Abstract
We have developed a novel microsphere-based genotyping method for 46 mucosal human papillomavirus (HPV) types. HPV DNA was amplified by PCR using general primers and typed by hybridization to HPV type-specific probes coupled to sortable microspheres based on the Luminex xMAP technology. Hybridization to each probe was specific for each HPV type without cross-hybridization and sensitive enough to allow typing of HPV contained in clinical specimens. The method was validated with direct sequencing and the Roche Linear Array genotyping method.
TEXT
About 50 human papillomavirus (HPV) types are associated with infections of the genital, anal, and oropharyngeal mucosae (2, 8, 10). A few of these are known to be high-risk oncogenic types and the cause of cervical cancer (16), other anogenital cancers, and head and neck malignancies (3, 8). Detection of high-risk HPV types collectively is being considered as a screening method for cervical cancer, with the promise of improving the sensitivity and cost-effectiveness of cervical cancer screening programs (6). In recent years, there is also mounting evidence for the utility of type-specific identification of HPV type 16 (HVP16) and HPV18, as these two types are significantly associated with persistent infection and lesion progression, thus conferring a higher risk for cancer than other oncogenic types (15). As HPV16 and -18 account for 70% of the cervical cancers worldwide (2), two HPV vaccines targeting these types have been developed and shown to be highly efficacious in preventing both persistent infection with the types and the associated dysplastic changes in the cervical epithelium that lead to malignant transformation (12, 22). Since these vaccines are type specific, it is important to know the distribution of the various HPV types in a population, as well as to have a surveillance system in place to monitor vaccine efficacy over time and any unexpected shifts in the frequency of HPV types not covered by the vaccines. The above highlights the need for and the importance of using type-specific tests for HPV.
Numerous HPV typing methods based on a variety of detection platforms have been described in the literature, and some are commercially available (reviewed in reference 19). At the Canadian National Microbiology Laboratory (NML), we have developed a Luminex-based method (NML Luminex assay) to meet our need for sensitive and extensive typing of HPV in the context of epidemiological and molecular surveillance studies at the national level. The assay is based on the xMAP platform (Luminex Corporation, Austin, TX), and it detects 46 mucosal types. In this report, we describe its design and performance.
HPV DNA either cloned in plasmids (for validation of the probes) or from clinical cervical cancer specimens (for assay validation) was amplified by a nested PCR method using the PGMY primers for the first step (5, 11) and the GP5+/GP6+ primers (7) for the second step. The GP6+ primer (Invitrogen, Burlington, ON, Canada) carried a 5′ biotin label and phosphorothioate bonds in the first 4 nucleotides (nt) on the 5′ end. Exact PCR conditions, which are important for maximum sensitivity of HPV DNA detection, are shown in Table S1 in the supplemental material.
For the detection of the PCR products by the xMAP technology, microspheres labeled with different ratios of red and infrared fluorophores (Luminex Corporation, Austin, TX) were coupled, according to the manufacturer's instructions, to HPV type-specific probes carrying a 5′-C12 amino modification that reacts with the carboxyl groups on the microspheres. For hybridization, PCR products in a total volume of 17 μl in a 96-well PCR microplate (Fisher, Ottawa, ON, Canada) were denatured at 95°C for 10 min; 33 μl of the microsphere mix (15 microspheres/μl for each set) was then added. The samples were incubated at the hybridization temperature of 60°C for 10 min, and after addition of 25 μl of a 0.04-μg/μl solution of streptavidin-phycoerythrin (PE) (Invitrogen) in 1× tetramethyl ammonium chloride (TMAC) (Sigma), they were incubated for 5 more minutes at 60°C. The optimum concentration of streptavidin-PE was determined as the one giving the highest signal-to-background ratio. The hybridization temperature of 60°C was chosen because it eliminated cross-hybridization among probes. Hybridization was analyzed on a Luminex Liquid Chip 200 flow cytometer (Qiagen) using the Luminex IS software (Luminex). The analysis was carried out at 60°C with a maximum volume of 50 μl of sample and a minimum count of 100 microspheres per type, with settings of 8,300 and 16,500 for the lower and upper gates, respectively, as recommended by the manufacturer. In order to increase the hybridization of the PCR products to the probes, we removed the nonlabeled strand of the PCR product using bacteriophage T7 gene 6 exonuclease (New England BioLabs, Pickering, ON, Canada) according to the method described previously (17). T7 exonuclease is a 5′→3′ processive enzyme that rapidly degrades one of the strands on a duplex DNA molecule (14). In order to protect the GP6+ strand carrying the biotin label and selectively digest only the GP5+ strand, the first 4 nucleotides at the 5′ end of the molecule were modified to include phosphorothioate bonds between the deoxyribose moieties instead of the usual phosphodiester bonds (17). Optimal digestion conditions were determined by incubating 40 units of T7 exonuclease with 100 μl of PCR product for various times and then measuring the fluorescence on the Luminex system. T7 exonuclease digestion increased the hybridization signal by about 2-fold, reaching a plateau from about 20 min to about 60 min of incubation. We chose for subsequent experiments 40 min of incubation. Under these conditions, the limit of the detection by Luminex, after PCR amplification, was between 5 and 10 ng of HPV16 DNA.
