Abstract
Immigrants from high-burden countries and HIV-coinfected individuals are risk groups for tuberculosis (TB) in countries with low TB incidence. Therefore, we studied their role in transmission of Mycobacterium tuberculosis in Switzerland. We included all TB patients from the Swiss HIV Cohort and a sample of patients from the national TB registry. We identified molecular clusters by spoligotyping and mycobacterial interspersed repetitive-unit–variable-number tandem-repeat (MIRU-VNTR) analysis and used weighted logistic regression adjusted for age and sex to identify risk factors for clustering, taking sampling proportions into account. In total, we analyzed 520 TB cases diagnosed between 2000 and 2008; 401 were foreign born, and 113 were HIV coinfected. The Euro-American M. tuberculosis lineage dominated throughout the study period (378 strains; 72.7%), with no evidence for another lineage, such as the Beijing genotype, emerging. We identified 35 molecular clusters with 90 patients, indicating recent transmission; 31 clusters involved foreign-born patients, and 15 involved HIV-infected patients. Birth origin was not associated with clustering (adjusted odds ratio [aOR], 1.58; 95% confidence interval [CI], 0.73 to 3.43; P = 0.25, comparing Swiss-born with foreign-born patients), but clustering was reduced in HIV-infected patients (aOR, 0.49; 95% CI, 0.26 to 0.93; P = 0.030). Cavitary disease, male sex, and younger age were all associated with molecular clustering. In conclusion, most TB patients in Switzerland were foreign born, but transmission of M. tuberculosis was not more common among immigrants and was reduced in HIV-infected patients followed up in the national HIV cohort study. Continued access to health services and clinical follow-up will be essential to control TB in this population.
INTRODUCTION
Despite major improvements in treatment and control, tuberculosis (TB) caused by Mycobacterium tuberculosis remains a major global public health problem (32). Control efforts have been thwarted in some regions following the advent of the human immunodeficiency virus (HIV) epidemic (17). HIV infection is the strongest risk factor for TB, substantially increasing the lifetime risk of progression from infection with M. tuberculosis to active disease (24). Among the estimated 9.4 million new TB patients emerging in 2009, 11 to 13% were estimated to be HIV infected (32).
In Europe, TB incidence has stabilized, but notification rates vary across the region (11). Countries from the Balkans have higher rates of TB than most Western European countries, and the highest rates are reported from Eastern Europe (11). In most European countries more than one-half of all TB cases occur among foreign-born individuals (25). In high-income countries such as Switzerland, TB incidence and mortality have been declining for more than a century (12). Migrants from high-TB-burden countries and HIV-infected populations are risk groups (1, 12); however, their role in transmission of M. tuberculosis within the host country is unclear.
We aimed to study M. tuberculosis transmission in Switzerland over a 9-year period with a focus on the role of immigrants and HIV-infected individuals.
MATERIALS AND METHODS
Study setting.
The Swiss Molecular Epidemiology of Tuberculosis (SMET) study is a collaborative project between the Swiss HIV Cohort Study (SHCS), the National Center for Mycobacteria, diagnostic microbiology laboratories, departments of respiratory medicine and public health, and the Federal Office of Public Health (FOPH). The overarching aims were to examine the genetic population structure of M. tuberculosis and the associations between strain variation, patient origin, and clinical characteristics in HIV-infected TB patients compared to HIV-negative TB patients in Switzerland. Further information on the SMET project is available at www.tb-network.ch. All participating sites are listed in the Acknowledgments.
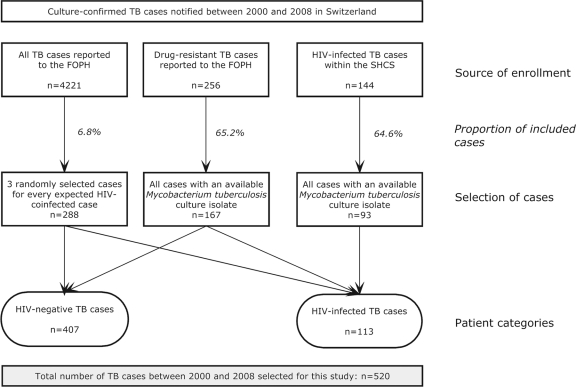
The SHCS is a prospective observational study of HIV-infected individuals followed up in HIV outpatient clinics in Switzerland, which has been described in detail previously (23). All HIV-infected patients diagnosed with TB between 2000 and 2008 whose M. tuberculosis complex (MTBC) isolates were available were included in the SMET study (Fig. 1). Furthermore, a random sample of 288 from the 4,221 culture-confirmed TB cases reported to the National TB Surveillance Registry during the same period were included (approximately 3 cases for 1 HIV-infected TB case from the SHCS). Finally, all reported drug-resistant TB cases were included. We accepted MTBC isolates cultured from both respiratory and nonrespiratory specimens.
Fig 1.
