Abstract
The use of telaprevir and boceprevir, both protease inhibitors (PI), as part of the specifically targeted antiviral therapy for hepatitis C (STAT-C) has significantly improved sustained virologic response (SVR) rates. However, different clinical studies have also identified several mutations associated with viral resistance to both PIs. In the absence of selective pressure, drug-resistant hepatitis C virus (HCV) mutants are generally present at low frequency, making mutation detection challenging. Here, we describe a mismatch amplification mutation assay (MAMA) PCR method for the specific detection of naturally occurring drug-resistant HCV mutants. MAMA PCR successfully identified the corresponding HCV variants, while conventional methods such as direct sequencing, endpoint limiting dilution (EPLD), and bacterial cloning were not sensitive enough to detect circulating drug-resistant mutants in clinical specimens. Ultradeep pyrosequencing was used to confirm the presence of the corresponding HCV mutants. In treatment-naïve patients, the frequency of all resistant variants was below 1%. Deep amplicon sequencing allowed a detailed analysis of the structure of the viral population among these patients, showing that the evolution of the NS3 is limited to a rather small sequence space. Monitoring of HCV drug resistance before and during treatment is likely to provide important information for management of patients undergoing anti-HCV therapy.
INTRODUCTION
Hepatitis C virus (HCV) is a positive-polarity, single-stranded RNA virus belonging to the genus Hepacivirus in the family Flaviviridae (17). Globally, approximately 130 million people have been already infected and approximately 3 million new infections occur annually (2), many of which develop into severe liver disease such as cirrhosis and hepatocellular carcinoma (8, 11).
Treatment regimens for chronic hepatitis C have significantly improved during the last decade, resulting in higher sustained virologic response (SVR) rates. The dual anti-HCV therapy is based on administration of long-acting pegylated alpha interferon (IFN) and ribavirin (RBV). Unfortunately, this therapeutic strategy is effective in only ∼50% of patients infected with HCV genotype 1, although much higher rates are reached in individuals infected with other viral genotypes (1, 27). Consensus interferon, a synthetic recombinant type I IFN derived from the most common amino acids found in IFN-α subtypes, has been shown to be useful in the management of patients who have previously failed to respond to the conventional therapy (12). In spite of the improved SVR, a number of adverse reactions to the IFN/RBV therapy are known, including dose- and treatment-limiting reactions such as depression, hematological “cytopenias,” thyroid dysfunction, and skin rash, making the treatment not well tolerated in many cases. Consequently, in addition to specialized nurse practitioner services, access to psychological, endocrinal, hematological, and possibly dermatological services is required for patients undergoing anti-HCV treatment (27). Thus, it is very hard for individuals to undergo this kind of treatment, which is often accompanied by burdensome side effects and, sorrowfully, is unsuccessful in roughly half of cases. Therefore, the emergence of novel agents with potentially higher antiviral activity and milder side effects is of utmost importance for the appropriate management of HCV cases.
The development of specifically targeted antiviral therapies for hepatitis C (STAT-C) is expected to significantly expand the “pool” of antiviral drugs available for HCV control. The advent of several direct-acting antiviral (DAA) agents, such as HCV-specific polymerase and protease inhibitors (PI), in the near future is anticipated with high expectations and hopes of improved SVR rates. Two different linear peptidomimetic ketoamides, boceprevir and telaprevir, have been recently approved for HCV treatment by the US Food and Drug Administration (7). Clinical studies conducted among treatment-naïve patients infected with genotype 1 showed that triple therapy with the PIs, IFN, and RBV significantly improved SVR in comparison with the standard dual-treatment regimen. Moreover, it has been suggested that the new regimen might lead to a shorter duration of treatment among those patients achieving a rapid virologic response (13). However, the emergence of drug-resistant variants, due to a high viral replication rate and the presence of an error-prone RNA polymerase with no proofreading activity, is a major issue with STAT-C (30). Thus, minor variants with a resistant phenotype outcompete wild-type viruses in the presence of a given drug, becoming the predominant species during the course of treatment. This remodeling of the structure of the viral population has been extensively investigated, and different studies have identified diverse mutations associated with viral resistance to both approved PIs (24, 25). The mutations associated with telaprevir in vivo that are most frequently observed are single changes at positions V36A/M, T54A, R155K/T, and A156V/T/S or combinations at positions 36/155 or positions 36/156 (24). Boceprevir-resistant mutations included changes at positions V55A and V170A in addition to the previously known telaprevir mutations at positions V36A/M, T54A/S, R155K/T, and A156S (25). Development of drug-resistant HCV mutants generally occurs shortly after starting therapy, suggesting that generation of such viral variants is the result of purifying selection of preexisting resistant viruses, consequently leading to treatment failure (16). Thus, monitoring of resistant HCV mutants among individuals undergoing anti-HCV therapy is of importance to define the course of treatment. However, in the absence of selective pressure (antiviral therapy), HCV variants bearing mutations conferring resistance are generally present at a very low frequency within the viral population, making mutation detection extremely challenging. Mismatch amplification mutation assay (MAMA) PCR is a sensitive methodology that has been widely used for the detection of single nucleotide polymorphisms (SNPs) in a variety of settings (6, 23).
