Abstract
Zygomycetes of the order Mucorales can cause life-threatening infections in humans. These mucormycoses are emerging and associated with a rapid tissue destruction and high mortality. The resistance of Mucorales to antimycotic substances varies between and within clinically important genera such as Mucor, Rhizopus, and Lichtheimia. Thus, an accurate diagnosis before onset of antimycotic therapy is recommended. Matrix-assisted laser desorption ionization (MALDI)–time of flight (TOF) mass spectrometry (MS) is a potentially powerful tool to rapidly identify infectious agents on the species level. We investigated the potential of MALDI-TOF MS to differentiate Lichtheimia species, one of the most important agents of mucormycoses. Using the Bruker Daltonics FlexAnalysis (version 3.0) software package, a spectral database library with m/z ratios of 2,000 to 20,000 Da was created for 19 type and reference strains of clinically relevant Zygomycetes of the order Mucorales (12 species in 7 genera). The database was tested for accuracy by use of 34 clinical and environmental isolates of Lichtheimia comprising a total of five species. Our data demonstrate that MALDI-TOF MS can be used to clearly discriminate Lichtheimia species from other pathogenic species of the Mucorales. Furthermore, the method is suitable to discriminate species within the genus. The reliability and robustness of the MALDI-TOF-based identification are evidenced by high score values (above 2.3) for the designation to a certain species and by moderate score values (below 2.0) for the discrimination between clinically relevant (Lichtheimia corymbifera, L. ramosa, and L. ornata) and irrelevant (L. hyalospora and L. sphaerocystis) species. In total, all 34 strains were unequivocally identified by MALDI-TOF MS with score values of >1.8 down to the generic level, 32 out of 34 of the Lichtheimia isolates (except CNM-CM 5399 and FSU 10566) were identified accurately with score values of >2 (probable species identification), and 25 of 34 isolates were identified to the species level with score values of >2.3 (highly probable species identification). The MALDI-TOF MS-based method reported here was found to be reproducible and accurate, with low consumable costs and minimal preparation time.
INTRODUCTION
Among the basal lineages of terrestrial fungi (formerly Zygomycetes), the Entomophthorales and Mucorales are known to cause infections in humans which are named entomophthoromycoses and mucormycoses, respectively. Whereas entomophthoromycoses are characterized by local infections of the skin and the gastrointestinal tract, mucormycoses comprise deep and systemic infections of the rhinocerebral and bronchorespiratory tract (20). Although both types of mycoses (formerly summarized as zygomycoses) are regarded to be comparatively uncommon, the number of patients with mucormycoses has increased during the last decades (21). These infections are associated with rapid infarct of the blood vessels and high mortality (13). Mucormycosis-inducing pathogens belong to the Mucorales, e.g., Rhizopus, Apophysomyces, Mucor, Cunninghamella, and Lichtheimia (formerly, Mycocladus, Absidia) (13, 20, 26).
Susceptibility of different zygomycetes to antifungal drugs varies considerably (2, 3, 4, 8). Therefore, fast and accurate identification of the pathogen is crucial for estimation of the incidence for mucormycoses caused by Lichtheimia species in monitoring surveys (e.g., the survey of Skiada et al. [23]) and to enrich repositories (e.g., Fungiscope) with clinical specimens. The conventional diagnosis includes the identification based on the morphology of the cultivated strains or on histopathology (7, 16). Both methods require considerable experience for correct identification of genera and species. Alternatively, molecular identification based on PCR using universal or taxon-specific primers can be used (7, 26). However, the purification of DNA followed by PCR-mediated detection is labor- and time-consuming, is highly dependent on the specificity of the oligonucleotide primers, and often requires subsequent sequencing of the PCR amplicons. Thus, identification by PCR is not easily adaptable to routine analysis in diagnostic laboratories. The requirements for a fast and accurate identification increasingly necessitate the development of more rapid, broad-spectrum identification strategies for clinical use. Diagnostic methodologies based on matrix-assisted laser desorption ionization (MALDI)–time of flight (TOF) mass spectrometry (MS) have successfully been used in recent years to discriminate clinically relevant Ascomycetes, e.g., Candida, Aspergillus, and Penicillium (1, 6, 15, 17, 24). Moreover, MALDI-TOF MS was shown to be suitable for routine identification of bacteria with an accuracy of >95% (9, 22). Importantly, it can also be used for cultivation-independent identification of bacteria in blood samples (19). However, the suitability of MALDI-TOF MS for the differentiation of zygomycetes has not been described yet.
Here we present the identification of mucoralean fungi with MALDI-TOF MS using the genus Lichtheimia as an example. The genus comprises three clinically relevant species (Lichtheimia corymbifera, L. ramosa, and L. ornata) and two species (L. hyalospora and L. sphaerocystis) which have not been associated with infections (5). Although Lichtheimia spp. are believed to be rare causative agents of infections, their abundance in clinical environments may be underestimated due to a lack of recognition (14). Recently, Lichtheimia was reported to be the second (with Rhizopus being the first) most common causative agent of zygomycosis in Europe (23). Furthermore, Lichtheimia spp. were commonly isolated in Germany from the lungs of white stork chicks with fungal pneumonia (18), suggesting frequent occurrence in the environment. Its complexity and the well-defined species designation render the Lichtheimia genus to be an ideal candidate for testing the high resolution and the diagnostic power of MALDI-TOF MS. Our results show that MALDI-TOF MS is a reliable, reproducible, fast, accurate, and cost-effective method for the identification of clinically important zygomycetes.
MATERIALS AND METHODS
Strains and cultivation.
