Abstract
“Classical” Whipple's disease (cWD) is caused by Tropheryma whipplei and is characterized by arthropathy, weight loss, and diarrhea. T. whipplei infectious endocarditis (TWIE) is rarely reported, either in the context of cWD or as isolated TWIE without signs of systemic infection. The frequency of TWIE is unknown, and systematic studies are lacking. Here, we performed an observational cohort study on the incidence of T. whipplei infection in explanted heart valves in two German university centers. Cardiac valves from 1,135 patients were analyzed for bacterial infection using conventional culture techniques, PCR amplification of the bacterial 16S rRNA gene, and subsequent sequencing. T. whipplei-positive heart valves were confirmed by specific PCR, fluorescence in situ hybridization, immunohistochemistry, histological examination, and culture for T. whipplei. Bacterial endocarditis was diagnosed in 255 patients, with streptococci, staphylococci, and enterococci being the main pathogens. T. whipplei was the fourth most frequent pathogen, found in 16 (6.3%) cases, and clearly outnumbered Bartonella quintana, Coxiella burnetii, and members of the HACEK group (Haemophilus species, Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, and Kingella kingae). In this cohort, T. whipplei was the most commonly found pathogen associated with culture-negative infective endocarditis.
INTRODUCTION
Classical Whipple's disease (cWD), first described in 1907, is a chronic multisystemic infectious disease caused by Tropheryma whipplei (31). The typical clinical manifestations of cWD are weight loss, diarrhea, and polyarthralgia (33). Since cWD is rare despite the ubiquitous presence of the pathogen in the environment (21) and the frequent contact of healthy subjects with T. whipplei (9, 27), immunological and genetic host factors seem to predispose subjects to the manifestation of infection (24, 27).
The spectrum of known manifestations of T. whipplei infections has expanded during past years. Besides patients with cWD, T. whipplei has been detected in healthy carriers (9), in individuals with symptomatic but self-limiting infections (30), and in patients with chronic infections restricted to single organs (18). Infectious endocarditis (IE) due to T. whipplei has been reported in the form of case studies or small case series in more than 100 patients, some of them with no signs of cWD (1–3, 5–8, 12–14, 17–19, 22, 23, 29, 34, 35). Since T. whipplei-induced IE (TWIE) often does not meet the major clinical criteria for IE (8), diagnosis is achieved only after invasive procedures by analysis of the explanted heart valves using PCR, culture, and/or (immuno)histology (1–3, 5–8, 12–14, 17–19, 22, 23, 29, 34, 35). Due to the difficulties of diagnosis, no systematic study on the incidence of TWIE has been performed to date.
Here, we present an observational cohort study on the frequency of TWIE at two university centers in Germany. During an 8-year period (2000 to 2007), 16 patients with TWIE—and among those, 14 with isolated TWIE—were identified, which made T. whipplei the most common pathogen associated with culture-negative endocarditis.
MATERIALS AND METHODS
Study population and inclusion criteria.
The three cohorts of patients and the workflow of this observational study are displayed in Fig. 1. We analyzed cardiac valves from patients with valve destruction that required surgery for hemodynamic reasons and/or for the treatment of clinically defined or suspected infective endocarditis (IE). Within this cohort, we describe a total of 16 TWIE patients, 14 with isolated TWIE, only 3 of whom were reported previously (12, 13, 22).
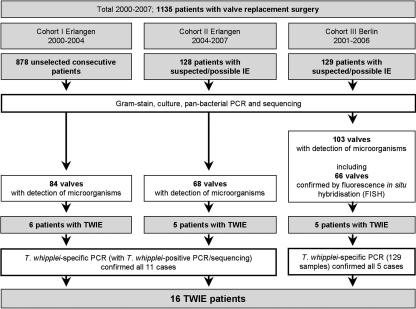
Fig 1.
Details of the study cohorts.
The 16 cases were identified at the University Hospitals of Erlangen and Berlin (total of 1,135 patients) by analysis of explanted heart valves, which had been sent to the microbiological diagnostic laboratories for routine examination (Gram stain, culture, PCR, and, in Berlin, fluorescence in situ hybridization [FISH]). For all patients, valve replacement was necessary due to clinical presentation and valve dysfunction. Cohort I (Erlangen, from 2000 to August 2004) was comprised of 878 patients, which were not selected for suspected IE and which represented 82% of all valve resections performed. Cohort II (Erlangen, September 2004 to January 2007) consisted of 128 preselected patients with suspected or possible IE (based on the clinical presentation of the patient and/or macroscopic appearance of the valves during surgery) (Fig. 1). Cohort III (Berlin, January 2001 to December 2006) included 129 patients with signs of IE. For all patients with proven bacterial infection of the valve, data on clinical or intraoperative signs of vegetations were collected in retrospect.