Unique probes, 30 nucleotides (nt) in length with a 5′-C12 amino linker modification (kindly provided by the NML DNA Core Laboratory), were targeted at the region of the L1 gene comprised between the GP5+ and GP6+ primers (7). Unsuitable probes (not sensitive or not specific) were redesigned typically by shifting their position 10 nucleotides to the right or to the left along the variable region of the GP5+/GP6+ fragment. The final set of probes is shown in Table 1. Sensitivity and specificity for each type were determined by using DNA from genomic or L1 clones from defined HPV types, procured as described in Table S2 in the supplemental material. For all assays, four negative controls containing only host cell DNA were run alongside the samples. The average background fluorescence of each bead in the controls was subtracted from the fluorescence of each bead of the samples. This correction normalizes the background variations that occur between runs and between microsphere types. For example, while in one experiment the average background (all microspheres of 8 negative controls) was 12 ± 47, the backgrounds for the microspheres for HPV33 and -72 were 66 ± 18 and 318 ± 45, respectively. After correction, the average background became 0.4 ± 8.5 overall and 0.4 ± 9.0 and 0.6 ± 12 for the HPV33 and -72 microspheres, respectively. This correction avoided the need for a bead washing step used in other Luminex procedures (13, 18, 20, 21, 23). A fluorescence signal greater than 100 fluorescence units (FU) after correction was chosen as threshold for positivity.
Table 1.
Probes used to detect 46 mucosal HPV types by the NML Luminex method
| HPV type | Oligonucleotide sequence, 5′ to 3′ |
|---|---|
| 6 | CATCTTCCACATACACCAATTCTGATTATA |
| 11 | ACTATGTGCATCTGTGTCTAAATCTGCTAC |
| 13 | GTGTGTGCAGCCACTACATCATCTCTTTCA |
| 16 | AAATATGTCATTATGTGCTGCCATATCTAC |
| 18 | ATATGTGCTTCTACACAGTCTCCTGTACCT |
| 26 | CCTTACCATTAGTACATTATCTGCAGCATC |
| 30 | ATCTGCAACCACACAAACGTTATCCACATA |
| 31 | CAATATGTCTGTTTGTGCTGCAATTGCAAA |
| 32 | ACTGTAACAACTGAAGACACATACAAGTCT |
| 33 | TAATATGACTTTATGCACACAAGTAACTAG |
| 34 | TAGGTACACAATCCACAAGTACAACTGCAC |
| 35 | AAATATGTCTGTGTGTTCTGCTGTGTCTTC |
| 39 | ATCTACCTCTATAGAGTCTTCCATACCTTC |
| 40 | CTTATGTGCTGCCACACAGTCCCCCACACC |
| 42 | TCTGGTGATACATATACAGCTGCTAATTTT |
| 43 | AAACTTAACGTTATGTGCCTCTACTGACCC |
| 44 | AAACATGACAATATGTGCTGCCACTACACA |
| 45 | TAATTTAACATTATGTGCCTCTACACAAAA |
| 51 | GCCACTGCTGCGGTTTCCCCACATTTACTC |
| 52 | GACTTTATGTGCTGAGGTTAAAAAGGAAAG |
| 53 | CGCAACCACACAGTCTATGTCTACATATAA |
| 54 | ACAGCATCCACGCAGGATAGCTTTAATAAT |
| 56 | CATGACTATTAGTACTGCTACCAGAACAGT |
| 58 | ATGACATTATGCACTGAAGTAACTAAGGAA |
| 59 | CTTTCTGTGTGTGCTTCTACTACTTCTTCT |
| 61 | CATTTGTACTGCTACATCCCCCCCTGTATC |
| 62 | ACCGCCTCCACTGCTGCAGCAGAATACACG |
| 66 | GACTATTAATGCAGCTAAAAGCACATTAAC |
| 67 | TCTGAGGAAAAATCAGAGGCTACATACAAA |
| 68 | ATTGTCCACTACTACAGACTCTACTGTACC |