A total of 53 fungal strains were used in this study (Table 1) and deposited in the Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands (CBS); the Mold Collection of the Spanish National Center for Microbiology, Instituto de Salud Carlos III, Spain (CNM-CM); the strain collection of the Institute for Bacteriology and Mycology, Faculty of Veterinary Medicine at the University of Leipzig (IBML), and the Jena Microbial Resource Collection (FSU). Forty-six strains of different Lichtheimia species were used, including 34 clinical isolates, with 21 and 13 isolates being from human and animal hosts, respectively. Rhizopus oryzae and Mucor circinelloides f. circinelloides were used as negative controls/outgroups in Fig. 1, Fig. 2, and Table 2. The species designation was determined or confirmed via molecular identification on the basis of the internal transcribed spacer 1 (ITS1)-5.8S-ITS2 ribosomal DNA (rDNA) sequence. The fungal strains were cultivated in liquid Sabouraud glucose (2%) medium (SIFIN, Berlin, Germany) supplemented with penicillin (100 units/ml) and streptomycin (100 μg/ml) for 24 h at 22°C on a shaker (200 rpm).
Table 1.
Fifty-three strains investigated with present molecular identification based on MALDI-TOF analysesa
| Species | Strain | Type designation | Equivalent strain designation | Substrate | Country |
|---|---|---|---|---|---|
| Lichtheimia corymbifera | CBS 429.75* | NT | FSU 9682 | Soil | Afghanistan |
| L. corymbifera | CBS 519.71* | T of Absidia griseola | FSU 10164 | Kurone developed during the manufacture of soy sauce (koji) | Japan |
| L. corymbifera | CNM-CM 3346 | FSU 10239 | Human, sputum | Spain | |
| L. corymbifera | CNM-CM 3415 | FSU 10240 | Human, ear swab | Spain | |
| L. corymbifera | CNM-CM 4671 | FSU 10243 | Human, sputum | Spain | |
| L. corymbifera | CNM-CM 4738 | FSU 10244 | Human, bronchoalveolar lavage fluid | Spain | |
| L. corymbifera | CNM-CM 5039 | FSU 10247 | Human, peritoneal drainage | Spain | |
| L. corymbifera | CNM-CM 5538 | FSU 10252 | Human, sputum | Spain | |
| L. corymbifera | CNM-CM 5637 | FSU 10253 | Human, skin | Spain | |
| L. corymbifera | CNM-CM 5738 | FSU 10255 | Human, abscess | Spain | |
| L. corymbifera | CNM-CM 5861 | FSU 10257 | Human, cutaneous wound | Spain | |
| L. corymbifera | FSU 6250 | Human, scale | Germany | ||
| L. corymbifera | FSU 10563 | 623 | Stork, chick | Germany | |
| L. corymbifera | FSU 10564 | 829 | Stork, chick | Germany | |
| L. corymbifera | FSU 10565 | 612B | Stork, chick | Germany | |
| L. corymbifera | FSU 10567 | 909B | Stork, chick | Germany | |
| L. corymbifera | IBML 004-M10012 | FSU 10178 | Cattle, gut | Germany | |
| L. corymbifera | IBML 006-M10005 | FSU 10179 | Horse, gut | Germany | |
| L. corymbifera | IBML 007-D10005 | FSU 10180 | Horse, gut | Germany | |
| L. ramosa | CBS 103.35* | T of Absidia gracilis | FSU 9927 | Fruit, Musa sapientum | NA |
| L. ramosa | CBS 582.65* | NT | FSU 10166 | Seed, Theobroma cacao | Ghana |
| L. ramosa | CNM-CM 3013 | FSU 10238 | Human, wound | Spain | |
| L. ramosa | CNM-CM 4228 | FSU 10241 | Human, face skin | Spain | |
| L. ramosa | CNM-CM 4849 | FSU 10245 | Human, skin biopsy | Spain | |
| L. ramosa | CNM-CM 5111 | FSU 10248 | Human, sputum | Spain | |
| L. ramosa | CNM-CM 5396 | FSU 10258 | Human, bronchoalveolar lavage fluid | Spain | |
| L. ramosa | CNM-CM 5398 | FSU 10250 | Human, bronchioaspirate | Spain | |
| L. ramosa | CNM-CM 5399 | FSU 10251 | Human, bronchioaspirate | Spain | |