The diagnosis of IE in our setting was based on the analysis of cardiac valve tissue that had been submitted for routine microbiological analysis due to the following reasons: (i) clinical suspicion of IE according to Duke criteria; (ii) the verification of IE by pathological examination, evidence of IE by echocardiography (floating vegetations), and/or intraoperative detection of macroscopically infected tissue; or (iii) destructed cardiac valves that necessitated valve resection. The diagnosis of TWIE required evidence of T. whipplei within the valve tissue by at least two independent T. whipplei-specific molecular techniques and/or histological methods and the absence of other IE-inducing pathogens. TWIE in the absence of any other clinical symptoms of cWD (e.g., arthritis or gastrointestinal signs) was defined as isolated TWIE.
Diagnostic procedures. (i) Culture of valve specimens.
Valve specimens were cultured on agar plates under aerobic (chocolate agar and tryptone soya agar with 5% sheep blood [Oxoid, Cambridge, United Kingdom]) and anaerobic (tryptone soya agar, 5% sheep blood, hemin, and vitamin K [Oxoid]) conditions as well as in aerobic and anaerobic liquid enrichment cultures. Grown bacteria were identified by an analysis of their biochemical profiles using the Vitek 2 or API system (bioMérieux, Nürtingen, Germany). In two cases, T. whipplei was cultured from snap-frozen valve tissue in axenic medium as described previously (32).
(ii) PCR.
DNA was extracted directly from explanted cardiac valve tissue. Bacterial 16S rRNA genes were amplified by using broad-range PCR (31) followed by sequence analysis. T. whipplei sequences were further confirmed by one T. whipplei-specific PCR in Berlin (22) and by two different T. whipplei-specific PCRs in Erlangen (23S rRNA gene and 16S-23S rRNA intergenic spacer [12]). In both centers, PCR was followed by sequence analysis and a GenBank BLAST search.
(iii) FISH.
Valve specimens were fixed immediately after explantation. Embedding, sectioning, and FISH was performed, as described previously (22), with probe EUB338 for the broad-range detection of bacteria and probe nonEUB338 to exclude nonsense hybridization. T. whipplei was simultaneously detected with the species-specific probe RE-WHIP3 (5′-TATTGCAACCCTCTGTACCA-3′). Actinomyces odontolyticus exhibiting one mismatch at the probe-binding site was included in every experiment as a negative control. Positive FISH results were confirmed by T. whipplei-specific PCR (22) on adjacent tissue sections.
(iv) Histopathology and immunohistochemistry.
Heart valves were stained with hematoxylin-eosin (HE) and periodic acid-Schiff stain (PAS). T. whipplei-specific immunohistochemistry (IHC) was performed as described previously (19). Valves colonized by Enterococcus faecalis, Staphylococcus epidermidis, Lactobacillus paracasei, Staphylococcus aureus, and Streptococcus oralis served as negative controls.
RESULTS
Diagnosis of IE.
In cohort I, 84 of 878 heart valves from unselected patients (9.5%) were positive for bacteria, as detected by bacterial 16S rRNA gene amplification and sequencing (Fig. 1). In cohort II, 68 valve specimens (53.1%) derived from 128 patients that were preselected for suspected or possible IE were positive by PCR for causative bacteria. In cohort III, 103 valves from 129 patients with signs of IE were positive by PCR (79.8%), 66 of which were confirmed by panbacterial, genus-, or species-specific FISH. All FISH results were consistent with findings by 16S rRNA gene amplification and sequencing.
Thus, between 2000 and 2007, for a total of 255 patients, bacterial colonization was diagnosed by comprehensive conventional and molecular microbiological analyses of 1,135 cardiac valves.
Frequency of T. whipplei in IE.
PCR and sequencing, FISH, and culture of cardiac valve tissues revealed a spectrum of pathogens that are typically the main causes of IE, with streptococci, staphylococci, and enterococci found in 93 (36.5%), 93 (36.5%), and 30 (11.8%) of the 255 positive cases of cohorts I, II, and III, respectively. Unexpectedly, T. whipplei was found in the valve tissues from 16 of the 255 patients with bacterium-positive cardiac valves (6.3%), without the detection of other bacteria (Fig. 1). Within the subgroup of patients with clinically suspected IE (cohort II plus cohort III; 257 patients), T. whipplei was detected in 10 of 171 bacterium-positive valves (5.8%), thus at a rate similar to that of the entire collection of explanted valves. Therefore, T. whipplei was the fourth most common pathogen and the most frequently detected fastidious pathogen of classical culture-negative IE in our patients. Internal transcribed spacer (ITS) variants were determined for 11 cases of TWIE and revealed variants 1 (6 cases), 2 (4 cases), and 7 (1 case) (12).