Enrollment strategy of tuberculosis (TB) patients diagnosed between 2000 and 2008 that were included in this study. The overarching aim of the project was to examine the genetic population structure of Mycobacterium tuberculosis and the associations between strain variation, patient origin, and clinical characteristics in HIV-infected compared to HIV-negative TB patients in Switzerland. Further information on this project is available at www.tb-network.ch. The overall proportion of included patients during the study period was 12.3% of all culture-confirmed TB cases in Switzerland. Among HIV-negative cases, 146 (35.9%) were drug resistant, and among HIV-infected cases, 21 (18.6%) were drug resistant, as reported to the Federal Office of Public Health (FOPH; National TB Surveillance Registry). SHCS, Swiss HIV Cohort Study.
Clinical data collection.
We obtained clinical data by standardized questionnaires sent to the treating physicians and extracted relevant data from the SHCS database. We collected sociodemographic data (age, sex, origin of birth, citizenship, legal status, immunosuppressive therapy, risk factors for TB), laboratory parameters (sputum smear result and, for HIV-infected cases, CD4 cell count and plasma HIV RNA level), and clinical information (site of disease, radiography findings). A positive smear result was defined as a sputum or bronchial specimen positive for acid-fast bacilli. Chest radiography parameters were consolidation, cavitations, enlarged intrathoracic lymph nodes, and pleural thickening. Drug resistance was defined as any resistance to isoniazid, rifampin, or ethambutol as reported to the FOPH. Most TB cases in Switzerland are treated under the guidance of experienced infectious and respiratory disease specialists, and the clinical data were of high quality.
We designed the questionnaire according to accepted standards and then piloted and modified it. Completeness of data was monitored by the study coordinator. Data were entered in a dedicated database, which included bound checking, check digits (additional number added to a unique identifier to check for errors when entering identification numbers), and fixed taxonomy. We compared randomly selected questionnaires with the database entries.
Molecular analysis.
Mycobacterial isolates were cultured and DNA was extracted according to standard laboratory procedures. We used spacer oligonucleotide typing (spoligotyping) and 24-locus mycobacterial interspersed repetitive unit–variable-number tandem-repeat (MIRU-VNTR) analysis to identify molecular clusters as previously described (27). We analyzed data using the MIRU-VNTRplus online tool (http://www.miru-vntrplus.org). Molecular clusters were defined as a group of completely identical isolates in the spoligotyping and MIRU-VNTR pattern. We determined the main M. tuberculosis lineages by single nucleotide polymorphisms (SNPs) using multiplex real-time PCR with fluorescence-labeled probes (TaqMan; Applied Biosystems) adopted from previous studies (7, 13, 14, 18). Region of difference (RD) deletion PCRs were performed for RD702 and RD711, which define the West African lineages (14).
Statistical analyses.
We used chi-square tests or Fisher's exact tests to test the statistical significance of differences between groups in binary variables and the Wilcoxon rank sum test for continuous variables. The clustering proportion was calculated by the “n” method and expressed as the number of patients in clusters divided by the total number of individuals (16). We used weighted logistic regression models to obtain age- and sex-adjusted odds ratios (aOR) of the probability of belonging to a molecular cluster. As our study sample included, by design, a high proportion of HIV-infected patients and patients with drug-resistant TB (Fig. 1), we computed appropriate weights to take sampling proportions into account. Furthermore, we restricted the analysis to patients randomly sampled from the TB registry to examine the clustering proportion in the random sample. All analyses were performed in Stata version 11.1 (Stata Corporation, College Station, TX).
Ethics approval.
The study was approved by the ethics committee of the Canton of Bern, Switzerland. Informed consent was obtained from all patients enrolled in the SHCS. For patients outside the SHCS, informed consent was obtained by the treating physicians. In some cases informed consent could not be obtained from the patient because he or she could not be located or was known to have died. For these cases we obtained permission from the Federal Expert Commission on Confidentiality in Medical Research to use the data provided by the treating physician.
RESULTS
Patient characteristics.
A total of 520 TB cases with an available MTBC strain were diagnosed between 2000 and 2008 and included in the study (Fig. 1). Patient characteristics are summarized in Table 1. A total of 224 (43.1%) patients were born in Europe, and the most frequent countries of origin were Serbia (27 cases, 5.2%) and Italy and Portugal, with 15 cases each (2.9%).
Table 1.
