacillus cereus is an aerobic or facultative anaerobic, spore-forming, Gram-positive rod that is ubiquitously found throughout the natural environment (i.e., in the soil). Bacillus species can also commonly be found in the hospital environment (i.e., dressings, intravenous catheters, and linens), on personal products (i.e., contact lens solution), and even in the mucous membranes of healthy adults (2, 7, 12, 14, 16). Sometimes dismissed as a contaminant, the organism has been identified as a pathogen in a wide variety of illnesses (7). Broadly divided, the organism is responsible for gastrointestinal and nongastrointestinal diseases (7). Among the gastrointestinal illnesses, Bacillus cereus is classically responsible for the onset of nausea and profuse vomiting after consuming rice (or other starchy food) secondary to its preformed, heat-stable emetic toxin. Nongastrointestinal Bacillus cereus infections have caused serious and sometimes fatal illnesses, such as pneumonia, meningitis, encephalitis, and brain abscesses (7).
Bacillus cereus is a rare cause of endocarditis, and when found to be the causative pathogen, is typically associated with preexisting valvular disease (rheumatic heart disease) (17), prosthetic heart valves (3, 4, 10, 11, 13, 19), a history of intravenous drug abuse (6, 15, 18), pacemakers (1, 12), or leukemia (5) (Table 1).
Table 1.
Summary of data for the cases of Bacillus cereus endocarditisa
| Yr reported | Reference | Risk factor | Valve affected | Antibiotic treatment | Surgery | Outcome |
|---|---|---|---|---|---|---|
| 1974 | 6 | IVDA | Tricuspid | Lm, Cm | No | Recovered |
| 1978 | 3 | Mechanical valve | Mitral | Tm, Chl | No | Died |
| 1979 | 15 | IVDA | NR | Naf | NR | Recovered |
| 1979 | 15 | IVDA | NR | Cm | NR | Recovered |
| 1979 | 15 | IVDA | NR | Cm | NR | Recovered |
| 1979 | 15 | IVDA | No vegetations by ECHO | Chl, Gent, Em | No | Recovered |
| 1979 | 17 | RHD | Aortic | Pen, Gent, Stm | No | Died |
| 1979 | 18 | IVDA | No vegetations by ECHO | Cm, Kana | No | Recovered |
| 1982 | 11 | Porcine valve | Aortic | Cm | Yes | Recovered |
| 1987 | 12 | Hx rheumatic fever, pacemaker | Right ventricular pacing wire | Cm | Yes | Recovered |
| 1992 | 13 | Mechanical valve | Aortic | Vm | Yes | Recovered |
| 1994 | 19 | Mechanical valve | Mitral | Ami, Mino | Yes | Recovered |
| 1998 | 10 | Mechanical valve | Mitral | Vm, Gent, Rif | Yes | Recovered |
| 1999 | 4 | Mechanical valve | Mitral | Vm, Gent, Rif | Yes | Recovered |
| 2005 | 5 | Leukemia | Mitral | Pen, Vm, Cipro | No | Died |
| 2008 | 1 | Pacemaker | Pacing wire | Cef | Yes | Recovered |
| 2010 | None | Aortic | Dapto, Amp, Ctrx | Yes | Recovered |
IVDA, intravenous drug abuse; Lm, lincomycin; Cm, clindamycin; Tm, tobramycin; Chl, chloramphenicol; NR, not reported; Naf, nafcillin; ECHO, echocardiogram; Gent, gentamicin; Em, erythromycin; RHD, rheumatic heart disease; Pen, penicillin; Stm, streptomycin; Kana, kanamycin; Vm, vancomycin; Ami, amikacin; Mino, minocycline; Rif, rifampin; Cipro, ciprofloxacin; Cef, cefazolin; Dapto, daptomycin; Amp, ampicillin; Ctrx, ceftriaxone.
To our knowledge, there have been no prior case reports of native valve Bacillus cereus endocarditis in a patient without these risk factors.