Other pathogens known to cause blood-culture-negative IE, such as Bartonella quintana, Coxiella burnetii, and bacteria of the HACEK group, were identified in only three (1.2%), two (0.8%), and two (0.8%) cases, respectively. Other pathogens identified were Gram-negative rods in six cases (2.4%), Granulicatella-Abiotrophia spp. in four cases (1.6%), Candida spp. in two cases (0.8%), Propionibacterium acnes in two cases (0.8%), and as Lactobacillus paracasei and Gemella haemolysans in one case each (0.4%).
To compare the values from the different diagnostic methods, we further analyzed the data from cohort I only. Within this group of 99 patients with clinically diagnosed endocarditis, peripheral blood cultures, PCR analyses, and cultures of valve tissue yielded positive results in 26, 84, and 26 cases, respectively.
Verification of PCR results.
To corroborate the unexpectedly high incidence of T. whipplei infection, the detection of T. whipplei was confirmed by specific PCR for all samples (Table 1) and, for 11 cases where sufficient material was available, by histological examination, periodic acid-Schiff stain (PAS), or T. whipplei-specific immunohistochemistry, culture, and/or FISH.
Table 1.
Characteristics of TWIE patientsa
| Patient | Sex | Age at diagnosis (yr) | Valve | Presence of gastrointestinal symptoms | Test result |
||
|---|---|---|---|---|---|---|---|
| PCR valve | PAS valve | T. whipplei IHC valve | |||||
| 1b | M | 69 | AV | Nege | Pos | Pos | ND |
| 2c | M | 76 | AV/XE | Neg | Pos | Pos | Pos |
| 3 | M | 65 | MV | Neg | Pos | Pos | Pos |
| 4 | F | 60 | AV | Neg | Pos | Pos | ND |
| 5 | M | 69 | AV | Neg | Pos | ND | ND |
| 6 | M | 72 | AV | Neg | Pos | ND | ND |
| 7d | F | 77 | AV | Neg | Pos | Pos | Pos |
| 8 | M | 63 | AV | Neg | Pos | Pos | Pos |
| 9 | M | 72 | AV | Neg | Pos | Pos | Pos |
| 10 | M | 46 | AV | Neg | Pos | ND | ND |
| 11 | M | 69 | AV | Neg | Pos | Pos | Pos |
| 12 | F | 57 | MV | Neg | Pos | Pos | ND |
| 13 | M | 56 | AV | Neg | Pos | Pos | Pos |
| 14 | M | 59 | AV | Neg | Pos | ND | ND |
| 15 | F | 79 | AV | Posf | Pos | ND | ND |
| 16 | F | 73 | MV | Posf | Pos | ND | ND |
IHC, immunohistochemistry; M, male; F, female; AV, aortic valve; MV, mitral valve; XE, xenograft; ND, not determined, since no appropriate material was available; Pos, positive; Neg, negative.
Patient previously reported in a case report (13).
Patient previously reported in a case report (12).
Patient previously reported in a case report (22).
PAS-negative duodenal biopsy specimens.
PAS-positive duodenal biopsy specimens.
Histology.
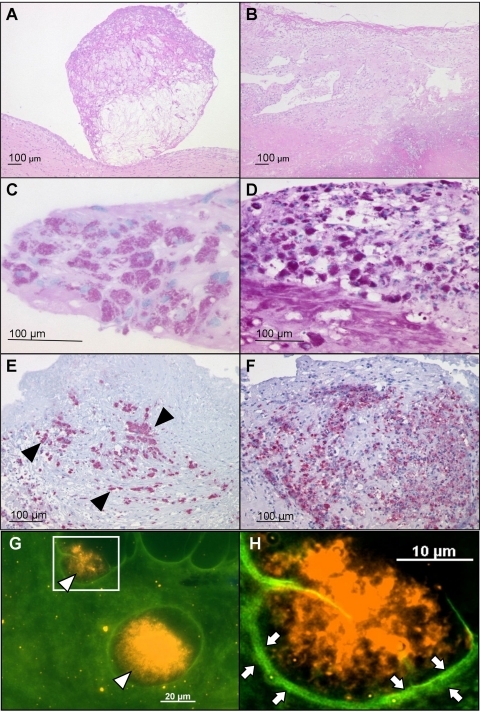
To provide evidence for a causal link between the presence of T. whipplei and the macroscopically observed valve damage, histological analyses were performed. Inflammatory infiltrates with PAS-positive macrophages were seen in all 10 T. whipplei-positive valves analyzed (Table 1 and Fig. 2C and D). Nine valves exhibited polypoid vegetations (Fig. 2A), and seven valves contained granulation tissue. One valve showed a fibrinous exudate. Prominent fibrosis (10 valves) (Fig. 2B) and calcifications (5 valves) pointed to previous degenerative valve disease.