Sociodemographic and clinical characteristics of included TB cases and comparison between clustered and nonclustered (unique) TB cases, Switzerland, 2000 to 2008
| Characteristic | Value for: |
||
|---|---|---|---|
| All cases (n = 520) | Clustered cases (n = 90) | Unclustered cases (n = 430) | |
| Median age (yr) at TB diagnosis (IQR) | 36.5 (28–51) | 36 (27–45) | 37 (29–53) |
| No. (%) of males | 254 (48.9) | 47 (52.2) | 207 (48.1) |
| No. (%) born in: | |||
| Switzerland | 119 (22.9) | 23 (25.6) | 96 (22.3) |
| Other Europe | 106 (20.4) | 17 (18.9) | 89 (20.7) |
| Sub-Saharan Africa | 132 (25.4) | 33 (36.7) | 99 (23.0) |
| Asia | 120 (23.1) | 12 (13.3) | 108 (25.1) |
| Central/South America | 20 (3.8) | 3 (3.3) | 17 (4.0) |
| Other regions/unknown | 23 (4.4) | 2 (2.2) | 21 (4.9) |
| No. (%) of: | |||
| Swiss nationals | 130 (25.0) | 19 (21.1) | 111 (25.8) |
| Foreigners, residents | 140 (26.9) | 18 (20.0) | 122 (28.4) |
| Foreigners, othera | 59 (11.3) | 13 (14.4) | 46 (10.7) |
| Asylum seekers/refugees | 101 (19.4) | 21 (23.3) | 80 (18.6) |
| Unknown | 90 (17.3) | 19 (21.1) | 71 (16.5) |
| No. (%) | |||
| HIV infected | 113 (21.7) | 21 (23.3) | 92 (21.4) |
| HIV negative | 407 (78.3) | 69 (76.7) | 338 (78.6) |
| No. (%) with immunosuppression other than due to HIV infectionb | 32 (6.2) | 8 (8.9) | 24 (5.6) |
| No. (%) with: | |||
| Previous TB diagnosis | 50 (9.6) | 8 (8.9) | 42 (9.8) |
| No previous TB diagnosis | 300 (57.7) | 50 (55.6) | 250 (58.1) |
| Unknown | 170 (32.7) | 32 (35.6) | 138 (32.1) |
| No. (%) with positive smear result | 168 (32.3) | 38 (42.2) | 130 (30.2) |
| No. (%) with cavitary disease | 113 (21.7) | 27 (30.0) | 86 (20.0) |
| No. (%) with clinical manifestation | |||
| Pulmonary | 387 (74.4) | 72 (80.0) | 315 (73.3) |
| Extrapulmonary | 133 (25.6) | 18 (20.0) | 115 (26.7) |
| No. (%) with TB in family or social surroundings in last 2 yr | 38 (7.3) | 11 (12.2) | 27 (6.3) |
Visitors, students, etc.
Use of anti-tumor necrosis factor (anti-TNF) blockers, malignancy, organ transplantation, use of steroids or methotrexate.
Genetic diversity of M. tuberculosis.
The most frequent M. tuberculosis lineage was lineage 4 (also known as the Euro-American lineage) with 378 (72.7%) patients, followed by lineage 2 (east Asian lineage) with 54 patients (10.4%) and lineage 1 (Indo-Oceanic lineage) with 43 patients (8.3%). The distribution of lineages differed between foreign- and Swiss-born cases (P < 0.0001) (Table 2), with more patients with lineages other than lineage 4 among foreign-born cases. Among the strains of lineage 4, the ill-defined T spoligotype family was most common (30.2%), followed by the H family (21.7%) and the Latin American-Mediterranean family (20.6%). The distribution of strains across lineages did not change over the 9-year study period above what could be expected by chance (P = 0.58). Similarly, the variation in the proportion of clustered patients over time was not statistically significant (P = 0.61) (Table 3).
Table 2.
Distribution of the main Mycobacterium tuberculosis lineages among foreign-born and Swiss-born TB cases, Switzerland, 2000 to 2008
| Lineagea | No. (%) of patientsb |
|
|---|---|---|
| Foreign born | Swiss born | |
| 1 | 42 (10.5) | 1 (0.8) |
| 2 | 48 (12.0) | 6 (5.1) |
| 3 | 29 (7.2) | 4 (3.4) |
| 4 | 271 (67.6) | 107 (89.9) |
| Other | 11 (2.7) | 1 (0.8) |
| Total | 401 (100.0) | 119 (100.0) |
Lineage 1, Indo-Oceanic lineage; lineage 2, east Asian lineage (includes Beijing strains); lineage 3, CAS/Delhi lineage; lineage 4, Euro-American lineage; other, other lineages including West African lineages.
Fisher's exact test; P < 0.0001.
Table 3.
Distribution of M. tuberculosis strains across lineages and numbers of clustered patients, Switzerland, 2000–2008
| Case yr | No. of patients | No. (%) of patients with lineage: |
No. (%) of clustered patients | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | Other | |||
| 2000 | 48 | 1 (2.8) | 2 (4.2) | 1 (2.1) | 41 (85.4) | 3 (6.3) | 5 (10.4) |
| 2001 | 29 | 3 (10.3) | 1 (3.5) | 3 (10.3) | 22 (75.9) | 0 (0) | 5 (17.2) |
| 2002 | 36 | 4 (11.1) | 5 (13.9) | 0 (0) | 25 (69.4) | 2 (5.6) | 8 (22.2) |
| 2003 | 65 | 4 (6.2) | 7 (10.8) | 5 (7.7) | 47 (72.3) | 2 (3.1) | 10 (15.4) |
| 2004 | 76 | 7 (9.2) | 8 (10.5) | 6 (7.9) | 55 (72.4) | 0 (0) | 16 (21.1) |
| 2005 | 63 | 3 (4.8) | 9 (14.3) | 4 (6.4) | 46 (73.0) | 1 (1.6) | 10 (15.9) |
| 2006 | 72 | 8 (11.1) | 8 (11.1) | 5 (6.9) | 50 (69.4) | 1 (1.4) | 14 (19.4) |
| 2007 | 71 | 7 (9.9) | 8 (11.3) | 7 (9.9) | 49 (69.0) | 0 (0) | 12 (16.9) |
| 2008 | 60 | 6 (10.0) | 6 (10.0) | 2 (3.3) | 43 (71.7) | 3 (5.0) | 10 (16.7) |
| P valuea | 0.22 | 0.25 | 0.48 | 0.11 | 0.30 | 0.61 | |
| Total | 520 | 43 (8.3) | 54 (10.4) | 33 (6.4) | 378 (72.7) | 12 (2.3) | 90 (17.3) |
P values were determined by a linear test of trend using logistic regression.
