Abstract
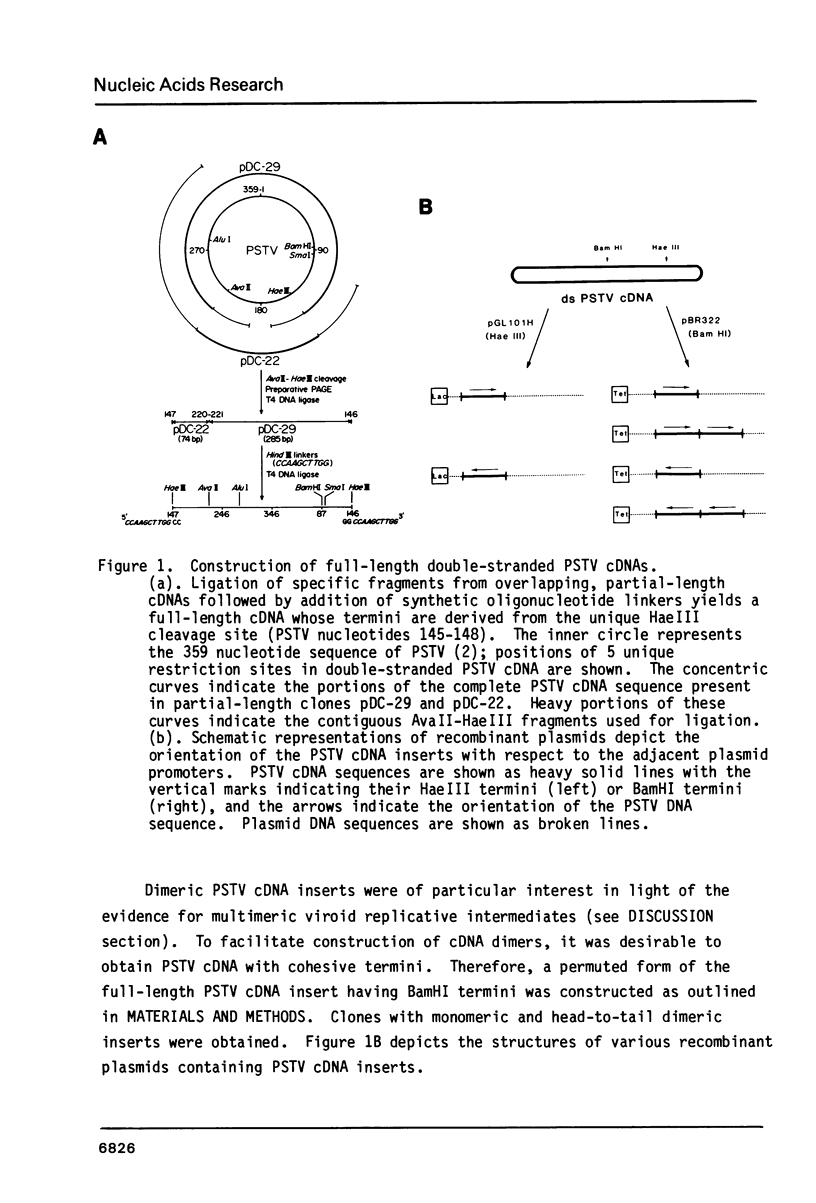
Contiguous restriction fragments from two cloned partial-length potato spindle tuber viroid (PSTV) cDNAs were used to construct recombinant DNAs containing full-length monomeric and dimeric PSTV cDNA. When five different PSTV cDNA plasmids and RNA isolated from E. coli cells harboring these plasmids were tested for infectivity on tomato, plasmid DNAs containing PSTV cDNA dimers were infectious. RNA transcripts containing the sequence of PSTV from these plasmids were also infectious. The sequences of the viroid progeny and the cloned DNA were identical. In vitro mutagenesis of infectious PSTV cDNAs will allow systematic investigation of the role of specific sequences in viroid replication and pathogenesis.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Branch A. D., Robertson H. D., Dickson E. Longer-than-unit-length viroid minus strands are present in RNA from infected plants. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6381–6385. doi: 10.1073/pnas.78.10.6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael G. G., McMaster G. K. The analysis of nucleic acids in gels using glyoxal and acridine orange. Methods Enzymol. 1980;65(1):380–391. doi: 10.1016/s0076-6879(80)65049-6. [DOI] [PubMed] [Google Scholar]
- Chan H. W., Israel M. A., Garon C. F., Rowe W. P., Martin M. A. Molecular cloning of polyoma virus DNA in Escherichia coli: lambda phage vector system. Science. 1979 Mar 2;203(4383):887–892. doi: 10.1126/science.217088. [DOI] [PubMed] [Google Scholar]
- Dickson E. A model for the involvement of viroids in RNA splicing. Virology. 1981 Nov;115(1):216–221. doi: 10.1016/0042-6822(81)90104-5. [DOI] [PubMed] [Google Scholar]
- Diener T. O. Are viroids escaped introns? Proc Natl Acad Sci U S A. 1981 Aug;78(8):5014–5015. doi: 10.1073/pnas.78.8.5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan J. B., Pastan I., de Crombrugghe B. Sequence rearrangement and duplication of double stranded fibronectin cDNA probably occurring during cDNA synthesis by AMV reverse transcriptase and Escherichia coli DNA polymerase I. Nucleic Acids Res. 1980 Jul 11;8(13):3055–3064. doi: 10.1093/nar/8.13.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried M., Klein B., Murray K., Greenaway P., Tooze J., Boll W., Weissmann C. Infectivity in mouse fibroblasts of polyoma DNA integrated into plasmid pBR322 or lambdoid phage DNA. Nature. 1979 Jun 28;279(5716):811–816. doi: 10.1038/279811a0. [DOI] [PubMed] [Google Scholar]