The goal of this work was to develop a rapid, highly specific, and sensitive assay suitable for the identification of the known telaprevir- and boceprevir-resistant HCV mutants on the basis of mismatch amplification mutation assay (MAMA) PCR. This method was validated in clinical samples from treatment-naïve HCV patients. Comparison with endpoint limiting dilution (EPLD) and bacterial cloning was carried out. Confirmation of HCV mutants by ultradeep amplicon pyrosequencing using GS FLX Titanium technology was also performed. The results suggest that monitoring of HCV resistance before and during treatment is feasible with MAMA PCR and deep amplicon sequencing. Early identification of drug-resistant HCV variants might provide important information for management of patients undergoing anti-HCV therapy.
MATERIALS AND METHODS
Patients.
Four patients with chronic HCV, aged 40 to 58 years, were enrolled in this study. All patients were anti-HCV treatment naïve. Ethical reviews were performed and informed consent approval was granted by the Ethical Committee of the Mexican Institute for Epidemiological Diagnosis and Reference (InDRE). Informed consent was obtained from all subjects. Patients' blood samples were collected by venipuncture and stored in PPT Vacutainer tubes (Becton Dickinson, Franklin Lakes, NJ) and processed according to the manufacturer's recommendations. The blood collection tubes were centrifuged at 1,800 × g for 20 min at room temperature, and plasma was then gently removed by pipetting. Plasma and blood samples (200 μl) from all subjects were stored at −70°C until use. Patients' characteristics are summarized in Table 1.
Table 1.
Patient characteristics
| Patient | Gender | Age (yr) | HCV genotype | IL28B genotype |
|
|---|---|---|---|---|---|
| rs8099917 | rs12979860 | ||||
| 1 | Female | 40 | 1b | TG | TC |
| 2 | Female | 58 | 1b | TT | TT |
| 3 | Male | 46 | 1b | TT | CC |
| 4 | Female | 54 | 1b | TT | TT |
IL28B genotyping.
IL28B genotyping was performed by melt-MAMA PCR as reported elsewhere (9). Briefly, DNA was extracted from whole blood (200 μl) using a QIAamp DNA blood kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. The purified material (50 ng) was subjected to melt-MAMA PCR for the identification of IL28B SNPs. PCR amplification was carried out in a final reaction volume of 20 μl using LightCycler 480 SYBR green I Master (Roche Applied Science, Indianapolis, IN) and primers (0.5 μM each). The PCR was carried out on a LightCycler 480 system (Roche) under the following conditions: pre-PCR for 5 min at 95°C, followed by 40 amplification cycles of 95°C for 5 s, 60°C for 5 s, and 72°C for 15 s. PCR amplicons were resolved by melting curve (MC) analysis starting at 70°C, with incremental steps of 1°C. SNP analysis was performed using LightCycler 480 software (Roche).
Design of HCV NS3 mutant-specific primers.
A comprehensive nucleotide alignment of the HCV NS3 region containing representative sequences was obtained from the Los Alamos National Laboratory HCV database (http://hcv.lanl.gov/content/sequence/NEWALIGN/align.html). The optimized alignment for genotype 1, particularly for subtypes 1a and 1b, was then analyzed using the Lasergene DNA and protein analysis package, version 8.0.2 (DNASTAR Inc., Madison, WI), in order to design specific primer sets for each mutant (mutant-specific primers) based on their respective nucleotide patterns (Table 2). The 3′ ends in all mutant-specific primers were designed to carry the complementary nucleotide to the corresponding mutation. Additionally, one adjacent mismatch, at the penultimate nucleotide position on the 3′ end, was also incorporated into the mutant-specific primer in order to prevent extension and subsequent amplification of wild-type viruses, increasing primer specificity and enhancing discrimination between mutants and wild-type viruses.
Table 2.