Lineages are as defined for Table 2.
Molecular clusters.
We identified 35 clusters; 20 clusters (57.1%) involved foreign-born cases only, 4 involved Swiss-born cases only (11.4%), and 11 (31.4%) were mixed. The clusters included 90 patients, for a proportion of clustered patients of 17.3%. The mean cluster size was 2.6 cases (range, 2 to 8 cases), and the median interval between the first and the most recent TB case in a cluster was 38.8 months (interquartile range [IQR], 12.7 to 63.3 months).
Predictors of clustering.
Younger age and male sex were associated with clustering, but associations failed to reach statistical significance (Table 4). There was little evidence for an association with birth origin. For example, the aOR comparing patients born in sub-Saharan Africa with Swiss-born patients was 1.03 (95% confidence interval [CI], 0.40 to 2.66). The aOR comparing Swiss-born with any foreign-born patients was 1.58 (95% CI, 0.73 to 3.43; P = 0.25). Of note, clustering was reduced in HIV-infected patients (aOR, 0.49; 95% CI, 0.26 to 0.93; P = 0.030), and this remained when additionally adjusting for positive smear result and presence of cavitary disease (aOR, 0.49; 95% CI, 0.25 to 0.97; P = 0.040). A positive smear result, cavitary disease, pulmonary TB, and recent TB within the family or social surroundings were all associated with clustering (Table 4).
Table 4.
Sociodemographic and clinical predictors of molecular clustering in TB cases, Switzerland, 2000 to 2008a
| Characteristic | Unadjusted result |
Adjusted result |
||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Age (yr) at TB diagnosis | 0.21 | 0.29 | ||
| 16–30 | 1 | 1 | ||
| 30–49 | 0.60 (0.30–1.19) | 0.63 (0.32–1.27) | ||
| ≥50 | 0.57 (0.28–1.15) | 0.60 (0.29–1.22) | ||
| Sex | 0.078 | 0.10 | ||
| Male | 1 | 1 | ||
| Female | 0.60 | 0.62 (0.35–1.10) | ||
| Origin of birth | 0.067 | 0.086 | ||
| Switzerland | 1 | 1 | ||
| Other Europe | 0.87 (0.39–1.96) | 0.74 (0.31–1.75) | ||
| Sub-Saharan Africa | 1.73 (0.79–3.78) | 1.03 (0.40–2.66) | ||
| Other regions/unknown | 0.57 (0.25–1.32) | 0.37 (0.14–0.98) | ||
| Legal status | 0.13 | 0.13 | ||
| Swiss nationals | 1 | 1 | ||
| Foreigners, residents | 0.67 (0.30–1.52) | 0.46 (0.18–1.17) | ||
| Foreigners, otherb | 1.98 (0.81–4.82) | 1.48 (0.56–3.92) | ||
| Asylum seekers/refugees | 1.57 (0.69–3.56) | 0.90 (0.32–2.51) | ||
| Unknown | 1.62 (0.61–4.30) | 1.32 (0.49–3.52) | ||
| HIV status | 0.055 | 0.030 | ||
| Negative | 1 | 1 | ||
| Infected | 0.55 (0.30–1.01) | 0.49 (0.26–0.93) | ||
| Immunosuppression other than due to HIV infectionc | 0.26 | 0.078 | ||
| No | 1 | 1 | ||
| Yes | 1.73 (0.67–4.50) | 2.54 (0.90–7.17) | ||
| Smear result | 0.007 | 0.012 | ||
| Negative | 1 | 1 | ||
| Positive | 2.26 (1.25–4.09) | 2.16 (1.18–3.96) | ||
| Cavitary TB | 0.005 | 0.012 | ||
| No | 1 | 1 | ||
| Yes | 2.48 (1.32–4.64) | 2.27 (1.20–4.28) | ||
| Pulmonary TB | 0.011 | 0.015 | ||
| No | 1 | 1 | ||
| Yes | 2.73 (1.26–5.89) | 2.63 (1.21–5.74) | ||
| TB in family or social surroundings in last 2 yr | 0.031 | 0.049 | ||
| No | 1 | 1 | ||
| Yes | 2.90 (1.10–7.63) | 2.75 (1.01–7.51) | ||
Results are from weighted logistic regression models, unadjusted and adjusted for age and sex. CI, confidence interval; OR, odds ratio.
Visitors, students, etc.
Use of anti-TNF blockers, malignancy, organ transplantation, use of steroids or methotrexate.
Clusters involving immigrants.
Among the 20 clusters of foreign-born cases, 8 involved patients born in the same country, 9 were cases from neighboring countries or countries of the same region, and 3 were cases that originated from different continents. Of the 230 foreign-born TB cases with a reported immigration date, 161 (70.0%) were diagnosed with TB and started on treatment more than 1 year after immigration to Switzerland, with a median interval between immigration and diagnosis of 3.7 years (IQR, 0.7 to 9.6).