Fig 2.
Histology and FISH of exemplary heart valves of patients with isolated T. whipplei IE. (A and B) HE stainings; (C and D) PAS; (E and F) T. whipplei-specific immunohistochemistry; (G and H) FISH with the specific probe RE-WHIP3. (A) Polypoid vegetation. (B) Fibrotic valve with an infiltrate of foamy macrophages. (C and D) Red-stained PAS-positive macrophages in the stroma. (E and F) Numerous T. whipplei-infected macrophages stained red (exemplary ones are marked with black arrowheads) (alkaline phosphatase–anti-alkaline phosphatase [APAAP] method using Fast Red). (G) FISH with the specific probe RE-WHIP3 of the cardiac valve of a patient with isolated T. whipplei IE. The overlay of the fluorescein isothiocyanate (FITC) and Cy3 filter sets shows clusters of T. whipplei FISH-positive cells (orange, with open arrowheads) in the green autofluorescent background of the tissue. (H) Inset of panel G at a higher magnification. Fibrosis surrounding the bacteria is visible (bright green fluorescence and open arrows).
Immunohistochemistry.
Anti-T. whipplei immunostaining revealed positive results for all 7 samples tested (Table 1 and Fig. 2E and F, where T. whipplei-infected cells are colored red in both stainings). PAS and immunohistochemistry were negative for valve specimens colonized with other bacteria.
FISH.
T. whipplei-specific in situ hybridization which detects rRNA of presumably living bacteria was positive for 1 out of 5 samples (patient 7) (Table 1) and showed T. whipplei in clusters surrounded by fibrotic tissue (Fig. 2G and H, where T. whipplei RNA is shown as orange fluorescence in the green autofluorescent background of the tissue). For this FISH-positive sample, a confirmatory T. whipplei-specific PCR from adjacent sections was positive.
Culture.
T. whipplei was grown from 2 out of 4 samples in axenic medium, as described previously (patients 10 and 11) (Table 1) (32), although the tissue was not optimally preserved for this purpose (e.g., tiny pieces stored frozen for >12 months).
Thus, the primary PCR-based diagnosis of TWIE was confirmed by species-specific PCR in all 16 cases. Eleven cases of TWIE were verified by additional tests: 10 cases by PAS (7 of these 10 cases were also IHC positive, 1 was also FISH positive, and 1 was also culture positive) and 1 additional case by cultivation of T. whipplei.
Clinical characteristics of patients with TWIE.
The clinical data prior to the valve explantation were recorded by using a standardized questionnaire with feedback from 15 of 16 TWIE patients. Patients with TWIE were 66.38 ± 9.0 years old (mean ± standard deviation [SD]) at the time of diagnosis of TWIE. The aortic valve was affected in 13 of the 16 TWIE cases, and the mitral valve was affected in 3 cases.
Clinical symptoms and outcomes.
Two of the TWIE patients suffered from intestinal symptoms typically associated with cWD, which was corroborated by a PAS-positive gastrointestinal biopsy specimen (patients 15 and 16; 0.8% of all identified IE patients) (Table 1). Of 14 isolated TWIE patients without intestinal symptoms (5.5% of all identified IE patients), only one had a gastroscopy, but no PAS-positive macrophages were detected in the duodenum (patient 1) (Table 1) (13).
Only one case of TWIE formally fulfilled the Duke criteria for the diagnosis of IE prior to cardiac valve explantation (patient 11) (Table 1). However, TWIE patients frequently suffered from valvular heart disease prior to the diagnosis of IE (13 patients; 87%), whereas inflammatory arthritis (2 patients; 13%), chronic diarrhea (2 patients; 13%), fever (3 patients; 20%), and weight loss (2 patients; 13%) were only rarely observed. The outcome was fatal for 5 of the 16 TWIE patients (Table 2), who either were untreated (1 of 2 patients), received different antibiotic regimens (1 of 3 patients), or were treated with ceftriaxone followed by trimethoprim-sulfamethoxazole (SXT), as recommended for cWD (3 of 11 patients).
Table 2.