| 69 | ACTGTATCTGCACAATCTGCATCTGCCACT |
| 70 | GTCTGCCTGCACCGAAACGGCCATACCTGC |
| 71 | ACCAAAACTGTTGAGTCTACATATAAAGCC |
| 72 | CAGCTTCTAATTTTCGTGAGTATCTTCGCC |
| 73 | TAGGTACACAGGCTAGTAGCTCTACTACAA |
| 74 | TAACATGACTGTGTGTGCTCCTACCTCACA |
| 81 | TACTATTTGCACAGCTACATCTGCTGCTGC |
| 82 | GCTGTTACTCCATCTGTTGCACAAACATTT |
| 83 | CAGCTGCTGCTACACAGGCTAATGAATACA |
| 84 | GCTACCAACACCGAATCAGAATATAAACCT |
| 85 | TGCAACTACTAATCCAGTTCCATCTATATA |
| 86 | TAATTTTACTATTAGTGCCGCTACCCAGAA |
| 87 | CAATTTTACTATTAGTGCTGCCACTCAAAC |
| 89 | GCTTCCCAGTCTGGCACAGAATAC |
| 90 | CACCAATATGACTATTTGTGCCACACAAAC |
| 91 | TAACTTAACCTTGTGTGCATCCACTGAGTC |
| 97 | TCTACACAAAATGGCGTAGCTACCACATAT |
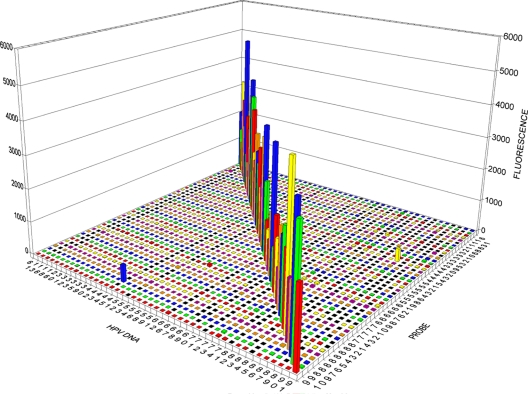
The results of a typical experiment are shown in Fig. 1. All 46 probes strongly hybridized only with the corresponding HPV DNA and not with HPV DNA of any other types. In the particular experiment shown in Fig. 1, the microspheres for HPV89, HPV72, and HPV81 showed fluorescence above 100 FU with probes for other types. This should be interpreted as random fluctuations, rather than systematic cross-reactivity, because the abnormal fluorescence reading was not reproducible in other experiments. This corresponds to a false-positive rate of 0.15% (3/1,980 measurements).
Fig 1.
Sensitivity and specificity of the 46 probes for their cognate HPV types. The probes are on the right axis, and the HPV DNAs are on the left axis. The vertical axis represents the fluorescence read for each microsphere carrying a specific HPV probe. The bars on the diagonal represent the hybridization of HPV DNA type with the intended cognate probe. The average background of four negative controls was subtracted for each microsphere, as described in the text.
Validation against clinical specimens was performed by comparing the results of the NML Luminex assay with direct sequencing of the amplified products utilizing 795 archived cervical specimens. These were amplified by nested PCR, and the products were typed with the NML Luminex assay and sequenced. The results showed a concordance of 97.7% between the two methods for the detection of HPV regardless of type. The sensitivity and specificity of the NML Luminex assay, using direct sequencing as a gold standard, were 98.8% (95% confidence interval [CI], 97.1 to 99.6) and 96.4% (95% CI, 96.4 to 93.8), respectively. Comparison of the distribution of HPV types detected by NML Luminex and direct sequencing methods is shown in Table 2. The direct sequencing method could not determine the sequences of 34 HPV positive samples, 32 of which were typeable by the Luminex method. There was no agreement on the HPV type detected for 13 of 429 (3.0%) samples positive by both methods. The NML Luminex assay detected a total of 793 HPV types versus 577 for direct sequencing. This discrepancy is due to the fact that direct sequencing cannot detect multiple HPV types present in the same sample.