Primer sequences
| Primer designation | Sequence |
|
|---|---|---|
| Mutant-specific primera | Opposite primer | |
| MAMA PCR | ||
| V36A-F | GTCGAGGGGGAGGTTCAAGTGCC | GGAATGACATCAGCATGCCTCGTGAC |
| V36M-F | GGTCGAGGGGGAGGTTCAAGTGATG | |
| T54A-F | CTGCATCAACGGCGTGTGTTGCG | CCGGCGCACCGGAATGACATC |
| V55A-F | CAACGGCGTGTGTTGGACCCG | |
| R155K-R | CCGGGTGCACACAGCAGCATT | AGGCATGCTGATGTCATTCCGGTG |
| R155T-R | CCGGGTGCACACAGCAGCAG | |
| A156S(T)-F | CCGTGGGCATCTTCAGGGCT | GTGCTCTTACCGCTGCCGGTG |
| A156S(C)-F | CCGTGGGCATCTTCAGGGCC | |
| A156T-R | CCCCGGGTGCACACAGCACT | AGGCATGCTGATGTCATTCCGGTG |
| A156V-R | CCCCGGGTGCACACAGCCA | |
| V170A-R | TGGTAGTTTCCATGGACTCAACGGGAG | |
| Ultradeep sequencing | ||
| NS3 universalb | ACGGCCTACGCCCAGCAGAC | GAGGAGTTGTCCGTGAACACCGG |
| NS3AMID1F2 | CGTATCGCCTCCCTCGCGCCATCAGACGAGTGCGTACGGCCTACGCCCAGCAGAC | |
| NS3AMID1R2 | CTATGCGCCTTGCCAGCCCGCTCAGACGAGTGCGTGAGGAGTTGTCCGTGAACACCGG | |
| NS3AMID2F2 | CGTATCGCCTCCCTCGCGCCATCAGACGCTCGACAACGGCCTACGCCCAGCAGAC | |
| NS3AMID2R2 | CTATGCGCCTTGCCAGCCCGCTCAGACGCTCGACAGAGGAGTTGTCCGTGAACACCGG | |
| NS3AMID3F2 | CGTATCGCCTCCCTCGCGCCATCAGAGACGCACTCACGGCCTACGCCCAGCAGAC | |
| NS3AMID3R2 | CTATGCGCCTTGCCAGCCCGCTCAGAGACGCACTCGAGGAGTTGTCCGTGAACACCGG | |
| NS3AMID4F2 | CGTATCGCCTCCCTCGCGCCATCAGAGCACTGTAGACGGCCTACGCCCAGCAGAC | |
| NS3AMID4R2 | CTATGCGCCTTGCCAGCCCGCTCAGAGCACTGTAGGAGGAGTTGTCCGTGAACACCGG | |
Sequences in boldface and italic represent nucleotide positions associated with mutation detection.
Nucleotide position with respect to reference sequence NC004102. Forward primer nucleotide position, 3429 to 3448; reverse primer nucleotide position, 3963 to 3985.
Artificial constructions bearing drug-resistant HCV mutants.
Initially, we constructed different plasmids bearing all known HCV mutations conferring resistance to both telaprevir and boceprevir. The strategy used to clone and mutate the corresponding nucleotide position has been reported by our group previously (9). Plasmids were purified and sequenced to confirm the presence of the corresponding mutations.
Bacterial cloning.
Total nucleic acid from all four clinical samples was extracted in a MagNAPure LC system (Roche) using a Total Nucleic Acid Isolation kit (Roche). cDNA synthesis was carried out using a SuperScript VILO cDNA Synthesis kit (Invitrogen, Carlsbad, CA). PCR amplification using specific HCV NS3 universal primers (Table 2) was carried out using a LightCycler 480 and the following PCR conditions: pre-PCR for 5 min at 95°C, followed by 40 amplification cycles of 95°C for 15 s, 50°C for 15 s, and 72°C for 35 s. PCR amplicons were resolved and analyzed as described above. The resulting PCR amplicons were purified using SizeSelect gels (Invitrogen) and inserted into the pCR2.1-TOPO plasmid vector, according to the instructions supplied with the TOPO TA cloning kit (Invitrogen). The plasmid vectors were used to transform One Shot electrocompetent Escherichia coli cells. Transformed cells were spread on selective plates and incubated for 8 h at 37°C; subsequently, multiple clones (30 to 45) from each patient were subjected to colony PCR using the corresponding universal primers. Amplicons from PCR-positive clones were subjected to Sanger sequencing.
Endpoint limiting dilution PCR (EPLD).