Treatments and outcomes for patients with TWIEa
| Patient | Yr of resection | Treatment | Postoperative outcome and follow-up |
|---|---|---|---|
| 1 | 2000 | 1 yr SXT | Well until July 2000; death following urosepsis and endocarditis due to MRSA |
| 2 | 2000 | Ciprofloxacin, 1 yr SXT | Lost during follow-up after cerebral hemorrhage 3 mo after surgery |
| 3 | 2001 | Ceftriaxone 2 wk i.v.; 1 yr SXT | Well in outcome and at follow-up (6 yr) |
| 4 | 2002 | Ceftriaxone 2 wk i.v.; 1 yr SXT | Well in outcome and at follow-up (6 yr) |
| 5 | 2003 | Ceftriaxone 2 wk i.v.; 1 yr SXT | Well until April 2006 but death due to cardiac arrest |
| 6 | 2003 | None | Well in outcome and follow-up (4 yr) |
| 7 | 2003 | None | Death in 2003 due to cardiac arrest |
| 8 | 2003 | Before surgery, 6 wk of moxifloxacin and amoxicillin; after surgery, different regimens, including caspofungin, ceftazidime, ciprofloxacin, erythromycin, fluconazole, imipenem, linezolid, moxifloxacin, piperacillin, sulbactam, tazobactam, and vancomycin | Death in 2003 due to embolic ischemia of different organs |
| 9 | 2004 | Ceftriaxone 2 wk i.v.; 3 mo doxycycline and 1 yr SXT | Well in outcome and at follow-up (4 yr) |
| 10 | 2005 | Ceftriaxone 4 wk i.v. | Well in outcome and at follow-up (3 yr) |
| 11 | 2005 | 2 wk gentamicin, penicillin plus amoxicillin-sulbactam, and 1 yr SXT | Death in 2006 due to cardiac failure |
| 12 | 2005 | Penicillin, vancomycin, and vancomycin-fosfomycin 6 wk before and after surgery | Well in outcome and at follow-up (4 yr) |
| 13 | 2005 | Before valve resection, 2 wk penicillin-gentamicin; 12 wk penicillin; after valve resection, vancomycin-penicillin, followed by 6 wk penicillin | Well in postoperative outcome; lost during follow-up |
| 14 | 2006 | Ceftriaxone 2 wk i.v.; 1 yr SXT | Well in outcome and at follow-up (3 yr) |
| 15 | 2006 | Ceftriaxone 2 wk i.v.; 1 yr SXT | Well in outcome and at follow-up (3 yr) |
| 16 | 2007 | Ceftriaxone 6 wk, SXT 6 mo | Well in postoperative outcome; lost during follow-up |
Numbering of patients according to Table 1. SXT, trimethoprim-sulfamethoxazole; MRSA, methicillin-resistant Staphylococcus aureus; i.v., intravenous.
DISCUSSION
The microbiological diagnosis of IE is based mainly on blood cultures, which may fail due to previous antimicrobial treatment or infection with fastidious microorganisms, resulting in up to 31% of culture-negative cases (4, 16). Thus, molecular methods for the identification of microorganisms that are difficult to grow are particularly important (11, 16, 28, 34), and the detection of causative pathogens by molecular methods has been suggested to be added to the main Duke criteria for IE (20, 26).
We report on a surprisingly high incidence of TWIE among patients who underwent cardiac surgery for heart valve disease at two German university hospitals. Berlin represents a metropolitan region in Northern Germany, and the Charité-Universitätsmedizin Berlin houses the German reference laboratory for the diagnosis of Whipple's disease, whereas the Universitätsklinikum Erlangen is located in Southern Germany within the metropolitan area of Nuremberg and is a center for the diagnosis and treatment of cardiac diseases. Despite the fact that the incidences of TWIE were very similar in Erlangen cohort II and Berlin cohort III, a regional bias cannot be excluded, as previously discussed (8), since only patients from Germany were investigated. Differences in the regional distribution of TWIE are likely, since cWD is more common in Caucasians, and most of the cases have been reported from Germany, Switzerland, and Eastern France.
T. whipplei was detected in 16 of 255 cases in which IE was diagnosed by cultivation and detection by molecular or histological methods from cardiac valve tissue (6.3%). Importantly, a comparably high incidence of TWIE (5.8%) was found when the analysis was restricted to those patients for whom IE was suspected prior to cardiac surgery (cohort II plus cohort III). This result defines T. whipplei as the most common fastidious pathogen of culture-negative IE in a large group of German patients.