| L. ramosa | CNM-CM 5400 | FSU 10242 | Human, bronchioaspirate | Spain | |
| L. ramosa | CNM-CM 5677 | FSU 10254 | Human, tracheal aspirate | Spain | |
| L. ramosa | FSU 6197* | T of Absidia idahoensis var. thermophila | As 3.4808 | Soil | China |
| L. ramosa | FSU 10156 | Human, stool | Germany | ||
| L. ramosa | FSU 10566 | 905A | Stork, chick | Germany | |
| L. ramosa | FSU 10568 | 909A | Stork, chick | Germany | |
| L. ramosa | IBML 003-D10007 | FSU 10175 | Cattle, gut | Germany | |
| L. ramosa | IBML 014-M10036 | Horse, fur and skin | Germany | ||
| L. ornata | CBS 291.66* | T of Absidia ornata | FSU 10165 | Bird, dung | India |
| L. ornata | CBS 958.68* | FSU 10167 | NA | NA | |
| L. ornata | CNM-CM 4978 | FSU 10246 | Human, wound | Spain | |
| L. hyalospora | CBS 100.28* | T of Absidia blakesleeana | FSU 10160 | Nut, Bertholletia excelsa | USA |
| L. hyalospora | CBS 102.36* | T of Absidia cristata | FSU 10161 | Stem, Manihot esculenta | Ghana |
| L. hyalospora | CBS 173.67* | NT of Tieghemella hyalospora | FSU 10163 | Fermented food, taosi | Philippines |
| L. hyalospora | CBS 518.71* | T of Absidia blakesleeana var. atrospora | FSU 10162 | Kurone developed during the manufacture of soy sauce (koji) | Japan |
| L. sphaerocystis | CBS 420.70* | T | FSU 10079 | NA | India |
| L. sphaerocystis | CBS 647.78 | FSU 10638 | Dung of mouse | India | |
| L. sphaerocystis | CBS 648.78 | FSU 10640 | Soil | India | |
| Apophysomyces elegans | CBS 476.78 | T | FSU 789 | Soil, mango orchard | India |
| Cokeromyces recurvatus | CBS 158.50 | T | FSU 793 | Rabbit, dung | USA |
| Mucor circinelloides f. circinelloides | CBS 195.68 | NT of M. circinelloides | FSU 10232 | Air | The Netherlands |
| Rhizomucor miehei | CBS 182.67 | T of Mucor miehei | FSU 9680 | Rotting plant, Parthenium argentatum | USA |
| Rhizopus oryzae | CBS 112.07* | T of R. oryzae | FSU 10159 | Human, lung | The Netherlands |
| R. microsporus var. microsporus | CBS 308.87 | FSU 5255 | Human, necrotic skin (hand after a spider bite) | Australia | |
| Syncephalastrum racemosum | DSM 859 | FSU 762 | NA | NA |
Ex-type strains are printed in bold and indicated by T (type strain) or NT (neotype strain). Thirteen strains (marked with asterisks) were used as representative type/reference material and included in the comparative phylogenetic study (as shown in Fig. 2). CBS, Centraalbureau voor Schimmelcultures Utrecht, The Netherlands; CNM-CM, Mold Collection of the Spanish National Center for Microbiology, Instituto de Salud Carlos III, Spain; IBML, Institute for Bacteriology and Mycology, Faculty of Veterinary Medicine at the University of Leipzig, Leipzig, Germany; FSU, Jena Microbial Resource Collection (formerly Fungal Reference Centre of the Friedrich Schiller University Jena, Germany); NA, not available.
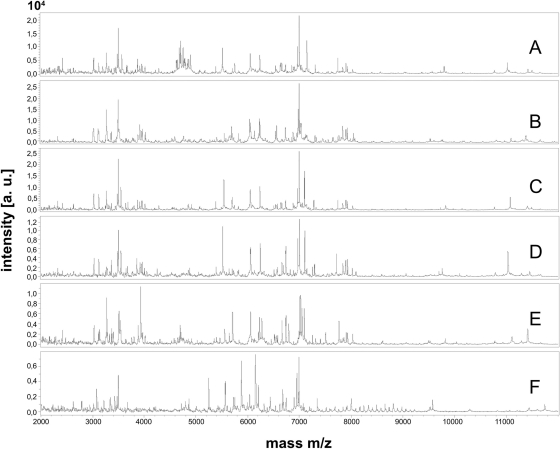
Fig 1.
MALDI-TOF spectra (m/z 2,000 to 12,000) of Lichtheimia corymbifera CBS 429.75NT (A), L. ramosa CBS 582.65NT (B), L. ornata CBS 291.66T (C), L. hyalospora CBS 173.67NT (D), L. sphaerocystis CBS 420.70T (E), and Rhizopus oryzae CBS 112.07 T (F). a.u., arbitrary units.
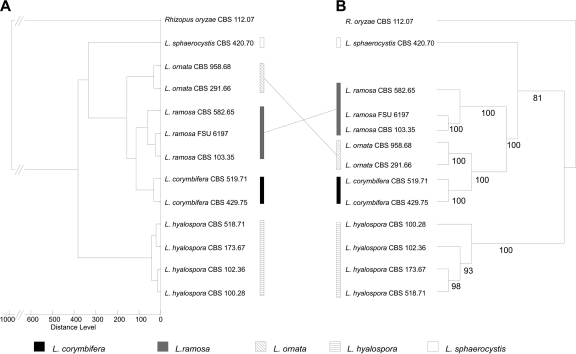
Fig 2.
Comparative distance analyses based on mass spectra (A) and combined nucleotide sequences of LSU, ITS, and the actin gene (B) using identical sets of strains. The phylogenetic relationships among the clinically relevant species are supported by bootstrap values of 100%; bootstrap values of >80% support phylogenetic relationships at deeper branches.
Table 2.