Analysis of the intrahost viral population was assessed by using an adaptation of our previously reported method (23). Briefly, cDNA synthesis was performed as mentioned above. cDNA log dilutions were prepared in quadruplicate for each sample. Subsequently, all cDNA dilutions were subjected to real-time PCR as mentioned above. The endpoint was defined as the last dilution where two out of four reactions were successfully amplified. Dilutions meeting these criteria are expected to have amplified from a single cDNA molecule. The original cDNA was then diluted in order to reach the endpoint dilution, and 96 individual PCRs were prepared. Under these conditions, ∼50% of the reactions were PCR positive. All PCR-positive clones were recovered and subjected to Sanger sequencing.
MAMA PCR.
cDNA from all clinical samples was subjected to MAMA PCR for the identification of all known drug-resistant HCV mutants by the use of the mutant-specific primers (Table 2). PCR amplification was carried out in a final reaction volume of 20 μl using LightCycler 480 SYBR green I Master (Roche) and the primers (1 μM each). The PCR was carried out under the following conditions: pre-PCR for 5 min at 95°C, followed by 40 amplification cycles of 95°C for 5 s, 60°C for 5 s, and 72°C for 15 s. PCR amplicons were resolved by MC analysis as described above. Mutant detection was performed by the identification of specific melting peaks using the LightCycler 480 software (Roche).
Sanger sequencing.
Direct amplicon sequencing from all PCR and bacterial clones was performed. For this purpose, an amplification-and-dilution approach was carried out. PCR products were diluted 1:20 in molecular analysis-grade water prior to sequencing. Both DNA strands were subjected to Sanger sequencing using BigDye version 3.1 chemistry (Applied Biosystems, Carlsbad, CA) following the manufacturer's recommendations and the corresponding forward or reverse primer. The resulting sequencing products were later processed on a 3130xl Genetic Analyzer (Applied Biosystems). Sequences were assembled and analyzed using SeqMan and MegAlign and the Lasergene DNA & protein analysis package, version 8.0.2 (DNASTAR).
Ultradeep pyrosequencing.
Amplicon deep sequencing was performed on a 454/Roche GS FLX platform following the manufacturer's instructions. Each sample was amplified independently with fusion primers, including the 454 primer keys (A and B for forward and reverse primers, respectively), a different multiple identifier (MID) for each sample, and the HCV NS3-specific primers (Table 2). The PCR products were resolved and purified by agarose gel electrophoresis on SizeSelect e-gels. The quality of the amplicons was assessed on a 3100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). Purified amplicons were quantified using a Quan-iT PicoGreen double-stranded DNA (dsDNA) assay kit (Invitrogen). PCR amplicons were mixed at equimolar concentrations and diluted to a final concentration of 107 molecules/μl prior to being subjected to emulsion PCR (emPCR). emPCR for both strands was performed following the instructions supplied with the kit. Enriched beads were subjected to pyrosequencing (Titanium chemistry) using the 454/Roche GS FLX instrument. The processing scheme was set up for full processing for amplicon libraries. The original sequence reads (raw data) were processed using the SFFFILE tools. Sequence reads belonging to each sample were identified and separated by the corresponding MID. The numbers of sequence reads per sample were 18,041, 18,155, 18,478, and 22,310 reads for patients 1, 2, 3, and 4, respectively. The sfffile tool (sffinfo) was used to obtain the fasta and qual files for each of the independent samples. Denoising of data sets was carried out using flow clustering as implemented in QIIME (5). Reads were separated by length in order to identify the coexistence of two or more mutations in individual variants. Thus, only long reads covering the entire length of the amplicon were included in the analysis.
Sequence analysis.
Multiple alignment was performed using MUSCLE version 3.8.31 (http://www.drive5.com/muscle/downloads.htm). Sequence reads were then analyzed using MEGA 5 (26). Median-joining network analysis was carried out as described elsewhere (22).
RESULTS
Consensus sequencing does not reflect the presence of drug-resistant HCV mutants in treatment-naïve patients.
Four HCV treatment-naïve individuals were tested for drug-resistant HCV mutants by direct amplicon sequencing (Sanger sequencing). All plasma samples were amplified using the HCV NS3 universal primers. The consensus sequences did not show the presence of any of the known drug-resistant HCV mutations (Table 3).
Table 3.