For the primary detection of bacterial DNA, broad-range 16S rRNA gene PCR and, in cohort III, broad-range bacterial FISH were used as routine methods, which exclude a methodological bias toward T. whipplei. To reinforce our PCR findings, we applied additional techniques, which confirmed the T. whipplei infections. In numerous confirmatory tests, only a few negative results were obtained with T. whipplei-specific culture and FISH. Both techniques are quite elaborate methods that require viable or rRNA-rich microorganisms, respectively. Inappropriate sample preservation or sampling error may have contributed to these negative results.
Previous reports suggested T. whipplei to be a cause of culture-negative IE (1–3, 5–8, 11–14, 16–19, 22, 23, 29, 34, 35). T. whipplei was detected in 3.5% (34), 4.3% (2), and 7.1% (14) of IE patients in much smaller series from Northern and Middle Europe and in 0.6% (16) and 2.6% (11), respectively, of IE patients in extensive French cohorts. Since T. whipplei cannot be routinely cultured and since serological tests are still lacking, TWIE will be missed unless molecular techniques are applied. PCR of cardiac valve tissue seems to be the most reliable method for the detection of T. whipplei (11, 18). In addition, an isolated form of TWIE seems to predominate in the German patients, since only 2 of the 16 TWIE patients had symptoms of cWD, and only 1 patient suffered from inflammatory arthritis. In previous reports, arthralgia was a much more prominent symptom of TWIE cases and thus might be a clinical hint toward a diagnosis of TWIE (8). Notably, only one of the TWIE patients fulfilled the Duke criteria for the diagnosis of IE, indicating that TWIE is slowly progressive and lacks markers of acute infection.
Our comprehensive study illustrates that T. whipplei is an important differential diagnosis for culture-negative IE, even in the absence of symptoms of cWD. These data support the suggestion that PCR analysis of cardiac valve tissue should be added to the main Duke criteria for the diagnosis of IE, provided that the data are interpreted within the clinical context and assays are strictly standardized (20, 26).
A reliable and fast diagnosis of TWIE appears to be critical, since the rate of lethality of TWIE has been reported to be 57% (8). Currently, the identification of T. whipplei as the causative agent will be missed in most cases of TWIE, and if it is diagnosed, the treatment recommendation is only empirical and based on the treatment of cWD (15). It is unclear whether treatment for 4 to 6 weeks, as recommended for most forms of IE, with either penicillin G, amoxicillin, ceftriaxone, rifampin, gentamicin, or vancomycin alone or in combination (15) is sufficient to clear an infection by T. whipplei. Alternatively, a cWD-specific treatment that consists of either intravenous ceftriaxone, meropenem, or penicillin G for 2 weeks followed by oral SXT for 12 months or of doxycycline plus hydroxychloroquine orally for at least 18 months (10, 33) may be necessary. Of our 16 patients with TWIE, 14 were treated with antibiotics, 11 of whom were treated with antibiotics recommended for cWD. During the follow-up period, ranging from 1 to 6 years, five patients died due to cardiovascular complications. Since our cohort was comprised of chronically ill and old patients, three patients died despite being treated as recommended for cWD. Although the therapeutic outcome was not within the primary scope of our study and was analyzed only in retrospect, we propose that TWIE should be treated with the same drugs and regimens as those recommended for cWD (33). However, prospective studies regarding the therapy of TWIE are currently not available and are challenging to conduct.
Previous studies from Southern Europe identified Bartonella spp., C. burnetii, Granulicatella spp., Abiotrophia spp., and HACEK microorganisms as major pathogens in culture-negative IE (11, 16). Similar to earlier reports from northern countries (2, 14, 34), these fastidious pathogens were considerably less prevalent in our series, since we identified B. quintana, C. burnetii, Granulicatella-Abiotrophia spp., and bacteria of the HACEK group in only three (1.2%), two (0.8%), four (1.6%), and two (0.8%) cases, respectively. Epidemiologic, geographic, and climatic conditions might influence the prevalence of infectious agents (4, 34). For example, the high rate of IE due to Q fever near Marseille correlated with an exposure to dust contaminated by C. burnetii from animal reservoirs (25), whereas Q fever in Germany is quite rare, especially in urban regions. Likewise, a north-south gradient for the incidence of Bartonella infections has been described (4).
In summary, we have reported the results of an observational cohort study on the frequency of TWIE in two university centers in Germany between 2000 and 2007. T. whipplei was identified as the fourth most frequently found pathogen in 16 (6.3%) cases. It is noteworthy that the diagnosis of TWIE will be missed by conventional bacterial culture techniques, which might explain the few cases reported in the past. Our findings suggest that T. whipplei might be a much more frequent cause of surgically treated culture-negative IE than previously thought.