Matrix of score values calculated for 46 strains of Lichtheimia against the type and reference strains of Lichtheimia corymbifera, L. ramosa, L. ornata, L. hyalospora, L. sphaerocystis, Rhizopus oryzae, and Mucor circinelloides used as comparative measures
| Strain | Score value |
||||||
|---|---|---|---|---|---|---|---|
| L. corymbifera CBS 429.75NT | L. ramosa CBS 582.65NT | L. ornata CBS 291.66T | L. hyalospora CBS 173.67NT | L. sphaerocystis CBS 420.70T | Rhizopus oryzae CBS 112.07T | Mucor circinelloides f. circinelloides CBS 195.68NT | |
| L. corymbifera CBS 429.75 | 3.000 | 1.756 | 2.388 | 1.881 | 1.474 | 0.229 | <0 |
| L. corymbifera CBS 519.71 | 2.720 | 2.036 | 2.429 | 1.754 | 1.470 | 0.190 | <0 |
| L. corymbifera CNM-CM 3346 | 2.717 | 1.700 | 2.347 | 1.525 | 1.266 | 0.468 | <0 |
| L. corymbifera CNM-CM 3415 | 2.605 | 1.951 | 2.407 | 1.632 | 1.429 | 0.556 | <0 |
| L. corymbifera CNM-CM 4671 | 2.646 | 1.993 | 2.356 | 1.795 | 1.179 | 0.306 | <0 |
| L. corymbifera CNM-CM 4738 | 2.637 | 2.046 | 2.377 | 1.778 | 1.562 | 0.160 | <0 |
| L. corymbifera CNM-CM 5039 | 2.769 | 1.473 | 2.122 | 1.775 | 1.368 | 0.718 | <0 |
| L. corymbifera CNM-CM 5538 | 2.688 | 1.983 | 2.354 | 1.641 | 1.506 | 0.452 | <0 |
| L. corymbifera CNM-CM 5637 | 2.653 | 2.007 | 2.365 | 1.783 | 1.339 | 0.373 | 0.085 |
| L. corymbifera CNM-CM 5738 | 2.578 | 2.030 | 2.268 | 1.658 | 1.574 | 0.576 | 0.036 |
| L. corymbifera CNM-CM 5861 | 2.608 | 1.903 | 2.283 | 1.709 | 1.298 | 0.381 | <0 |
| L. corymbifera FSU 6250 | 2.627 | 2.107 | 2.441 | 1.720 | 1.346 | 0.001 | <0 |
| L. corymbifera FSU 10563 | 2.700 | 2.094 | 2.358 | 1.853 | 1.483 | 0.221 | <0 |
| L. corymbifera FSU 10564 | 2.712 | 1.959 | 2.406 | 1.706 | 1.547 | 0.567 | <0 |
| L. corymbifera FSU 10565 | 2.670 | 1.988 | 2.349 | 1.833 | 1.244 | 0.509 | <0 |
| L. corymbifera FSU 10567 | 2.749 | 2.027 | 2.397 | 1.846 | 1.494 | 0.430 | <0 |
| L. corymbifera IBML 004-M10012 | 2.616 | 1.959 | 2.381 | 1.774 | 1.446 | 0.358 | <0 |
| L. corymbifera IBML 006-M10005 | 2.595 | 2.170 | 2.375 | 1.830 | 1.522 | 0.410 | <0 |
| L. corymbifera IBML 007-D10005 | 2.729 | 2.056 | 2.424 | 1.883 | 1.475 | 0.649 | <0 |
| L. ramosa CBS 103.35 | 2.000 | 2.300 | 2.014 | 1.773 | 1.428 | 0.364 | 0.203 |
| L. ramosa CBS 582.65 | 1.828 | 3.000 | 2.005 | 1.509 | 1.420 | 0.297 | 0.245 |
| L. ramosa CNM-CM 3013 | 1.915 | 2.039 | 1.865 | 1.760 | 1.145 | 0.567 | 0.423 |
| L. ramosa CNM-CM 4228 | 1.935 | 2.500 | 1.974 | 1.699 | 1.301 | 0.396 | <0 |
| L. ramosa CNM-CM 4849 | 1.781 | 2.381 | 1.988 | 1.725 | 1.447 | <0 | <0 |
| L. ramosa CNM-CM 5111 | 1.710 | 2.575 | 1.968 | 1.620 | 1.360 | 0.311 | <0 |
| L. ramosa CNM-CM 5396 | 1.822 | 2.016 | 1.893 | 1.691 | 1.307 | 0.110 | 0.401 |
| L. ramosa CNM-CM 5398 | 1.917 | 2.203 | 2.002 | 1.808 | 1.396 | 0.780 | 0.357 |
| L. ramosa CNM-CM 5399 | 1.831 | 1.856 | 1.960 | 1.841 | 1.125 | <0 | 0.225 |
| L. ramosa CNM-CM 5400 | 1.948 | 2.182 | 2.075 | 1.780 | 1.302 | 0.569 | 0.212 |
| L. ramosa CNM-CM 5677 | 1.916 | 2.055 | 2.072 | 1.832 | 1.350 | 0.806 | 0.274 |
| L. ramosa FSU 6197 | 2.084 | 2.253 | 2.091 | 1.860 | 1.370 | 0.480 | 0.308 |
| L. ramosa FSU 10156 | 1.914 | 2.025 | 1.967 | 1.540 | 1.271 | 0.442 | 0.171 |
| L. ramosa FSU 10566 | 2.046 | 1.887 | 2.101 | 1.818 | 1.262 | 0.153 | 0.228 |
| L. ramosa FSU 10568 | 2.048 | 2.330 | 2.155 | 1.801 | 1.009 | 0.705 | 0.824 |
| L. ramosa IBML 003-D10007 | 1.953 | 2.538 | 2.073 | 1.779 | 1.309 | 0.273 | <0 |
| L. ramosa IBML 014-M10036 | 1.736 | 2.466 | 1.880 | 1.624 | 1.299 | <0 | <0 |
| L. ornata CBS 291.66 | 2.387 | 2.059 | 3.000 | 2.051 | 1.734 | 0.469 | <0 |
| L. ornata CBS 958.68 | 2.268 | 2.102 | 2.829 | 1.997 | 1.734 | <0 | <0 |
| L. ornata CNM-CM 4978 | 2.170 | 1.853 | 2.705 | 1.824 | 1.568 | 0.590 | <0 |
| L. hyalospora CBS 100.28 | 1.698 | 1.575 | 2.074 | 2.596 | 1.558 | 0.005 | 0.395 |
| L. hyalospora CBS 102.36 | 1.776 | 1.269 | 2.050 | 2.712 | 1.511 | <0 | 0.270 |
| L. hyalospora CBS 173.67 | 1.804 | 1.670 | 2.049 | 3.000 | 1.477 | <0 | 0.708 |
| L. hyalospora CBS 518.71 | 1.758 | 1.570 | 1.980 | 2.856 | 1.566 | <0 | 0.404 |
| L. sphaerocystis CBS 420.70 | 1.436 | 1.424 | 1.692 | 1.481 | 3.000 | 0.172 | 0.351 |
| L. sphaerocystis CBS 647.78 | 1.044 | 0.757 | 1.095 | 0.521 | 2.308 | <0 | <0 |
| L. sphaerocystis CBS 648.78 | 0.564 | 0.418 | 0.748 | 0.941 | 2.073 | 0.233 | <0 |
| Rhizopus oryzae CBS 112.07 | 0.470 | 0.530 | 0.465 | 0.145 | 0.129 | 3.000 | <0 |
| Mucor circinelloides f. circinelloides CBS 195.68 | <0 | 0.378 | <0 | 0.046 | <0 | <0 | 3.000 |
Sample preparation for MALDI-TOF MS analysis.