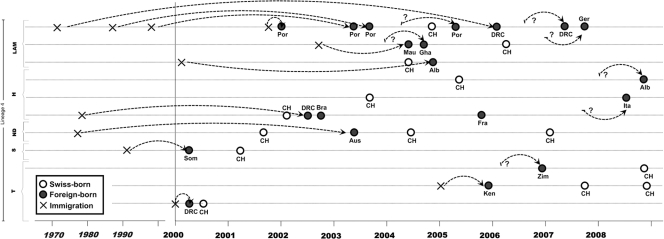
The 11 mixed clusters comprised 13 Swiss-born (10.9% of all Swiss-born cases) and 21 foreign-born (5.2% of all foreign-born cases) cases. Among the 21 foreign-born cases, 17 were from intermediate- or high-TB-burden countries (Portugal, Albania, Brazil, sub-Saharan Africa countries) and 4 were from Switzerland's neighboring countries (Austria, France, Germany, Italy) with low TB incidences (Fig. 2). In 6 of the 11 mixed clusters, a foreign-born patient was the first reported case.
Fig 2.
Distribution of clustered TB cases (n = 34) in the 11 mixed molecular clusters involving Swiss-born and foreign-born individuals (asylum seekers/refugees, foreigners, foreign employees, etc.), by time of TB diagnosis, immigration, birth country, and lineage/spoligotyping family. Alb, Albania; Aus, Austria; Bra, Brazil; CH, Switzerland; DRC, Democratic Republic of the Congo; Fra, France; Ger, Germany; Gha, Ghana; Ita, Italy; Ken, Kenya; Mau, Mauretania; Por, Portugal; Som, Somalia; Zim, Zimbabwe; H, Haarlem; LAM, Latin American-Mediterranean; ND, spoligotype family not designated; S, spoligotype family S; T, ill-defined spoligotype family T. Crosses indicate year at immigration; a question mark indicates that the immigration year was unknown.
Clusters involving HIV-infected patients.
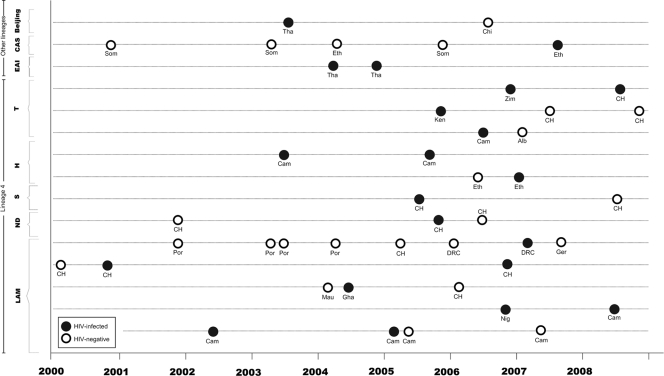
Of 113 HIV-infected TB cases, 21 cases (18.6% of all HIV-infected TB cases) were included in 15 different molecular clusters (Fig. 3). Nine of these 15 clusters (with nine patients or 8.0% of all HIV-coinfected patients) included one HIV-infected patient and at least one HIV-negative patient. The remaining six clusters (12 patients, 10.6%) involved two HIV-infected TB cases. Eight of the 15 clusters involved patients from the same geographical region (Fig. 3).
Fig 3.
Distribution of HIV-infected TB cases (n = 21) in the 15 molecular clusters that involve at least one HIV-infected TB case, by time of TB diagnosis, lineage/spoligotype family, and birth country. Abbreviations are as defined for Fig. 2 with the following additions: Cam, Cameroon; Chi, China; Eth, Ethiopia; Ger, Germany; Nig, Nigeria; Tha, Thailand; CAS, central Asian; EAI, East African-Indian.
Additional analysis.
Restricting the analysis to the group of randomly selected patients (n = 288) (Fig. 1), we found 42 clustered patients in 17 molecular clusters and a clustering proportion of 14.6% (95% CI, 10.7 to 19.2%).
DISCUSSION
In this nationwide molecular epidemiological study of TB in Switzerland over a 9-year period we found that recent transmission of TB accounted for about 17% of TB cases. There was no evidence for an increase in the clustering proportion over time and no evidence for the emergence of a particular M. tuberculosis lineage, for example, Beijing strains. We identified 35 molecular clusters, the majority of which involved foreign-born cases. Immigrants were not, however, more likely to be involved in recent transmissions than Swiss-born cases, and HIV-infected patients were less likely to be part of transmission clusters than non-HIV-infected patients.
A nationwide study from The Netherlands found a clustering proportion of 35% between 1993 and 1997 (30), while a more recent study from Norway reported a proportion of 20.6% between 1994 and 2005 (9). A study preceding the introduction of potent combination antiretroviral therapy (cART) found a clustering proportion of 24% among HIV-infected patients in Switzerland (26). We observed a substantially lower proportion of 10% for transmission between HIV-infected individuals, in line with a collaborative analysis of cohort studies from low-income and high-income countries, which found that the incidence of TB was reduced by 50% to 60% following the introduction of cART (5, 10). The clinical care and close follow-up provided in a clinical cohort (10) might have further reduced transmission and might explain the lower clustering in HIV-infected patients, compared to HIV-negative patients. Of note, TB among the HIV-infected population was at least partly due to transmissions from HIV-negative individuals, consistent with reports from countries with high TB and HIV burdens (2).