Table 2.
Distribution of HPV types detected by NML Luminex method and direct sequencing
| HPV type | NML Luminex |
Direct sequencing |
||||
|---|---|---|---|---|---|---|
| n | % of types | % of positive samples | n | % of types | % of positive samples | |
| Negative | 353 | NAa | NA | 361 | NA | NA |
| Any type | 442 | NA | NA | 434 | NA | NA |
| 6 | 43 | 7.5 | 5.4 | 39 | 4.9 | 4.9 |
| 11 | 12 | 2.1 | 1.5 | 11 | 1.4 | 1.4 |
| 13 | 0 | 0.0 | 0.0 | 0 | 0.0 | 0.0 |
| 16 | 87 | 15.1 | 10.9 | 68 | 8.6 | 8.6 |
| 18 | 26 | 4.5 | 3.3 | 15 | 1.9 | 1.9 |
| 26 | 0 | 0.0 | 0.0 | 0 | 0.0 | 0.0 |
| 30 | 2 | 0.3 | 0.3 | 0 | 0.0 | 0.0 |
| 31 | 29 | 5.0 | 3.6 | 29 | 3.7 | 3.6 |
| 32 | 3 | 0.5 | 0.4 | 2 | 0.3 | 0.3 |
| 33 | 11 | 1.9 | 1.4 | 14 | 1.8 | 1.8 |
| 35 | 8 | 1.4 | 1.0 | 2 | 0.3 | 0.3 |
| 39 | 22 | 3.8 | 2.8 | 16 | 2.0 | 2.0 |
| 40 | 9 | 1.6 | 1.1 | 5 | 0.6 | 0.6 |
| 42 | 13 | 2.3 | 1.6 | 5 | 0.6 | 0.6 |
| 43 | 0 | 0.0 | 0.0 | 1 | 0.1 | 0.1 |
| 44 | 3 | 0.5 | 0.4 | 1 | 0.1 | 0.1 |
| 45 | 12 | 2.1 | 1.5 | 10 | 1.3 | 1.3 |
| 51 | 16 | 2.8 | 2.0 | 9 | 1.1 | 1.1 |
| 52 | 33 | 5.7 | 4.2 | 17 | 2.1 | 2.1 |
| 53 | 25 | 4.3 | 3.1 | 12 | 1.5 | 1.5 |
| 54 | 11 | 1.9 | 1.4 | 8 | 1.0 | 1.0 |
| 56 | 10 | 1.7 | 1.3 | 4 | 0.5 | 0.5 |
| 58 | 28 | 4.9 | 3.5 | 25 | 3.2 | 3.1 |
| 59 | 11 | 1.9 | 1.4 | 5 | 0.6 | 0.6 |
| 61 | 7 | 1.2 | 0.9 | 1 | 0.1 | 0.1 |
| 62 | 26 | 4.5 | 3.3 | 17 | 2.1 | 2.1 |
| 66 | 39 | 6.8 | 4.9 | 30 | 3.8 | 3.8 |
| 67 | 7 | 1.2 | 0.9 | 8 | 1.0 | 1.0 |
| 68 | 2 | 0.3 | 0.3 | 4 | 0.5 | 0.5 |
| 69 | 3 | 0.5 | 0.4 | 2 | 0.3 | 0.3 |
| 70 | 11 | 1.9 | 1.4 | 10 | 1.3 | 1.3 |
| 71 | 3 | 0.5 | 0.4 | 0 | 0.0 | 0.0 |
| 72 | 5 | 0.9 | 0.6 | 4 | 0.5 | 0.5 |
| 73 | 5 | 0.9 | 0.6 | 3 | 0.4 | 0.4 |
| 74 | 1 | 0.2 | 0.1 | 0 | 0.0 | 0.0 |
| 81 | 8 | 1.4 | 1.0 | 6 | 0.8 | 0.8 |
| 82 | 7 | 1.2 | 0.9 | 5 | 0.6 | 0.6 |
| 83 | 11 | 1.9 | 1.4 | 5 | 0.6 | 0.6 |
| 84 | 12 | 2.1 | 1.5 | 1 | 0.1 | 0.1 |
| 85 | 2 | 0.3 | 0.3 | 2 | 0.3 | 0.3 |
| 86 | 1 | 0.2 | 0.1 | 1 | 0.1 | 0.1 |
| 87 | 2 | 0.3 | 0.3 | 3 | 0.4 | 0.4 |
| 89 | 8 | 1.4 | 1.0 | 4 | 0.5 | 0.5 |
| 90 | 3 | 0.5 | 0.4 | 1 | 0.1 | 0.1 |
| 91 | 1 | 0.2 | 0.1 | 0 | 0.0 | 0.0 |
| 97 | 0 | 0.0 | 0.0 | 0 | 0.0 | 0.0 |
| 102 | NA | NA | NA | 2 | 0.3 | 0.3 |
NA, not applicable.