Discussion.
Finland and Barnes reported the first cases of Gram-positive bacillus endocarditis in 1933 (two cases) and in 1951 (one case) at Boston City Hospital. Characterized as miscellaneous causes of endocarditis at that time, these first two cases occurred before the introduction of highly potent antibacterial drugs, and thus both patients died (9). Farrar's review in 1963 of nonpathogenic organisms of the genus Bacillus marked the beginning of the widespread recognition of nonanthrax Bacillus spp. as causing pathogenic human disease (8).
Bacillus cereus endocarditis was first reported in 1974 in an IV drug-abusing (IVDA) patient (6). Since then, five additional cases have been reported in the literature. Bacteremia in IVDA patients is likely acquired from skin colonization, contamination of the injection equipment, or organisms in the heroin itself. Tuazon et al. showed that Bacillus spp. could be cultured from 32% of heroin samples and 47% of injection drug paraphernalia (14). To date, all of the patients with IVDA-associated, native valve Bacillus cereus endocarditis received effective antibiotic therapy, and none of them died or required emergent valve replacement. The mantra has been that IVDA-associated endocarditis responds extremely well to antibiotic therapy alone, whereas that associated with prosthetic valves has much higher morbidity and mortality rates and usually requires surgical intervention. Our report describes the first case of native valve endocarditis in which a patient did not respond initially to antibiotic therapy alone and required emergent valve replacement due to hemodynamic instability.
Bacillus cereus endocarditis associated with prosthetic valves has been reported a total of six times. The first case, in 1978, was treated with antibiotic therapy alone, and the patient died secondary to septic emboli (3). Subsequently, all patients have been treated with valve replacement and made good recoveries; hence, it is recommended that prompt valve replacement surgery be considered to reduce high morbidity and mortality rates associated with Bacillus cereus prosthetic valve endocarditis (4).
In general, Bacillus cereus endocarditis appears to typically follow a benign course and generally responds to antibiotic treatment alone. Penicillins and cephalosporins are traditionally avoided as initial therapy given the high incidence of resistance to these agents. Out of the antimicrobial agents in routine use today, Bacillus cereus appears to be nearly uniformly sensitive to aminoglycosides, clindamycin, erythromycin, and vancomycin. Of the 17 cases reported, three have died. Those patients that died include one with rheumatic heart disease (RHD), another with leukemia, and the last with a mechanical valve. All of these cases were treated with antibiotics alone, and surgery for valve replacement was not performed.
Conclusion.
In our case report of native valve Bacillus cereus endocarditis, the etiology of septicemia and subsequent seeding of the aortic valve remains elusive. The patient lacked typical predisposing factors and had no signs or symptoms of immunodeficiency. Given the review of systems obtained from the patient and physical exam findings, possible portals of entry include infectious diarrhea or odontogenic infection, although both of these seem unlikely.
Historically, IVDA-associated Bacillus cereus endocarditis has responded well to antibiotics alone, without the need for surgical intervention. Our case highlights an aggressive case of native-valve Bacillus cereus endocarditis and the need for prompt consideration of valve replacement if the patient is hemodynamically unstable or is experiencing large numbers of septic emboli.
ACKNOWLEDGMENTS
We acknowledge Fran Smith of the Arnold Library at Straub Clinic & Hospital for her tireless work to secure reference materials for us to write the manuscript.
B.S.T. and M.J.B. have nothing to disclose. W.K.K.L. reports receiving lecturing fees from Pfizer, Johnson & Johnson, Cubist, Gilead, Astellas, and Merck and research support from Pfizer and Bio Cryst and is on the advisory board for Astellas.