- Ghosh P. K., Reddy V. B., Piatak M., Lebowitz P., Weissman S. M. Determination of RNA sequences by primer directed synthesis and sequencing of their cDNA transcripts. Methods Enzymol. 1980;65(1):580–595. doi: 10.1016/s0076-6879(80)65061-7. [DOI] [PubMed] [Google Scholar]
- Grill L. K., Semancik J. S. RNA sequences complementary to citrus exocortis viroid in nucleic acid preparations from infected Gynura aurantiaca. Proc Natl Acad Sci U S A. 1978 Feb;75(2):896–900. doi: 10.1073/pnas.75.2.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross H. J., Domdey H., Lossow C., Jank P., Raba M., Alberty H., Sänger H. L. Nucleotide sequence and secondary structure of potato spindle tuber viroid. Nature. 1978 May 18;273(5659):203–208. doi: 10.1038/273203a0. [DOI] [PubMed] [Google Scholar]
- Gross H. J., Krupp G., Domdey H., Raba M., Jank P., Lossow C., Alberty H., Ramm K., Sänger H. L. Nucleotide sequence and secondary structure of citrus exocortis and chrysanthemum stunt viroid. Eur J Biochem. 1982 Jan;121(2):249–257. doi: 10.1111/j.1432-1033.1982.tb05779.x. [DOI] [PubMed] [Google Scholar]
- Gross H. J., Liebl U., Alberty H., Krupp G., Domdey H., Ramm K., Sänger H. L. A severe and a mild potato spindle tuber viroid isolate differ in three nucleotide exchanges only. Biosci Rep. 1981 Mar;1(3):235–241. doi: 10.1007/BF01114910. [DOI] [PubMed] [Google Scholar]
- Gross H. J., Riesner D. Viroids: a class of subviral pathogens. Angew Chem Int Ed Engl. 1980;19(4):231–243. doi: 10.1002/anie.198002313. [DOI] [PubMed] [Google Scholar]
- Hadidi A., Cress D. E., Diener T. O. Nuclear DNA from uninfected or potato spindle tuber viroid-infected tomato plants contains no detectable sequences complementary to cloned double-stranded viroid cDNA. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6932–6935. doi: 10.1073/pnas.78.11.6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseloff J., Symons R. H. Chrysanthemum stunt viroid: primary sequence and secondary structure. Nucleic Acids Res. 1981 Jun 25;9(12):2741–2752. doi: 10.1093/nar/9.12.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidecker G., Messing J., Gronenborn B. A versatile primer for DNA sequencing in the M13mp2 cloning system. Gene. 1980 Jun;10(1):69–73. doi: 10.1016/0378-1119(80)90145-6. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Lebeurier G., Hirth L., Hohn B., Hohn T. In vivo recombination of cauliflower mosaic virus DNA. Proc Natl Acad Sci U S A. 1982 May;79(9):2932–2936. doi: 10.1073/pnas.79.9.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Gronenborn B., Müller-Hill B., Hans Hopschneider P. Filamentous coliphage M13 as a cloning vehicle: insertion of a HindII fragment of the lac regulatory region in M13 replicative form in vitro. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3642–3646. doi: 10.1073/pnas.74.9.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Owens R. A., Cress D. E. Molecular cloning and characterization of potato spindle tuber viroid cDNA sequences. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5302–5306. doi: 10.1073/pnas.77.9.5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens R. A., Diener T. O. RNA intermediates in potato spindle tuber viroid replication. Proc Natl Acad Sci U S A. 1982 Jan;79(1):113–117. doi: 10.1073/pnas.79.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens R. A., Diener T. O. Sensitive and rapid diagnosis of potato spindle tuber viroid disease by nucleic Acid hybridization. Science. 1981 Aug 7;213(4508):670–672. doi: 10.1126/science.213.4508.670. [DOI] [PubMed] [Google Scholar]
- Racaniello V. R., Baltimore D. Cloned poliovirus complementary DNA is infectious in mammalian cells. Science. 1981 Nov 20;214(4523):916–919. doi: 10.1126/science.6272391. [DOI] [PubMed] [Google Scholar]
- Schaffner W. Direct transfer of cloned genes from bacteria to mammalian cells. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2163–2167. doi: 10.1073/pnas.77.4.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortle D., DiMaio D., Nathans D. Directed mutagenesis. Annu Rev Genet. 1981;15:265–294. doi: 10.1146/annurev.ge.15.120181.001405. [DOI] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D. The bidirectional transfer of DNA and RNA to nitrocellulose or diazobenzyloxymethyl-paper. Anal Biochem. 1980 Nov 15;109(1):123–129. doi: 10.1016/0003-2697(80)90019-6. [DOI] [PubMed] [Google Scholar]
- Symons R. H. Avocado sunblotch viroid: primary sequence and proposed secondary structure. Nucleic Acids Res. 1981 Dec 11;9(23):6527–6537. doi: 10.1093/nar/9.23.6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi T., Palmieri M., Weissmann C. QB DNA-containing hybrid plasmids giving rise to QB phage formation in the bacterial host. Nature. 1978 Jul 20;274(5668):223–228. doi: 10.1038/274223a0. [DOI] [PubMed] [Google Scholar]
- Visvader J. E., Gould A. R., Bruening G. E., Symons R. H. Citrus exocortis viroid: nucleotide sequence and secondary structure of an Australian isolate. FEBS Lett. 1982 Jan 25;137(2):288–292. doi: 10.1016/0014-5793(82)80369-4. [DOI] [PubMed] [Google Scholar]
- van Wezenbeek P., Vos P., van Boom J., van Kammen A. Molecular cloning and characterization of a complete DNA copy of potato spindle tuber viroid RNA. Nucleic Acids Res. 1982 Dec 20;10(24):7947–7957. doi: 10.1093/nar/10.24.7947. [DOI] [PMC free article] [PubMed] [Google Scholar]