To test the ability of the NML Luminex assay to detect multiple types in the same sample, we amplified DNAs from different HPV types separately and then mixed them together in a single Luminex detection reaction. The total amount of DNA was kept constant to simulate the situation of clinical samples, in which a mixture of different DNAs is amplified to the maximum capacity of the PCR regardless of the number of types present. The results showed that at least 30 different types can be detected simultaneously by the Luminex method with no cross-hybridization except for some false positives for HPV type 72 in samples which contained more than 20 HPV types (data not shown).
The NML Luminex method was compared with the Linear Array method (Roche Molecular System Inc., Branchburg, NJ), a leading commercial genotyping method, utilizing 150 archival cervical specimens. The linear array HPV52 result, which may be due to cross-hybridization with types 33, 35, and 58, was confirmed by an HPV52-specific PCR method (4). The results showed that the NML Luminex assay was slightly more sensitive than Linear Array for detection of specimens positive for HPV but less sensitive for detecting multiple types (Table 3), but none of these differences were statistically significant. The lower sensitivity in detecting multiple types is likely due to limitations in the PCR method used, since we have shown that the NML Luminex assay can detect at least 30 different types of HPV simultaneously, if the DNAs are amplified separately and then mixed together. However, it is possible that a higher sensitivity for minute amounts of DNA of the reverse line blot platform may be the reason for the better performance of the Linear Array in detecting multiple types. A larger comparison study between NML Luminex and Linear Array may help in resolving this point.
Table 3.
Comparison of NML Luminex and Linear Array methods to detect multiple HPV infections
| Parameter | No. of samples |
|
|---|---|---|
| Luminex | Linear Array | |
| Positive for any type | 171 | 164 |
| Total HPV types detected | 63 | 75 |
| Single infections | 85 | 73 |
| Multiple infections | 47 | 47 |
| 2 types | 28 | 21 |
| 3 types | 9 | 14 |
| 4+ types | 10 | 12 |
In conclusion, the NML Luminex assay is a promising novel method for genotyping 46 mucosal HPV types associated with human infections with a high specificity and sensitivity. In our laboratory, we have used the NML Luminex assay for typing over 15,000 specimens from numerous HPV prevalence studies in Canada and elsewhere, with a number of manuscripts in preparation and two already published (1, 24). The use of T7 gene 6 exonuclease and the subtraction of the background for each bead eliminate the need for a washing step, which is required for other HPV typing methods based on Luminex. In addition, our method has the most comprehensive coverage of HPV types compared with other published methods to date: the method of Oh et al. (18) detects 15 HPV types, that of Schmitt et al. (20) covers 22 HPV types, the method of Jiang et al. (13) detects 26 HPV types, and a commercial method from Qiagen covers 18 HPV types (21). The limitations of our method are a lower sensitivity for multiple types compared to Linear Array and the inability of the PCR system to amplify certain variants of HPV68 (9). Future optimization of the PCR system may overcome these limitations.
Supplementary Material
Footnotes
Published ahead of print 23 November 2011
Supplemental material for this article may be found at http://jcm.asm.org/.