ACKNOWLEDGMENTS
The study was funded by European Union contract no. QLG1-CT-2002-01049; the German Research Foundation, Bonn, Germany (KFO 104, SFB633); the Interdisciplinary Centre of Clinical Research (IZKF) of the Universitätsklinikum Erlangen, Germany (project grant A14 to C.B.); and the Robert Koch Institute, Berlin, Germany.
We thank Diana Bösel, Nadine Gehrmann, Simone Spieckermann, and Annett Petrich for technical assistance.
Except for D.R., none of the contributing authors has a conflict of interest. D.R. declares a potential conflict of interest since he is the coinventor of two patents: (i) U.S. patent 7,410,787 B1, relating to Whipple's disease, Diagnosis of Whipple's Disease, deposited by Protisvalor Mediterranee, Marseilles, France, and (ii) U.S. patent 7,166,430 B2, relating to T. whipplei, Sequence of the Tropheryma whipplei Bacteria rpoB Gene and Oligonucleotide for Molecular Diagnosis of Whipple's Disease, deposited by Université de la Mediterranee (Aix-Marseille II), Marseille, France.
Footnotes
Published ahead of print 30 November 2011
REFERENCES
- 1.Aiouaz H, et al. 2005. Whipple's disease endocarditis: report of 5 cases and review of the literature. Rev. Med. Interne 26:784–790 (In French.) [DOI] [PubMed] [Google Scholar]
- 2.Bosshard PP, et al. 2003. Etiologic diagnosis of infective endocarditis by broad-range polymerase chain reaction: a 3-year experience. Clin. Infect. Dis. 37:167–172 [DOI] [PubMed] [Google Scholar]
- 3.Brondex A, Jobic Y. 8 January 2011, posting date Infective endocarditis as the only manifestation of Whipple's disease: an atypical presentation. Ann. Cardiol. Angeiol. [Epub ahead of print.] doi:10.1016/j.ancard.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Brouqui P, Raoult D. 2006. New insight into the diagnosis of fastidious bacterial endocarditis. FEMS Immunol. Med. Microbiol. 47:1–13 [DOI] [PubMed] [Google Scholar]
- 5.Chan V, et al. 30 August 2011, posting date Tropheryma whipplei aortic valve endocarditis without systemic Whipple's disease. Int. J. Infect. Dis. [Epub ahead of print.] doi:10.1016/j.ijid.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 6.Dreier J, Szabados F, von Herbay A, Kroger T, Kleesiek K. 2004. Tropheryma whipplei infection of an acellular porcine heart valve bioprosthesis in a patient who did not have intestinal Whipple's disease. J. Clin. Microbiol. 42:4487–4493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Escher R, et al. 2010. Endocarditis due to Tropheryma whipplei: rapid detection, limited genetic diversity, and long-term clinical outcome in a local experience. Clin. Microbiol. Infect. 16:1213–1222 [DOI] [PubMed] [Google Scholar]
- 8.Fenollar F, Lepidi H, Raoult D. 2001. Whipple's endocarditis: review of the literature and comparisons with Q fever, Bartonella infection, and blood culture-positive endocarditis. Clin. Infect. Dis. 33:1309–1316 [DOI] [PubMed] [Google Scholar]
- 9.Fenollar F, et al. 2008. Prevalence of asymptomatic Tropheryma whipplei carriage among humans and nonhuman primates. J. Infect. Dis. 197:880–887 [DOI] [PubMed] [Google Scholar]
- 10.Feurle GE, Junga NS, Marth T. 2010. Efficacy of ceftriaxone or meropenem as initial therapies in Whipple's disease. Gastroenterology 138:478–486 [DOI] [PubMed] [Google Scholar]
- 11.Fournier PE, et al. 2010. Comprehensive diagnostic strategy for blood culture-negative endocarditis: a prospective study of 819 new cases. Clin. Infect. Dis. 51:131–140 [DOI] [PubMed] [Google Scholar]
- 12.Geissdörfer W, Wittmann I, Röllinghoff M, Schoerner C, Bogdan C. 2001. Detection of a new 16S-23S rRNA spacer sequence variant (type 7) of Tropheryma whippelii in a patient with prosthetic aortic valve endocarditis. Eur. J. Clin. Microbiol. Infect. Dis. 20:762–763 [DOI] [PubMed] [Google Scholar]
- 13.Geissdörfer W, et al. 2001. A case of aortic valve disease associated with Tropheryma whippelii infection in the absence of other signs of Whipple's disease. Infection 29:44–47 [DOI] [PubMed] [Google Scholar]