One milliliter of each culture was centrifuged for 10 min at 6,500 × g. The pellet was resuspended in 1 ml of water (high-pressure liquid chromatography [HPLC] quality; Merck, Germany), mixed thoroughly, centrifuged for 5 min at 6,500 × g, resuspended in a mixture of 200 μl bidistilled water and 600 μl absolute ethanol, and stored in tubes (1.5 ml; Eppendorf, Germany) at −75°C overnight. After thawing and collection via centrifugation, the fungal cells were dried at 37°C for 1 h and mixed thoroughly with 50 μl of 70% formic acid (Merck, Germany) and 50 μl acetonitrile (Merck, Germany), followed by a centrifugation step for 2 min at 6,500 × g. A volume of 1 μl clear supernatant was placed onto a ground steel MALDI target plate (Bruker Daltonik GmbH, Germany) and allowed to dry at room temperature. Five MALDI target positions per strain were prepared in parallel. Subsequently, each sample was overlaid with 1 μl of matrix, a saturated solution of α-cyano-hydroxy-cinnamic acid in 2.5% trifluoroacetic acid and 50% acetonitrile in water (CHCA; Bruker Daltonik GmbH, Germany), and air dried at room temperature. All chemicals used (Merck, Germany) were designated to be especially suitable for HPLC- or MALDI-based techniques according to the recommendations of the manufacturer. This procedure, including fungal cultivation, was repeated to ensure reproducibility of results.
MALDI-TOF measurement and database creation.
Nineteen strains comprising 12 well-characterized strains of Lichtheimia species, including type strains of all five species of Lichtheimia and 7 additional strains of Apophysomyces elegans, Cokeromyces recurvatus, Mucor circinelloides f. circinelloides, Syncephalastrum racemosum, Rhizomucor miehei, Rhizopus microsporus var. microsporus, and R. oryzae, were used to create the database. The MALDI-TOF MS measurement was performed using a Microflex LT mass spectrometer (Bruker Daltonik GmbH, Germany). The method is proteomics based and displays the overall protein spectra mainly represented by ribosomal proteins. Spectra were recorded in linear positive mode within a mass range from 2,000 to 20,000 Da. Spectrum acquisition was performed automatically using the software FlexControl (Bruker Daltonik GmbH, Germany) in autoexecute mode by rastering the target spot with the default pattern, including spectrum collection 40 times with a laser frequency of 60 Hz. All collected spectra were subjected to analysis with the software package FlexAnalysis (version 3.0; Bruker Daltonik GmbH, Germany). The software performs steps of smoothing, normalization, baseline correction, identification of the most significant peaks, and comparison of all collected spectra to each other (m/z values). Five reference spectra were generated per sample, and finally, a master spectrum was created and transferred into our database.
Testing of the database using Lichtheimia strains.
To test the created database, spectra of 34 additional isolates of Lichtheimia species were generated as described above and identified using the database as a library for identification purposes. Results of the pattern-matching process were expressed as proposed by the manufacturer with scores ranging from 0 to 3.0. Interpretation of the log (score [S]) values was performed as described by Marklein et al. (17) and van Veen et al. (25). Log (score) values of >1.7 generally indicated relationships on the genus level, and values of >2.0 indicated relationships on the species level. In particular, the meaning of the score values is as follows: 2.300 to 3.000, highly probable species identification; 2.000 to 2.299, secure genus identification, probable species identification; 1.700 to 1.999, probable genus identification; and 0.000 to 1.699, identification not reliable. The dendrograms shown in Fig. 2 and 3 were generated by using the software MALDI Biotyper (version 2.0; Bruker Daltonik GmbH, Germany).
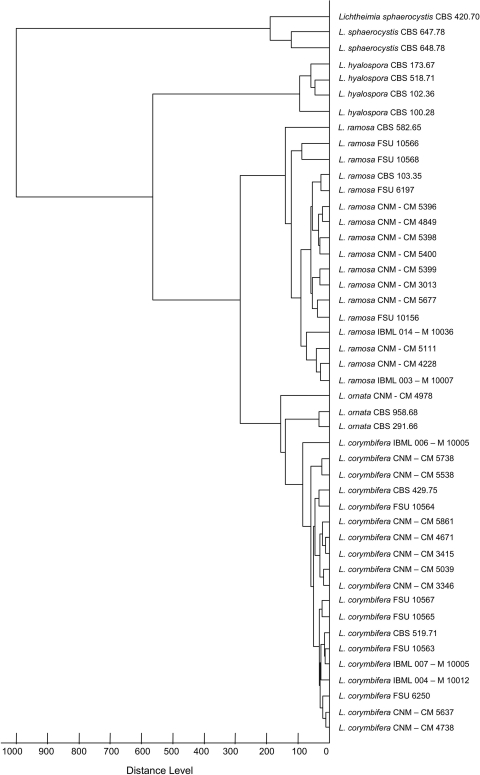
Fig 3.
Cladogram of a total of 46 isolates from Lichtheimia species based on mass spectra.
Phylogenetic multilocus analysis.