Footnotes
Published ahead of print 23 November 2011
REFERENCES
- 1.Abusin S, Bhimaraj A, Khadra S. 2008. Bacillus cereus endocarditis in a permanent pacemaker: a case report. Cases J. 1: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrie D, Wilson JA, Hoffman PN, Kramer JM. 1992. Bacillus cereus meningitis in two neurosurgical patients: an investigation into the source of the organism. J. Infect. 25: 291– 297 [DOI] [PubMed] [Google Scholar]
- 3.Block CS, Levy ML, Fritz VU. 1978. Bacillus cereus endocarditis. A case report. S. Afr. Med. J. 53: 556– 557 [PubMed] [Google Scholar]
- 4.Castedo E, Castro A, Martin P, Roda J, Montero CG. 1999. Bacillus cereus prosthetic valve endocarditis. Ann. Thorac Surg. 68: 2351– 2352 [DOI] [PubMed] [Google Scholar]
- 5.Cone LA, Dreisbach L, Potts BE, Comess BE, Burleigh WA. 2005. Fatal Bacillus cereus endocarditis masquerading as an anthrax-like infection in a patient with acute lymphoblastic leukemia: case report. J. Heart Valve Dis. 14: 37– 39 [PubMed] [Google Scholar]
- 6.Craig CP, Lee WS, Ho M. 1974. Letter: Bacillus cereus endocarditis in an addict. Ann. Intern. Med. 80: 418– 419 [DOI] [PubMed] [Google Scholar]
- 7.Drobniewski FA. 1993. Bacillus cereus and related species. Clin. Microbiol. Rev. 6: 324– 338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farrar WE., Jr 1963. Serious infections due to “non-pathogenic” organisms of the genus Bacillus. Review of their status as pathogens. Am. J. Med. 34: 134– 141 [DOI] [PubMed] [Google Scholar]
- 9.Finland M, Barnes MW. 1970. Changing etiology of bacterial endocarditis in the antibacterial era. Experiences at Boston City Hospital 1933-1965. Ann. Intern. Med. 72: 341– 348 [DOI] [PubMed] [Google Scholar]
- 10.Martin Cadenas P, et al. 1998. Endocarditis by Bacillus cereus 1 in prosthetic mitral valve. Enferm. Infecc. Microbiol. Clin. 16: 102– 104. (In Spanish.) [PubMed] [Google Scholar]
- 11.Oster HA, Kong TQ. 1982. Bacillus cereus endocarditis involving a prosthetic valve. South Med. J. 75: 508– 509 [DOI] [PubMed] [Google Scholar]
- 12.Sliman R, Rehm S, Shlaes DM. 1987. Serious infections caused by Bacillus species. Medicine 66: 218– 223 [DOI] [PubMed] [Google Scholar]
- 13.Steen MK, et al. 1992. Bacillus cereus endocarditis: report of a case and review. Clin. Infect. Dis. 14: 945– 946 [DOI] [PubMed] [Google Scholar]
- 14.Tuazon CU, Hill R, Sheagren JN. 1974. Microbiologic study of street heroin and injection paraphernalia. J. Infect. Dis. 129: 327– 329 [DOI] [PubMed] [Google Scholar]
- 15.Tuazon CU, et al. 1979. Serious infections from Bacillus sp. JAMA 241: 1137– 1140 [PubMed] [Google Scholar]
- 16.van Setten GB, Tervo T, Tarkkanen A. 1989. Acute keratitis and contamination of contact lens care systems with Bacillus cereus. Klin. Monbl Augenheilkd. 195: 28– 31 (In German) [PubMed] [Google Scholar]
- 17.Wanvarie S, Rochanawatanon M. 1979. Bacillus cereus endocarditis. J. Med. Assoc. Thai. 62: 34– 38 [PubMed] [Google Scholar]
- 18.Weller PF, Nicholson A, Braslow N. 1979. The spectrum of Bacillus bacteremias in heroin addicts. Arch. Intern. Med. 139: 293– 294 [PubMed] [Google Scholar]
- 19.Yamamura M, et al. 1994. A case of Bacillus cereus prosthetic valve endocarditis. Kyobu Geka 47: 232– 234 (In Japanese) [PubMed] [Google Scholar]