- 14.Grijalva M, Horvath R, Dendis M, Erny J, Benedik J. 2003. Molecular diagnosis of culture negative infective endocarditis: clinical validation in a group of surgically treated patients. Heart 89:263–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Habib G, et al. 2009. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): the Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC). Eur. Heart J. 30:2369–2413 [DOI] [PubMed] [Google Scholar]
- 16.Houpikian P, Raoult D. 2005. Blood culture-negative endocarditis in a reference center: etiologic diagnosis of 348 cases. Medicine (Baltimore) 84:162–173 [DOI] [PubMed] [Google Scholar]
- 17.Kolek M, Zaloudikova B, Freiberger T, Brat R. 2007. Aortic and mitral valve infective endocarditis caused by Tropheryma whipplei and with no gastrointestinal manifestations of Whipple's disease. Klin. Mikrobiol. Infekc. Lek. 13:213–216 (In Czech.) [PubMed] [Google Scholar]
- 18.Lagier JC, Lepidi H, Raoult D, Fenollar F. 2010. Systemic Tropheryma whipplei: clinical presentation of 142 patients with infections diagnosed or confirmed in a reference center. Medicine (Baltimore) 89:337–345 [DOI] [PubMed] [Google Scholar]
- 19.Lepidi H, et al. 2004. Cardiac valves in patients with Whipple endocarditis: microbiological, molecular, quantitative histologic, and immunohistochemical studies of 5 patients. J. Infect. Dis. 190:935–945 [DOI] [PubMed] [Google Scholar]
- 20.Lisby G, Gutschik E, Durack DT. 2002. Molecular methods for diagnosis of infective endocarditis. Infect. Dis. Clin. North Am. 16:393–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maiwald M, Schuhmacher F, Ditton HJ, von Herbay A. 1998. Environmental occurrence of the Whipple's disease bacterium (Tropheryma whippelii). Appl. Environ. Microbiol. 64:760–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mallmann C, et al. 2010. Fluorescence in situ hybridization to improve the diagnosis of endocarditis: a pilot study. Clin. Microbiol. Infect. 16:767–773 [DOI] [PubMed] [Google Scholar]
- 23.Marin M, et al. 2007. Tropheryma whipplei infective endocarditis as the only manifestation of Whipple's disease. J. Clin. Microbiol. 45:2078–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinetti M, et al. 2009. The HLA alleles DRB1*13 and DQB1*06 are associated to Whipple's disease. Gastroenterology 136:2289–2294 [DOI] [PubMed] [Google Scholar]
- 25.Maurin M, Raoult D. 1999. Q fever. Clin. Microbiol. Rev. 12:518–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Millar B, et al. 2001. Molecular diagnosis of infective endocarditis—a new Duke's criterion. Scand. J. Infect. Dis. 33:673–680 [DOI] [PubMed] [Google Scholar]
- 27.Moos V, et al. 2006. Reduced peripheral and mucosal Tropheryma whipplei-specific Th1 response in patients with Whipple's disease. J. Immunol. 177:2015–2022 [DOI] [PubMed] [Google Scholar]
- 28.Moter A, Musci M, Schmiedel D. 2010. Molecular methods for diagnosis of infective endocarditis. Curr. Infect. Dis. Rep. 12:244–252 [DOI] [PubMed] [Google Scholar]
- 29.Raoult D. 1999. Afebrile blood culture-negative endocarditis. Ann. Intern. Med. 131:144–146 [DOI] [PubMed] [Google Scholar]
- 30.Raoult D, et al. 2010. Tropheryma whipplei in children with gastroenteritis. Emerg. Infect. Dis. 16:776–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Relman DA, Schmidt TM, MacDermott RP, Falkow S. 1992. Identification of the uncultured bacillus of Whipple's disease. N. Engl. J. Med. 327:293–301 [DOI] [PubMed] [Google Scholar]
- 32.Renesto P, et al. 2003. Genome-based design of a cell-free culture medium for Tropheryma whipplei. Lancet 362:447–449 [DOI] [PubMed] [Google Scholar]
- 33.Schneider T, et al. 2008. Whipple's disease: new aspects of pathogenesis and treatment. Lancet Infect. Dis. 8:179–190 [DOI] [PubMed] [Google Scholar]
- 34.Voldstedlund M, Norum Pedersen L, Baandrup U, Klaaborg KE, Fuursted K. 2008. Broad-range PCR and sequencing in routine diagnosis of infective endocarditis. APMIS 116:190–198 [DOI] [PubMed] [Google Scholar]
- 35.Whistance RN, Elfarouki GW, Vohra HA, Livesey SA. 2011. A case of Tropheryma whipplei infective endocarditis of the aortic and mitral valves in association with psoriatic arthritis and lumbar discitis. J. Heart Valve Dis. 20:353–356 [PubMed] [Google Scholar]