Almost 90% of the 35 molecular clusters involved foreign-born cases. This finding suggests that TB in Switzerland is driven by immigrants in absolute terms; however, taking denominators into account, immigrants were not more likely to be involved in recent transmissions than Swiss-born patients. Only 11 clusters (31%) were mixed, but in half of these a foreign-born individual was the cluster's first case and half of the cases originated from a country with an intermediate to high TB burden, which makes them a priori more likely to be the origin of the cluster. Considering the low incidence of TB in the Swiss population (<3 per 100,000 people) we would have expected the majority of transmissions to be traceable to foreign-born cases. Cultural and social barriers reducing contacts between foreigners and the Swiss-born population may explain our findings, consistent with reports from other settings (3, 9, 20). We stress that only contact tracing can unambiguously define the directionality of transmission in each cluster.
Changes in the M. tuberculosis lineage distribution is likely when transmission is driven by foreign-born populations, who will introduce their lineage, or when a foreign strain with a higher potential of transmission is introduced. In our study the distribution of M. tuberculosis lineages differed significantly between foreign- and Swiss-born cases but overall the Euro-American lineage dominated throughout, with no evidence for the emergence of another lineage. In particular, we found no evidence for an increase in the east Asian lineage, which includes Beijing strains. Beijing strains may have a selective advantage over other strain lineages (22) and have been rapidly emerging in the Western Cape, South Africa (8), and the Canary Islands (6). Our observation is consistent with studies from Norway and Sweden, where a shift in the distribution of strain lineages was not observed, despite a high number of foreign-born TB cases (9, 15).
This is the first nationwide study on the molecular epidemiology of TB in Switzerland. Strengths of our study include the involvement of both the Swiss HIV Cohort Study and the National TB Surveillance Registry and the study period of 9 years, which is over twice the minimal recommended time period of 3 to 4 years (28). Sampling proportions were carefully assessed, allowing weighted analyses that reflected the national data. There are also several limitations. First, the size of the study was relatively small, with only 12.3% of all TB cases included in the study. A recent study of a national, comprehensive data set from The Netherlands showed that sampling biased the observed clustering proportion downwards but did not reduce the odds ratios for clustering. These odds ratios may therefore correctly be interpreted as risk factors for recent transmission (4). Second, transmission may also be underestimated when the expected size of clusters is small (16), as in our study. Finally, we did not confirm the molecular clusters by conventional contact tracing; however, the approach used in our study has been shown to be valid in numerous previous studies (21, 27, 29). Furthermore, contact tracing misses a considerable proportion of clustered TB cases (19).
In conclusion, our study supports the notion that transmission of M. tuberculosis is well contained in Switzerland. The public health approach to the control of TB includes early diagnosis through passive case finding, prompt and adequate treatment of infectious cases, and a national surveillance system (12). Transmission is particularly well contained in the HIV-infected population. The majority of TB patients were immigrants, but the risk of transmission in this group was comparable to that for patients born in the country. As migration from low-income countries with high infectious disease burdens is likely to increase in the future, access to health services, clinical follow-up, and adherence to treatment will continue to be essential to control TB in this population (31).
ACKNOWLEDGMENTS
We thank all tuberculosis patients for participating in this study, the treating physicians for providing clinical information, and the Microbiology Laboratories for providing strains. We are indebted to the National TB Surveillance Registry at the Federal Office of Public Health, as well as to Christa Butz and Yvonne Bongni from the Bernese Lung Association.
This work was supported by the Swiss National Science Foundation (grant 324730-12544), the Swiss HIV Cohort Study (grant 588), the Federal Office of Public Health (grant 09.007368), and the National Center for Mycobacteria. L.F. and M.E. are supported by the National Institute of Allergy and Infectious Diseases (IeDEA Southern Africa; grant 5U01-AI069924-5), and S.G. is supported by the National Institutes of Health (grant R01-AI090928-01) and the Swiss National Science Foundation (grant PP00A-119205). E.C.B. was supported by the University of Zürich, the European Community (PAR, FP7-HEALTH-2009-241476), and the Federal Office of Public Health (National Center for Mycobacteria). The Swiss HIV Cohort Study is supported by the Swiss National Science Foundation (grant 33CS30-134277). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
There are no conflicts of interest to declare.