The diagnostic resolution of the MALDI-TOF-generated dendrogram was validated with a multilocus phylogram using a representative set of 12 Lichtheimia strains and 1 strain of Rhizopus oryzae as the outgroup shown in Fig. 2. Nucleotide sequences for the nuclear large subunit (LSU) ribosomal DNA, the ITS region spanning ITS1-5.8S-ITS2, and the gene encoding actin were generated in one of our previous studies on Lichtheimia (5). The server version of MAFFT (www.ebi.ac.uk/Tools/mafft) was used for nucleotide sequence alignment. The combined alignment consists of nucleotide sequences of LSU, ITS, and actin with 648, 889, and 730 characters, respectively. Phylogenetic reconstruction was done using the online version of RaxML HPC-2 on Abe on the CIPRES portal (http://www.phylo.org). The GTRGAMMA model was used for the bootstrapping phase and the final tree. Bootstrap iterations were set to 1,000 replicates.
RESULTS
Comparison of phylogenies based on DNA sequence and mass spectrometric data.
Mass spectra were obtained from a total of 53 strains comprising 12 mucoralean species (Table 1). Seven species (each species represented by one strain) known to be clinically relevant (26) were used as outgroup taxa. These are Apophysomyces elegans, Cokeromyces recurvatus, Mucor circinelloides f. circinelloides, Syncephalastrum racemosum, Rhizomucor miehei, Rhizopus microsporus var. microsporus, and R. oryzae. The remaining 46 strains investigated in this study encompass the 5 species of the genus Lichtheimia, namely, L. corymbifera, L. ramosa, L. ornata, L. hyalospora, and L. sphaerocystis, comprising 19, 17, 3, 4, and 3 strains, respectively. The mass spectra showed clear differences (Fig. 1). The type strains of all five species of Lichtheimia (Fig. 1A to E) could be easily distinguished from all other clinically important mucoralean genera, like, e.g., Rhizopus oryzae (Fig. 1F). To test the topology-compliance of the cladograms derived from mass spectra, 12 well-characterized strains of Lichtheimia, including the type material from all 5 species (analyzed in Fig. 1), were subjected to comparative analyses using 28S (LSU) rDNA, ITS1-5.8S-ITS2, and the actin gene and Rhizopus oryzae as the outgroup (Fig. 2). The clear separation of all five Lichtheimia species, the split of L. ramosa into two subgroups, and the close genetic relatedness of the three clinical species as shown by Alastruey-Izquierdo et al. (5) could be confirmed in both analyses (Fig. 2A versus B). The phylogenetic tree based on a combined data set of ITS, LSU, and actin sequences revealed a robust branching pattern with bootstrap (BS) values of >80%. The clinically relevant species L. corymbifera, L. ramosa, and L. ornata form a monophyletic group supported by the maximum BS of 100% in the phylogenetic tree (Fig. 2B). Topologies of mass spectrometry-based cladograms and multigene genealogy were almost identical; only the positions of L. ramosa and L. ornata changed. In the MALDI-TOF MS analysis (Fig. 2A), L. ornata is basal to the clade consisting of L. ramosa and L. corymbifera, while L. ramosa appears to be paraphyletic to the monophyletic clade consisting of L. ornata and L. corymbifera in the phylogenetic analysis (Fig. 2B). This sister group relationship of L. ornata and L. corymbifera has previously been described on the basis of single-locus analyses for ITS and actin (5).
Identification of clinical isolates of Lichtheimia spp. based on comparison of MALDI-TOF mass spectra of the strains with type strain spectra.
The reliability of the log (score) values obtained by the MALDI-TOF analyses on Lichtheimia was validated in intergeneric comparisons using the mucoralean species Apophysomyces elegans, Mucor circinelloides f. circinelloides, Rhizopus microsporus var. microsporus, Rhizopus oryzae, Rhizomucor miehei, and Syncephalastrum racemosum, which were reported to be human pathogenic (13, 16, 21, 26). Rhizopus oryzae and Mucor circinelloides were used as representative outgroup taxa in Fig. 2 and 3. On average, score values were <0.5 when Lichtheimia spp. were compared with Apophysomyces elegans, Rhizopus microsporus var. microsporus, Rhizomucor miehei, and Syncephalastrum racemosum (data not shown). The highest intergeneric score value was 0.902 when Apophysomyces was compared with Syncephalastrum. Forty-six isolates of Lichtheimia (including 5 type strains) were subjected to iterative MALDI-TOF analysis at the intrageneric level (Table 2). Score values were <0.8 when the Lichtheimia isolates were compared to Rhizopus oryzae and Mucor circinelloides. Out of 41 Lichtheimia isolates subjected to MALDI-TOF-based species delimitation analyses, 18, 16, 2, 3, and 2 isolates were unambiguously designated to L. corymbifera, L. ramosa, L. ornata, L. hyalospora, and L. sphaerocystis with score values of >2.5, >1.8, >2.7, >2.5, and >2.0, respectively, compared to the type strains of L. corymbifera CBS 429.75, L. ramosa CBS 582.65, L. ornata CBS 291.66, L. hyalospora CBS 173.67, and L. sphaerocystis CBS 420.70, respectively. In contrast, interspecies variation did not exceed 2.5 and ranged from 1.1 to 2.4, 1.0 to 2.0, 1.5 to 2.3, 1.2 to 2.0, and 0.4 to 1.1 for L. corymbifera, L. ramosa, L. ornata, L. hyalospora, and L. sphaerocystis, respectively, providing moderate to low support for designation to species other than the original one. All strains (n = 19) of L. corymbifera were homogeneously identified with S values of >2.5 compared with the neotype (NT) strain CBS 429.75. It is proposed that only one top match be used for species identification, even if other matches fall into the highly probable species range. However, intraspecific variation appears to be higher in L. ramosa than in L. corymbifera. Four (all from clinical samples) of the 17 tested strains were identified with S values of >2.5, 11 strains gained score values of 2.0 > S < 2.5, and strains FSU 10566 and CNM-CM 5399 (recovered from stork chick and human bronchioaspirate, respectively) had score values of 1.8 > S < 2.0 compared to the type strain of L. ramosa CBS 582.65. On the other hand, all L. ornata (n = 3) isolates were identified correctly with S values of >2.7. In general, the clinically relevant species L. corymbifera, L. ramosa, and L. ornata were clearly discriminated from the nonclinically relevant type strains of L. hyalospora CBS 173.67 and L. sphaerocystis CBS 420.70, as indicated by low score values of 1.18 < S > 2.07. Comparison with L. sphaerocystis CBS 420.70 revealed S values of <1.5 in 31 out of a total of 39 strains from the clinically important species (Table 2). Two additionally tested strains of L. sphaerocystis gained score values of <1.1 compared to Lichtheimia type strains (Table 2). Taking into consideration the fact that log (score) values of >1.7 generally indicate relationships on the genus level (17, 25), L. sphaerocystis is taxonomically at the edge of the generic border. In total, 95% of all tested strains (39 out of 41) were identified to have score values of >2 (probable species identification) and 77% were identified to have score values of >2.3 (highly probable species identification) down to the species level. Just 2 isolates (FSU 10566 and CNM-CM 5399) showed moderate species identification, as indicated by score values of >1.8. A cladogram based on the mass spectra presents the correct placement in accordance with their species affiliation (Fig. 3).