REFERENCES
- 1. Andall-Brereton GM, et al. 2011. Human papilloma virus prevalence in a sample of women in the Caribbean island of Trinidad. Rev. Panam. Salud Publica 29:2220–2226 [Google Scholar]
- 2. Bosch FX, et al. 2008. Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine 26(Suppl. 10):K1–K16 [DOI] [PubMed] [Google Scholar]
- 3. Bouvard V, et al. 2009. A review of human carcinogens. Part B: biological agents. Lancet Oncol. 10:321–322 [DOI] [PubMed] [Google Scholar]
- 4. Coutlee F, et al. 2007. Confirmatory real-time PCR assay for human papillomavirus (HPV) type 52 infection in anogenital specimens screened for HPV infection with the linear array HPV genotyping test. J. Clin. Microbiol. 45:3821–3823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coutlee F, et al. 2006. Enhanced detection and typing of human papillomavirus (HPV) DNA in anogenital samples with PGMY primers and the linear array HPV genotyping test. J. Clin. Microbiol. 44:1998–2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cuzick J, et al. 2008. Overview of human papillomavirus-based and other novel options for cervical cancer screening in developed and developing countries. Vaccine 26(Suppl. 10):K29–K41 [DOI] [PubMed] [Google Scholar]
- 7. de Roda Husman AM, Walboomers JM, van den Brule AJ, Meijer CJ, Snijders PJ. 1995. The use of general primers GP5 and GP6 elongated at their 3′ ends with adjacent highly conserved sequences improves human papillomavirus detection by PCR. J. Gen. Virol. 76:1057–1062 [DOI] [PubMed] [Google Scholar]
- 8. D'Souza G, et al. 2007. Case-control study of human papillomavirus and oropharyngeal cancer. N. Engl. J. Med. 356:1944–1956 [DOI] [PubMed] [Google Scholar]
- 9. Eklund C, Zhou T, Dillner J. 2010. Global proficiency study of human papillomavirus genotyping. J. Clin. Microbiol. 48:4147–4155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Giuliano AR, et al. 2008. Epidemiology of human papillomavirus infection in men, cancers other than cervical and benign conditions. Vaccine 26(Suppl. 10):K17–K28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gravitt PE, et al. 2000. Improved amplification of genital human papillomaviruses. J. Clin. Microbiol. 38:357–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harper DM, et al. 2004. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet 364:1757–1765 [DOI] [PubMed] [Google Scholar]
- 13. Jiang HL, Zhu HH, Zhou LF, Chen F, Chen Z. 2006. Genotyping of human papillomavirus in cervical lesions by L1 consensus PCR and the Luminex xMAP system. J. Med. Microbiol. 55:715–720 [DOI] [PubMed] [Google Scholar]
- 14. Kerr C, Sadowski PD. 1972. Gene 6 exonuclease of bacteriophage T7. II. Mechanism of the reaction. J. Biol. Chem. 247:311–318 [PubMed] [Google Scholar]
- 15. Khan MJ, et al. 2005. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J. Natl. Cancer Inst. 97:1072–1079 [DOI] [PubMed] [Google Scholar]
- 16. Munoz N, et al. 2003. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N. Engl. J. Med. 348:518–527 [DOI] [PubMed] [Google Scholar]
- 17. Nikiforov TT, Rendle RB, Kotewicz ML, Rogers YH. 1994. The use of phosphorothioate primers and exonuclease hydrolysis for the preparation of single-stranded PCR products and their detection by solid-phase hybridization. PCR Methods Appl. 3:285–291 [DOI] [PubMed] [Google Scholar]
- 18. Oh Y, et al. 2007. Polymerase chain reaction-based fluorescent Luminex assay to detect the presence of human papillomavirus types. Cancer Sci. 98:549–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Poljak M, Kocjan BJ. 2010. Commercially available assays for multiplex detection of alpha human papillomaviruses. Expert Rev. Anti Infect. Ther. 8:1139–1162 [DOI] [PubMed] [Google Scholar]
- 20. Schmitt M, et al. 2006. Bead-based multiplex genotyping of human papillomaviruses. J. Clin. Microbiol. 44:504–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Seme K, et al. 2009. Digene HPV genotyping RH test RUO: comparative evaluation with INNO-LiPA HPV genotyping extra test for detection of 18 high-risk and probable high-risk human papillomavirus genotypes. J. Clin. Virol. 46:176–179 [DOI] [PubMed] [Google Scholar]
- 22. Villa LL, et al. 2005. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 6:271–278 [DOI] [PubMed] [Google Scholar]
- 23. Wallace J, Woda BA, Pihan G. 2005. Facile, comprehensive, high-throughput genotyping of human genital papillomaviruses using spectrally addressable liquid bead microarrays. J. Mol. Diagn. 7:72–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zehbe I, et al. 2011. Feasibility of self-sampling and human papillomavirus testing for cervical cancer screening in First Nation women from Northwest Ontario, Canada: a pilot study. BMJ Open 1(1):e000030 doi:10.1136/bmjopen-2010-000030 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.