Central coordinating team members were Lukas Fenner and Matthias Egger (Institute of Social and Preventive Medicine, Bern, Switzerland), Sebastien Gagneux and Marcel Tanner (Swiss Tropical and Public Health Institute, Basel, Switzerland), and Hansjakob Furrer (Inselspital Bern, Bern, Switzerland). The National Center for Mycobacteria member was Erik C. Böttger (Institute of Medical Microbiology, University of Zurich, Zurich, Switzerland). Microbiology laboratory members were Reno Frei (Clinical Microbiology, University Hospital Basel, Basel, Switzerland), Thomas Bodmer (Institute for Infectious Diseases, University of Bern, Bern, Switzerland), Beatrice Ninet and Jacques Schrenzel (Central Laboratory of Bacteriology, University Hospital Geneva, Geneva, Switzerland), Katia Jaton and Amalio Telenti (Institute of Microbiology, University Hospital of Lausanne, Lausanne, Switzerland), Hans Siegrist (ADMed Microbiology, La Chaux-de-Fonds, Switzerland), Gaby E. Pfyffer (Department of Medical Microbiology, Luzerner Kantonsspital, Lucerne, Switzerland), Thomas Bruderer (Centre for Laboratory Medicine, St.Gallen, Switzerland), Marisa Dolina (Cantonal Institute of Microbiology, Medical Bacteriology, Bellinzona, Switzerland), and Olivier Dubuis (Viollier AG Switzerland, Allschwil, Switzerland). Respiratory clinic members were Jean-Paul Janssens (University Hospital of Geneva) and Jesica Mazza Stalder (University Hospital of Lausanne). Federal Office of Public Health members were Peter Helbling and Ekkehardt Altpeter (Division of Communicable Diseases). The Union member was Hans L. Rieder (Institute of Social and Preventive Medicine, University of Zürich, and The Union, Paris, France). The members of the Swiss HIV Cohort Study were J. Barth, M. Battegay (University Hospital Basel), E. Bernasconi (St. Gallen), J. Böni, H. C. Bucher, C. Burton-Jeangros, A. Calmy (University Hospital of Geneva), M. Cavassini (University Hospital of Lausanne), C. Cellerai, M. Egger, L. Elzi, J. Fehr (University Hospital of Zurich), J. Fellay, M. Flepp, P. Francioli (president of the SHCS), H. Furrer (Inselspital Bern; chairman of the Clinical and Laboratory Committee), C. A. Fux, M. Gorgievski, H. Günthard (chairman of the Scientific Board), D. Haerry (deputy of Positive Council), B. Hasse, B. Hirschel (University Hospital of Geneva), H. H. Hirsch, B. Hirschel, M. Hoffmann (St. Gallen), I. Hösli, C. Kahlert, L. Kaiser, O. Keiser, C. Kind, T. Klimkait, H. Kovari, B. Ledergerber, A. P. Lugano (St. Gallen), G. Martinetti, B. Martinez de Tejada, K. Metzner, N. Müller, D. Nadal, G. Pantaleo, A. Rauch, S. Regenass, M. Rickenbach (head of the Data Center), C. Rudin (chairman of the Mother & Child Substudy), P. Schmid, D. Schultze, F. Schöni-Affolter, J. Schüpbach, R. Speck, P. Taffé, P. Tarr, A. Telenti, A. Trkola, P. Vernazza, R. Weber, and S. Yerly.
Footnotes
Published ahead of print 23 November 2011
Contributor Information
M. Battegay, (University Hospital Basel)
E. Bernasconi, (St. Gallen)
A. Calmy Burton-Jeangros, (University Hospital of Geneva).
M. Cavassini, (University Hospital of Lausanne)
J. Fehr, (University Hospital of Zurich)
H. Furrer, (Inselspital Bern; chairman of the Clinical and Laboratory Committee)
M. Hoffmann, (St. Gallen)
A. P. Lugano, (St. Gallen)
REFERENCES
- 1. Abgrall S, Del GP, Melica G, Costagliola D. 2010. HIV-associated tuberculosis and immigration in a high-income country: incidence trends and risk factors in recent years. AIDS 24: 763–771 [DOI] [PubMed] [Google Scholar]
- 2. Asiimwe BB, et al. 2009. DNA restriction fragment length polymorphism analysis of Mycobacterium tuberculosis isolates from HIV-seropositive and HIV-seronegative patients in Kampala, Uganda. BMC Infect. Dis. 9: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Borgdorff MW, et al. 1998. Analysis of tuberculosis transmission between nationalities in the Netherlands in the period 1993–1995 using DNA fingerprinting. Am. J. Epidemiol. 147: 187–195 [DOI] [PubMed] [Google Scholar]
- 4. Borgdorff MW, van den Hof S, Kalisvaart N, Kremer K, van Soolingen D. 2011. Influence of sampling on clustering and associations with risk factors in the molecular epidemiology of tuberculosis. Am. J. Epidemiol. 174: 243–251 [DOI] [PubMed] [Google Scholar]
- 5. Brinkhof MW, et al. 2007. Tuberculosis after initiation of antiretroviral therapy in low-income and high-income countries. Clin. Infect. Dis. 45: 1518–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Caminero JA, et al. 2001. Epidemiological evidence of the spread of a Mycobacterium tuberculosis strain of the Beijing genotype on Gran Canaria island. Am. J. Respir. Crit. Care Med. 164: 1165–1170 [DOI] [PubMed] [Google Scholar]
- 7. Comas I, Homolka S, Niemann S, Gagneux S. 2009. Genotyping of genetically monomorphic bacteria: DNA sequencing in Mycobacterium tuberculosis highlights the limitations of current methodologies. PLoS One 4: e7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cowley D, et al. 2008. Recent and rapid emergence of W-Beijing strains of Mycobacterium tuberculosis in Cape Town, South Africa. Clin. Infect. Dis. 47: 1252–1259 [DOI] [PubMed] [Google Scholar]