DISCUSSION
We showed that MALDI-TOF mass spectrometry is a convenient technique and powerful tool for the identification of human-pathogenic Mucorales at both intergeneric and intrageneric levels. However, a standardized sample preparation equally applied to all strains is crucial for reproducible measurement. Multiple techniques for the cultivation and the sample preparation for mass spectrometry of filamentous fungi have been published (1, 15). However, direct comparison of the spectra obtained by different protocols is not possible (9, 25). Therefore, a standardized procedure for sample preparation which should be applied to all mucoralean fungi subjected to MALDI-TOF analyses is proposed in this study.
Mass spectrum-based distance cladograms are phylogeny compliant compared to the phylogeny published for Lichtheimia by Alastruey-Izquierdo et al. (5). At the intergeneric level, the three clinically most important species, Rhizopus oryzae, Mucor circinelloides, and Lichtheimia corymbifera, could be clearly identified. In contrast, L. ramosa exhibits higher intraspecific heterogeneity, which is concordant with the findings of previous analyses (5, 11, 12). The identification of the pathogen and knowledge of the susceptibility to antifungal drugs are crucial for efficient treatment and therapy. There are significant differences in susceptibility to itraconazole, posaconazole, terbinafine, and amphotericin B between Rhizopus species and Lichtheimia species (2, 3, 4, 8). While Rhizopus species appear to be more resistant to amphotericin B than Mucor species (8), Lichtheimia species respond well to amphotericin B (4). Among the species of Lichtheimia, L. ramosa showed slightly higher MICs than the other species for all drugs (4). Therefore, in the future, identification at the species level may be helpful for determination of the antifungal therapy based on the susceptibility profile of the individual pathogen. The high resolution and discriminative power of MALDI-TOF mass spectrometry facilitate differentiation of closely related species. Thus, it represents an important prerequisite for future refinements and monitoring of efficiency control in antifungal therapy strategies, on the one hand, and for the detection of fungi with mycotoxin-producing or allergy-causing potential, on the other hand. The current procedure requires isolation and cultivation of the fungus prior to species determination. Direct identification of Lichtheimia in biopsy material has not been established yet. If the necessary mass spectrometer is available, MALDI-TOF is the fastest (5.1 min of hands-on time/identification) and cheapest ($0.50/sample) strategy among all the other cultivation-dependent methods of molecular detection (e.g., PCR, restriction fragment length polymorphism analysis, DNA fingerprinting) for fungal identification (10).
In summary, MALDI-TOF MS has been implemented as a rapid, simple, cost-effective, and high-throughput proteomic technique for routine identification of Lichtheimia isolates in our laboratory. Although five reference spectra were generated for the creation of master spectra, two spots (equivalent to two technical replications) would be sufficient for reliable species identification in routine diagnosis, because of the high reproducibility of the spectra. MALDI-TOF mass spectrometry yielded 100% accurate identification on the genus level (spectral scores, ≥1.8) for 41 strains of Lichtheimia. The technique has a high accuracy for microbial identification in general and performs as well as or better than conventional techniques. The performance can be significantly improved when more spectra of appropriate reference strains are added to the database (25). The data set presented in this study can be used as a reference set which can be included in the database of any MALDI-TOF equipment. Furthermore, the protocol for sample preparation used in this study provides a robust approach which eliminates the strong influences of culture media and culture conditions, as previously discussed for bacteria, yeasts and filamentous fungi (6, 7, 14, 15, 17, 19, 22, 24, 25). MALDI-TOF MS revolutionizes the identification of pathogens in clinical laboratories but requires a good knowledge of taxonomy when this technique is implemented in a routine clinical biology laboratory (9).
ACKNOWLEDGMENTS
We thank Ru-yong Zheng (Key Laboratory of Systematic Mycology and Lichenology, Institute of Microbiology, Chinese Academy of Sciences, Beijing, China) for providing strain As 3.4808 (FSU 6197).
This work was supported by bilateral grants (VO772/7-1 and VO772/9-1) of the Deutsche Forschungsgemeinschaft to K.V.