Drug-resistant HCV mutant detection by MAMA PCR and standard cloning and sequencing methods
| Patient no. and detection method | Mutation |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| V36 |
T54 (A) | V55 (A) | R155 |
A156 |
V170 (A) | |||||
| A | M | K | S | T | T | V | ||||
| 1 | ||||||||||
| MAMA PCR | + | − | − | − | − | + | − | − | + | − |
| Consensus sequencing | − | − | − | − | − | − | − | − | − | − |
| EPLD | − | − | − | − | − | − | − | − | − | − |
| Bacterial cloning | − | − | − | − | − | − | − | − | − | − |
| 2 | ||||||||||
| MAMA PCR | + | − | + | + | − | − | − | − | − | − |
| Consensus sequencing | − | − | − | − | − | − | − | − | − | − |
| EPLD | − | − | − | − | − | − | − | − | − | − |
| Bacterial cloning | − | − | − | − | − | − | − | − | − | − |
| 3 | ||||||||||
| MAMA PCR | + | − | − | − | − | − | − | − | − | − |
| Consensus sequencing | − | − | − | − | − | − | − | − | − | − |
| EPLD | − | − | − | − | − | − | − | − | − | − |
| Bacterial cloning | − | − | − | − | − | − | − | − | − | − |
| 4 | ||||||||||
| MAMA PCR | + | − | + | − | − | − | − | + | − | − |
| Consensus sequencing | − | − | − | − | − | − | − | − | − | − |
| EPLD | − | − | − | − | − | − | − | − | − | − |
| Bacterial cloning | − | − | − | − | − | − | − | − | − | − |
MAMA PCR allows the identification of circulating drug-resistant HCV mutants in clinical samples.
Our group has successfully designed several methods based on MAMA PCR for the identification of different SNPs in multiple settings (9, 23). Using this approach, we designed MAMA PCR primers for the identification of the known drug-resistant HCV mutants. Primers bearing one single mismatch position at the penultimate position (Table 2) were designed according to the guidelines reported by others (18). Implementation of the corresponding PCR protocol was carried out using artificial constructions bearing the appropriate nucleotide mutations. The validation process showed that the primers were able to differentiate between wild-type viruses and HCV mutants under experimental conditions using the plasmids mimicking the nucleotide changes observed among drug-resistant variants (data not shown). The analytical sensitivity of the MAMA PCR assay ranged between 10 and 20 copies per reaction. Evaluation of clinical specimens was conducted using samples from four different HCV treatment-naïve cases (Table 1). MAMA PCR identified several drug-resistant HCV mutants in this set of specimens (Table 3). Three patients harbored multiple mutants, while only one individual showed the presence of a single mutant. Three different drug-resistant HCV mutations (V36A, R155S, and A156V) were identified in virus from patient 1. Likewise, patient 2 showed several drug-resistant variants, including V36A, T54A, and V55A. Mutations found in virus from patient 4 included V36A, T54A, and A156T. Patient 3 was the only one to exhibit a single drug-resistant mutation (V36A).
Conventional EPLD and bacterial cloning lack the sensitivity to accurately detect the presence of low-frequency drug-resistant HCV mutants.
Two different approaches were used to confirm the presence of the corresponding mutants in these clinical samples. First, an adaptation of the endpoint limiting dilution protocol was used to assess the intrahost variation in the NS3 region (23). Multiple PCR clones (30, 37, 45, and 32 for patients 1, 2, 3, and 4, respectively) were identified and sequenced. None of the PCR clones analyzed showed the presence of any of the known drug-resistant HCV mutants (Table 3). As an alternative, bacterial cloning was used to identify the circulation of the aforementioned mutants. Multiple bacterial colonies (30 per patient) were subjected to colony PCR. All PCR-positive colonies were subsequently sequenced. Similarly to EPLD, bacterial cloning analysis did not show the presence of the reported HCV mutants (Table 3). Thus, it seems that the conventional methods used to address the structure of the viral population might not be sensitive enough to identify the circulation of drug-resistant HCV mutants in clinical samples before the start of therapy when the corresponding mutants are expected to be present at a very low frequency.
Ultradeep sequencing accurately reflects the presence of circulating HCV mutants in clinical samples.
Ultradeep pyrosequencing was used as an alternative to overcome the limitations of consensus sequencing, EPLD, and bacterial cloning. All four samples were subjected to amplicon ultradeep sequencing using the Titanium chemistry to warrant long reads. Ultradeep sequencing successfully identified the low-frequency HCV mutant variants present in these clinical samples (Table 3). The results of the MAMA PCR and ultradeep sequencing analyses were concordant, with the exception of the results determined for patient 1, where MAMA PCR identified mutation R155S but ultradeep sequencing did not detect the mutation.