- 9. Dahle UR, Eldholm V, Winje BA, Mannsåker T, Heldal E. 2007. Impact of immigration on the molecular epidemiology of Mycobacterium tuberculosis in a low-incidence country. Am. J. Respir. Crit. Care Med. 176: 930–935 [DOI] [PubMed] [Google Scholar]
- 10. Elzi L, et al. 2007. Reducing tuberculosis incidence by tuberculin skin testing, preventive treatment, and antiretroviral therapy in an area of low tuberculosis transmission. Clin. Infect. Dis. 44: 94–102 [DOI] [PubMed] [Google Scholar]
- 11. Falzon D, et al. 2008. Stopping TB in Europe: some progress but still not there. Euro. Surveill. 13 [DOI] [PubMed] [Google Scholar]
- 12.Federal Office of Public Health 2011. Tuberkulose in der Schweiz 2005–2009. Bull. BAG 10: 205–213 (Erratum, 13:277) [Google Scholar]
- 13. Fenner L, et al. 2011. “Pseudo-Beijing”: evidence for convergent evolution in the direct repeat region of Mycobacterium tuberculosis. PLoS One 6: e24737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gagneux S, et al. 2006. Variable host-pathogen compatibility in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 103: 2869–2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ghebremichael S, et al. 2010. Drug resistant Mycobacterium tuberculosis of the Beijing genotype does not spread in Sweden. PLoS One 5: e10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Glynn JR, Vynnycky E, Fine PE. 1999. Influence of sampling on estimates of clustering and recent transmission of Mycobacterium tuberculosis derived from DNA fingerprinting techniques. Am. J. Epidemiol. 149: 366–371 [DOI] [PubMed] [Google Scholar]
- 17. Harries AD, et al. 2010. The HIV-associated tuberculosis epidemic—when will we act? Lancet 375: 1906–1919 [DOI] [PubMed] [Google Scholar]
- 18. Hershberg R, et al. 2008. High functional diversity in Mycobacterium tuberculosis driven by genetic drift and human demography. PLoS Biol. 6: e311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lambregts-van Weezenbeek CSB, et al. 2003. Tuberculosis contact investigation and DNA fingerprint surveillance in The Netherlands: 6 years' experience with nation-wide cluster feedback and cluster monitoring. Int. J. Tuberc. Lung Dis. 7(suppl. 3): S463–S470 [PubMed] [Google Scholar]
- 20. Lillebaek TÅ, et al. 2001. Risk of Mycobacterium tuberculosis transmission in a low-incidence country due to immigration from high-incidence areas. J. Clin. Microbiol. 39: 855–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oelemann MC, et al. 2007. Assessment of an optimized mycobacterial interspersed repetitive-unit-variable-number tandem-repeat typing system combined with spoligotyping for population-based molecular epidemiology studies of tuberculosis. J. Clin. Microbiol. 45: 691–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Parwati I, van Crevel R, van Soolingen D. 2010. Possible underlying mechanisms for successful emergence of the Mycobcterium tuberculosis genotype strains. Lancet Infect. Dis. 10: 103–111 [DOI] [PubMed] [Google Scholar]
- 23. Schoeni-Affolter F, et al. 2010. Cohort profile: the Swiss HIV Cohort study. Int. J. Epidemiol. 39: 1179–1189 [DOI] [PubMed] [Google Scholar]
- 24. Selwyn PA, et al. 1992. Clinical manifestations and predictors of disease progression in drug users with human immunodeficiency virus infection. N. Engl. J. Med. 327: 1697–1703 [DOI] [PubMed] [Google Scholar]
- 25.Stop TB Partnership 2006. The global plan to stop TB 2006–2015. World Health Organization, Geneva, Switzerland [Google Scholar]
- 26. Sudre P, et al. 1999. Molecular epidemiology of tuberculosis among HIV-infected persons in Switzerland: a countrywide 9-year cohort study. Swiss HIV Cohort Study. Infection 27: 323–330 [DOI] [PubMed] [Google Scholar]
- 27. Supply P, et al. 2006. Proposal for standardization of optimized mycobacterial interspersed repetitive unit-variable-number tandem repeat typing of Mycobacterium tuberculosis. J. Clin. Microbiol. 44: 4498–4510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van der Spuy GD, van Helden PD, Warren RM. 2009. Effect of study duration on the interpretation of tuberculosis molecular epidemiology investigations. Tuberculosis 89: 238–242 [DOI] [PubMed] [Google Scholar]
- 29. van Deutekom H, et al. 2005. Molecular typing of Mycobacterium tuberculosis by mycobacterial interspersed repetitive unit-variable-number tandem repeat analysis, a more accurate method for identifying epidemiological links between patients with tuberculosis. J. Clin. Microbiol. 43: 4473–4479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van Soolingen D, et al. 1999. Molecular epidemiology of tuberculosis in the Netherlands: a nationwide study from 1993 through 1997. J. Infect. Dis. 180: 726–736 [DOI] [PubMed] [Google Scholar]
- 31. Wolff H, et al. 2010. Undocumented migrants in Switzerland: geographical origin versus legal status as risk factor for tuberculosis. J. Immigr. Minor. Health 12: 18–23 [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization 2010. Global tuberculosis control: epidemiology, strategy, financing. WHO report 2010. World Health Organization, Geneva, Switzerland [Google Scholar]