Footnotes
Published ahead of print 30 November 2011
REFERENCES
- 1. Alanio A, et al. 2011. Matrix-assisted laser desorption ionization time-of-flight mass spectrometry for fast and accurate identification of clinically relevant Aspergillus species. Clin. Microbiol. Infect. 17: 750–755 [DOI] [PubMed] [Google Scholar]
- 2. Alastruey-Izquierdo A, et al. 2009. Activity of posaconazole and other antifungal agents against Mucorales strains identified by sequencing of internal transcribed spacers. Antimicrob. Agents Chemother. 53: 1686–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alastruey-Izquierdo A, et al. 2009. In vitro activity of antifungals against Zygomycetes. Clin. Microbiol. Infect. 15 Suppl 5: 71–76 [DOI] [PubMed] [Google Scholar]
- 4. Alastruey-Izquierdo A, Cuesta I, Walther G, Cuenca-Estrella M, Rodriguez-Tudela JL. 2010. Antifungal susceptibility profile of human-pathogenic species of Lichtheimia. Antimicrob. Agents Chemother. 54: 3058–3060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alastruey-Izquierdo A, et al. 2010. Species recognition and clinical relevance of the zygomycetous genus Lichtheimia (syn. Absidia pro parte, Mycocladus). J. Clin. Microbiol. 48: 2154–2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bader O, et al. 2010. Improved clinical laboratory identification of human pathogenic yeasts by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin. Microbiol. Infect. 17: 1359–1365 [DOI] [PubMed] [Google Scholar]
- 7. Dannaoui E. 2009. Molecular tools for identification of Zygomycetes and the diagnosis of zygomycosis. Clin. Microbiol. Infect. 15 Suppl 5: 66–70 [DOI] [PubMed] [Google Scholar]
- 8. Dannaoui E, et al. 2003. In vitro susceptibilities of zygomycetes to conventional and new antifungals. J. Antimicrob. Chemother. 51: 45–52 [DOI] [PubMed] [Google Scholar]
- 9. De Bel A, et al. 2010. Correct implementation of matrix-assisted laser desorption ionization–time of flight mass spectrometry in routine clinical microbiology. J. Clin. Microbiol. 48: 1991–1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dhiman N, Hall L, Wohlfiel SL, Buckwalter SP, Wengenack NL. 2011. Performance and cost analysis of matrix-assisted laser desorption ionization–time of flight mass spectrometry for routine identification of yeast. J. Clin. Microbiol. 49: 1614–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ellis JJ, Hesseltine GW. 1966. Species of Absidia with ovoid sporangiospores. II. Sabouraudia 5: 59–77 [PubMed] [Google Scholar]
- 12. Garcia-Hermoso D, et al. 2009. Molecular and phenotypic evaluation of Lichtheimia corymbifera (formerly Absidia corymbifera) complex isolates associated with human mucormycosis: rehabilitation of L. ramosa. J. Clin. Microbiol. 47: 3862–3870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Greenberg RN, Scott LJ, Vaughn HH, Ribes JA. 2004. Zygomycosis (mucormycosis): emerging clinical importance and new treatments. Curr. Opin. Infect. Dis. 17: 517–525 [DOI] [PubMed] [Google Scholar]
- 14. Grosse-Herrenthey A, et al. 2008. Challenging the problem of clostridial identification with matrix-assisted laser desorption and ionization-time-of-flight mass spectrometry (MALDI-TOF MS). Anaerobe 14: 242–249 [DOI] [PubMed] [Google Scholar]
- 15. Hettick JM, et al. 2008. Discrimination of Penicillium isolates by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry fingerprinting. Rapid Commun. Mass Spectrom. 22: 2555–2560 [DOI] [PubMed] [Google Scholar]
- 16. Lass-Flörl C. 2009. Zygomycosis: conventional laboratory diagnosis. Clin. Microbiol. Infect. 15 Suppl 5: 60–65 [DOI] [PubMed] [Google Scholar]
- 17. Marklein G, et al. 2009. Matrix-assisted laser desorption ionization–time of flight mass spectrometry for fast and reliable identification of clinical yeast isolates. J. Clin. Microbiol. 47: 2912–2917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Olias P, Gruber AD, Winfried B, Hafez HM, Lierz M. 2010. Fungal pneumonia as a major cause of mortality in white stork (Ciconia ciconia) chicks. Avian Dis. 54: 94–98 [DOI] [PubMed] [Google Scholar]
- 19. Prod'hom G, Bizzini A, Durussel C, Bille J, Greub G. 2010. Matrix-assisted laser desorption ionization–time of flight mass spectrometry for direct bacterial identification from positive blood culture pellets. J. Clin. Microbiol. 48: 1481–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ribes JA, Vanover-Sams CL, Baker DJ. 2000. Zygomycetes in human disease. Clin. Microbiol. Rev. 13: 236–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roden MM, et al. 2005. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin. Infect. Dis. 41: 634–653 [DOI] [PubMed] [Google Scholar]
- 22. Seng P, et al. 2009. Ongoing revolution in bacteriology: routine identification of bacteria by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin. Infect. Dis. 49: 543–551 [DOI] [PubMed] [Google Scholar]
- 23. Skiada A, et al. 2011. Zygomycosis in Europe: analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007. Clin. Microbiol. Infect. 17: 1859–1867 [DOI] [PubMed] [Google Scholar]
- 24. Stevenson LG, Drake SK, Shea YR, Zelazny AM, Murray PR. 2010. Evaluation of matrix-assisted laser desorption ionization–time of flight mass spectrometry for identification of clinically important yeast species. J. Clin. Microbiol. 48: 3482–3486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Van Veen SQ, Claas EC, Kuijper EJ. 2010. High-throughput identification of bacteria and yeast by matrix-assisted laser desorption ionization–time of flight mass spectrometry in conventional medical microbiology laboratories. J. Clin. Microbiol. 48: 900–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Voigt K, Cigelnik E, O'Donnell K. 1999. Phylogeny and PCR identification of clinically important Zygomycetes based on nuclear ribosomal-DNA sequence data. J. Clin. Microbiol. 37: 3957–3964 [DOI] [PMC free article] [PubMed] [Google Scholar]