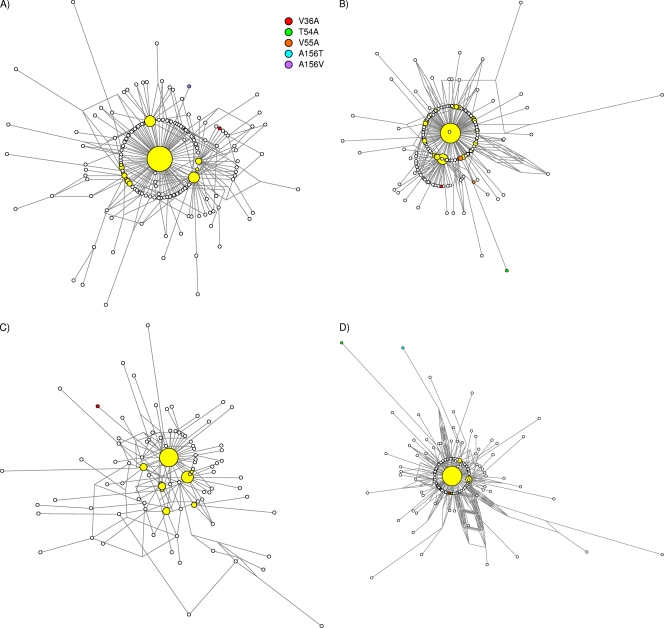
Median-joining network analysis was used to assess the structure of the viral population in these four specimens (Fig. 1). The details of the architecture of the networks, characterized by a major variant at the center of the network surrounded by several minor species, were similar for all four patients. The major variants (in the master sequence) in each patient (44%, 36%, 26%, and 44% for patients 1, 2, 3, and 4, respectively) unquestionably represented the most predominant species. Other secondary variants ranged between 2% and 11%. All minor variants were genetically close to the master sequence; the average nucleotide distances within each population were 3.2, 3.7, 5.5, and 3.7 for patients 1, 2, 3, and 4, respectively. In general, the variants bearing drug-resistant mutants included very few additional nucleotide substitutions. The exception was patient 4, for whom the variants that included mutations T54A and A156T were more distant than the average for that particular viral population. No combinatory mutations within the same variant were observed in any of the patients.
Fig 1.
Median-joining network analysis. Median-joining network analysis was conducted to assess the architecture of the viral population in each patient (A, patient 1; B, patient 2; C, patient 3; D, patient 4). Sequence reads from ultradeep amplicon sequencing were aligned, and the frequency of each was recorded. Thus, in the network, each node represents a unique haplotype within the viral population. The size of the node reflects the frequency (expressed as a percentage) of each haplotype. White nodes illustrate variants with a frequency ≤ 1%. The length of the link represents the nucleotide differences between two different haplotypes. Drug-resistant HCV mutants are depicted in different colors. Yellow nodes depict the major variants. The main haplotypes (major variants) were located at the center of network in all viral populations.
DISCUSSION
Here, we have described a MAMA PCR method for the specific detection of drug-resistant HCV mutants and the implementation of ultradeep sequencing to analyze the complexity of the viral population. The results showed that the MAMA PCR was superior to both EPLD and bacterial cloning for mutation detection. Comparison against ultradeep pyrosequencing showed concordance between the two methods. However, MAMA PCR was able to detect mutation R155S in one sample whereas the same mutant was not detected by any other method, including ultradeep sequencing.
Different PIs have been developed and evaluated in clinical trials. As a result, boceprevir and telaprevir have both been approved to be added to the existing IFN/RBV therapy (7). Incorporation of PIs into the conventional HCV treatment has improved SVR in both treatment-naïve and -experienced individuals and, at least in some cases, shortened treatment duration (3, 7, 15, 20, 31). However, the rapid emergence of drug-resistant mutants jeopardizes the effectiveness of STAT-C. Thus, monitoring of HCV drug-resistant variants is important to determine the course of therapy.
The simplicity of MAMA PCR methods enormously facilitates implementation in clinical and research laboratories. Here, MAMA PCR was found to be more sensitive than consensus sequencing, EPLD, and conventional bacterial cloning for the detection of drug-resistant HCV mutants. Additionally, the simplicity of MAMA PCR makes it superior to other methodologies known to be time-consuming, cumbersome, and costly. Thus, MAMA PCR is a viable alternative for HCV mutant detection (10). One of the most important shortcomings of MAMA PCR is the fact that multiple mutations occurring in the same viral variant cannot be identified. While the detection capabilities of MAMA PCR, as well as its quantitative properties when the method is properly adapted (10, 18), are unquestionable, the unfeasibility of differentiation of combinatory and single mutations taking place within the same molecule is a major drawback. Thus, usage of more advanced technology such as amplicon deep sequencing is more appropriate for a thorough evaluation of the viral population. The second major issue with MAMA PCR is the unspecific priming that can occur due to nucleotide substitution at the primer annealing site in highly variable regions. The performance of MAMA primers heavily relied on the artificial mutations incorporated at the 3′ end. These mismatches significantly enhance discrimination between wild-type and mutant viruses. Thus, when nucleotide substitutions take place at the artificially mutated nucleotide position, the discriminatory power of the MAMA primer may be significantly altered. In the case of HCV RNA polymerase, where proofreading is missing, the occurrence of such changes is, at least to some degree, plausible. Here, one mutant (R155S) was identified by MAMA PCR amplification but not by ultradeep sequencing. While the depth (coverage) might have played some role in the detection of this particular minor variant, we certainly cannot rule out mispriming. Similarly, some variants, including mutations V36M, R155K/T, A156T, and V170A, were not detected by either MAMA PCR or deep sequencing. Thus, the analytical performance of the specific MAMA primers for these mutants is still unknown. More clinical specimens bearing these mutations should be tested to further confirm the usefulness of such primers.
Ultradeep sequencing is a powerful technology that allows analysis of the viral population in great detail. Several reports have described the usage of this methodology in the study of HCV intrahost viral evolution (4, 14, 19, 28, 29). With reads covering the entire length of the amplicon, analysis of combinatory mutations, if present, can be accomplished. Besides identification of double mutants, ultradeep sequencing allows a more detailed portrayal of the viral population architecture. In this set of specimens, a major variant occupying the vast majority of the sequence space of the NS3 region in the population was observed. HCV PI-resistant mutations were organized around the master sequence. In general, all mutations, including the one conferring the drug-resistant phenotype, were only 1 to 2 nucleotides apart from the major variant. This could imply that the NS3 gene may be less tolerant to nucleotide substitutions in comparison to other genomic regions such as the HVR1 or the NS5A (21). Thus, changes occurring in this part of the viral genome might impose a rather high fitness cost. Additionally, no double mutants were identified among these patients. This might imply that double mutants can inflict a higher fitness cost in the absence of selective pressure.
In summary, we report two different methodologies, with several advantages over consensus sequencing, conventional EPLD, and bacterial cloning, for the identification of drug-resistant HCV mutants. The usage of these technologies should help improve understanding of the dynamics of drug-resistant HCV mutants.
Footnotes
Published ahead of print 23 November 2011
REFERENCES
- 1. Alavian SM, Behnava B, Tabatabaei SV. 2010. Comparative efficacy and overall safety of different doses of consensus interferon for treatment of chronic HCV infection: a systematic review and meta-analysis. Eur. J. Clin. Pharmacol. 66:1071–1079 [DOI] [PubMed] [Google Scholar]
- 2. Alter MJ. 2007. Epidemiology of hepatitis C virus infection. World J. Gastroenterol. 13:2436–2441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bacon BR, et al. 2011. Boceprevir for previously treated chronic HCV genotype 1 infection. N. Engl. J. Med. 364:1207–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bull RA, et al. 2011. Sequential bottlenecks drive viral evolution in early acute hepatitis C virus infection. PLoS Pathog. 7:e1002243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Caporaso JG, et al. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7:335–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cha RS, Zarbl H, Keohavong P, Thilly WG. 1992. Mismatch amplification mutation assay (MAMA): application to the c-H-ras gene. PCR Methods Appl. 2:14–20 [DOI] [PubMed] [Google Scholar]
- 7. Dore GJ, Matthews GV, Rockstroh J. 2011. Future of hepatitis C therapy: development of direct-acting antivirals. Curr. Opin. HIV AIDS 6:508–513 [DOI] [PubMed] [Google Scholar]
- 8. Ferlay J, et al. (ed). 2008, posting date GLOBOCAN 2008: cancer incidence and mortality worldwide. IARC CancerBase no. 10. International Agency for Research on Cancer, Lyon, France: http://globocan.iarc.fr/ [Google Scholar]
- 9. Fonseca-Coronado S, et al. 2011. Interleukin-28B genotyping by melt-mismatch amplification mutation assay PCR analysis using single nucleotide polymorphisms rs12979860 and rs8099917, a useful tool for prediction of therapy response in hepatitis C patients. J. Clin. Microbiol. 49:2706–2710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Franco S, et al. 2011. Natural prevalence of HCV minority variants that are highly resistant to NS3/4A protease inhibitors. J. Viral Hepat. 18:e578–e582 [DOI] [PubMed] [Google Scholar]
- 11. Global Burden Of Hepatitis Working Group C 2004. Global burden of disease (GBD) for hepatitis C. J. Clin. Pharmacol. 44:20–29 [DOI] [PubMed] [Google Scholar]
- 12. Gonzalez SA, Keeffe EB. 2009. Management of chronic hepatitis C treatment failures: role of consensus interferon. Biologics 3:141–150 [PMC free article] [PubMed] [Google Scholar]
- 13. Hézode C, et al. 2009. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N. Engl. J. Med. 360:1839–1850 [DOI] [PubMed] [Google Scholar]
- 14. Hiraga N, et al. 29 May 2011, posting date Rapid emergence of telaprevir resistant hepatitis C virus strain from wildtype clone in vivo. Hepatology. doi:10.1002/hep.24460. [Epub ahead of print [DOI] [PubMed] [Google Scholar]
- 15. Jacobson IM, et al. 2011. Telaprevir for previously untreated chronic hepatitis C virus infection. N. Engl. J. Med. 364:2405–2416 [DOI] [PubMed] [Google Scholar]
- 16. Kronenberger B, Zeuzem S. 2009. Treatment of chronic hepatitis C: anticipated impact of resistance in patients treated with protease inhibitors. Curr. Gastroenterol. Rep. 11:15–21 [DOI] [PubMed] [Google Scholar]
- 17. Lemon SM, Walker CM, Alter MJ, Yi M. 2007. Hepatitis C virus, p 1253–1304 In Knipe, Howley D. M. (ed), P. M. Fields virology, vol 1 Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 18. Li B, Kadura I, Fu DJ, Watson DE. 2004. Genotyping with TaqMAMA. Genomics 83:311–320 [DOI] [PubMed] [Google Scholar]
- 19. Merani S, et al. 2011. Effect of immune pressure on hepatitis C virus evolution: insights from a single-source outbreak. Hepatology 53:396–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Poordad F, et al. 2011. Boceprevir for untreated chronic HCV genotype 1 infection. N. Engl. J. Med. 364:1195–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ramachandran S, et al. 2011. Temporal variations in the hepatitis C virus intrahost population during chronic infection. J. Virol. 85:6369–6380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rivera-Osorio P, et al. 2011. Molecular epidemiology of autochthonous dengue virus strains circulating in Mexico. J. Clin. Microbiol. 49:3370–3374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rodríguez-Castillo A, Vaughan G, Ramirez-Gonzalez JE, Escobar-Gutierrez A. 2010. Simultaneous cocirculation of both European varicella-zoster virus genotypes (E1 and E2) in Mexico city. J. Clin. Microbiol. 48:1712–1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sarrazin C, et al. 2007. Dynamic hepatitis C virus genotypic and phenotypic changes in patients treated with the protease inhibitor telaprevir. Gastroenterology 132:1767–1777 [DOI] [PubMed] [Google Scholar]
- 25. Susser S, et al. 2009. Characterization of resistance to the protease inhibitor boceprevir in hepatitis C virus-infected patients. Hepatology 50:1709–1718 [DOI] [PubMed] [Google Scholar]
- 26. Tamura K, et al. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Teoh NC, Farrell GC, Chan HL. 2010. Individualisation of antiviral therapy for chronic hepatitis C. J. Gastroenterol. Hepatol. 25:1206–1216 [DOI] [PubMed] [Google Scholar]
- 28. Verbinnen T, et al. 2010. Tracking the evolution of multiple in vitro hepatitis C virus replicon variants under protease inhibitor selection pressure by 454 deep sequencing. J. Virol. 84:11124–11133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang GP, Sherrill-Mix SA, Chang KM, Quince C, Bushman FD. 2010. Hepatitis C virus transmission bottlenecks analyzed by deep sequencing. J. Virol. 84:6218–6228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yi M, et al. 2006. Mutations conferring resistance to SCH6, a novel hepatitis C virus NS3/4A protease inhibitor. Reduced RNA replication fitness and partial rescue by second-site mutations. J. Biol. Chem. 281:8205–8215 [DOI] [PubMed] [Google Scholar]
- 31. Zeuzem S, et al. 2011. Telaprevir for retreatment of HCV infection. N. Engl. J. Med. 364:2417–2428 [DOI] [PubMed] [Google Scholar]