Abstract
Pulsed-field gel electrophoresis (PFGE) analysis demonstrated that while 76% of patients had only one genotype of campylobacter, 10% carried two different but related genotypes (Dice coefficients > 0.78), and 14% carried at least two unrelated genotypes (Dice coefficients < 0.65). This supports the clustering of Campylobacter isolates with similar PFGE patterns, highlights the need to analyze multiple isolates from both sources and patients, and confirms that caution should be exercised before epidemiological links between patients or sources are dismissed.
TEXT
Campylobacteriosis is the most frequently reported disease in New Zealand, with an annual rate of 180 cases per 100,000 in 2010 (1). Genotyping of Campylobacter isolates using methods such as pulsed-field gel electrophoresis (PFGE) and multilocus sequence typing (MLST) has proven useful in better understanding this disease (7, 11). This paper describes the analysis by PFGE of more than one isolate of Campylobacter from each of 49 human cases of campylobacteriosis. Between April 2009 and February 2010, 673 clinical isolates of Campylobacter were obtained from clinical laboratories in Christchurch, New Zealand, as previously described (6). Isolates were identified as Campylobacter jejuni, C. coli, or C. lari using PCR assays (2, 16), and PFGE analysis was performed as previously described (6), using the restriction enzymes SmaI and KpnI. PFGE patterns which were indistinguishable (no discernible difference in pattern) were assigned the same PFGE pattern name. A small number of isolates produced only a single band as the result of unrestricted genomic DNA migrating into the agarose gel, when the restriction sites were masked by DNA modifications. These were reported as NO CUT. Pattern similarity was determined by calculation of Dice coefficients (4) based on the total number of bands present in the SmaI and KpnI patterns. SmaI patterns had between 1 and 11 bands (average, 7), while KpnI patterns had between 6 and 14 bands (average, 10), which results in a greater weighting for the KpnI pattern in Dice calculations. Patterns yielding pairwise Dice coefficients greater than 0.75 were classified as related patterns, while PFGE patterns with Dice coefficients less than 0.65 were classified as different.
The 673 isolates analyzed came from 603 different patients. There were 49 patients who had between two and five Campylobacter isolates obtained separately, yielding a total of 119 isolates. Some of these patients were food preparation workers who were requested not to return to work until Campylobacter could no longer be isolated from their feces. While they were requested to provide samples a week apart, some people actually provided multiple samples on 1 day, or subsequent days, while others provided samples up to 2 months apart. In other cases, samples were tested by a community laboratory, and then following hospitalization of the patients, another sample was tested by the hospital laboratory.
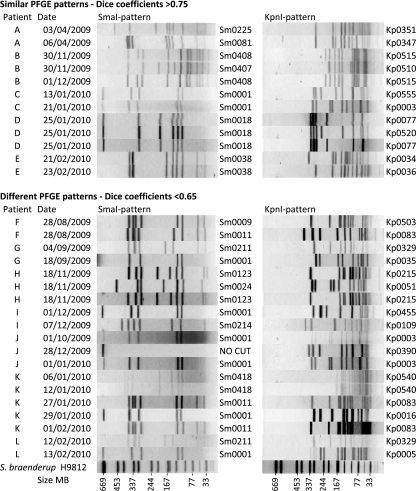
In all cases, only one species of Campylobacter was identified from each patient, with 45 of the patients being infected with C. jejuni, three with C. coli, and one with C. lari. For 76% (95% confidence interval, 61 to 87%) of patients, only one PFGE type was recovered when between two and four isolates were genotyped from each patient. These 37 patients had 34 different PFGE genotypes, with isolates provided up to a month apart. For the other 12 patients (24%), more than one PFGE genotype was recovered (Fig. 1). For five of these patients, two different but related PFGE patterns were identified. These patterns clearly differed but often with only one enzyme and then with four or fewer different bands, suggesting that the PFGE patterns were potentially related (Dice coefficients between 0.79 and 0.88 for isolates from the same patient).
Fig 1.
Pulsed-field gel electrophoresis (PFGE) patterns of campylobacter isolates from patients with more than one genotype of campylobacter. All isolates were Campylobacter jejuni except for those from patient B, all three of which were Campylobacter coli.
Another seven patients also had at least two PFGE patterns, but in these cases the PFGE patterns had a greater number of differences and would not normally be considered related (Dice coefficients of 0.22 to 0.63). Six of these patients had two different PFGE patterns, while for one patient, five isolates were analyzed with three different PFGE patterns (Fig. 1). This patient was a 1-year-old child living in a rural area who was reported to have eaten chicken feces. The same genotype (Sm0418:Kp0540) was recovered from this patient from the initial sample and from a sample provided 6 days later. Then, 3 weeks after the first sample, additional samples were tested, two of which were of the same genotype and one of which was of a different genotype. It is likely that this child was exposed to multiple sources of Campylobacter, and the reported consumption of chicken feces may not have been an isolated event.
The observation of multiple genotypes of Campylobacter from the same patient may have a number of explanations. A patient may have been infected with a single genotype of Campylobacter, but mutation in the course of infection may result in more than one genotype. Closely related genotypes would be expected in this situation, and this has been observed following the passage of C. jejuni through chickens (9, 10). Alternatively, the source of infection may contain more than one genotype of Campylobacter, which may be closely related or quite different. Multiple genotypes of Campylobacter have been reported from the same animal in chicken, bovine, and other animal sources (3, 8, 14, 15). An outbreak in Scotland linked to undercooked chicken pâté found at least four genotypes of Campylobacter, with three patients being coinfected with two different strains of Campylobacter (5). Analysis of Campylobacter isolates from the waterborne Walkerton outbreak in Canada found at least 12 genotypes of Campylobacter among human patients in the outbreak (3). Multiple genotypes may also be the consequence of different infection events. Ongoing water contamination, repeated contact with multiple animals, or consumption of multiple contaminated foods may all result in infection with different types of Campylobacter.
The isolation of multiple strains of Campylobacter from patients adds a further layer of complexity to the epidemiology of campylobacteriosis. This study provides support for grouping related PFGE patterns, and this grouping is necessary if clusters of cases with a common source are to be identified. The matching of PFGE types with sources on the basis of similar patterns is also therefore justified. In both these situations, the use of two enzymes, as in this study, reduces the chances of similar patterns occurring by chance in isolates with no genetic similarity. Some SmaI patterns in particular are very common, with relatively few bands. The Sm0001 pattern, for example, has just five bands, and among 673 isolates, 12% exhibited this pattern. KpnI analysis, however, discriminated these isolates into 19 different KpnI patterns, some of which are quite divergent.
In the case of different PFGE patterns, isolating and genotyping more than one isolate from each patient or source may be necessary for clusters of cases to be identified. It is, however, an expensive exercise, and based on this small study, it may not add value in three quarters of cases. One use of genotyping data is in attribution studies, comparing genotypes from different sources (12). In this study, where different isolates were recovered from the same patient and these isolates had previously been isolated from an animal source, they were in all cases from the same source (either poultry or ruminant). Therefore, even if only one isolate was recovered from each patient, the same conclusion might be made as to source attribution.
A previous study in the United Kingdom found that four of 53 patients had two genotypes of C. jejuni (13). While PFGE analysis of these strains was performed only with SmaI, using our Dice coefficient criteria, some patients had related strains, while others had different strains. While the number of patients in our study with either related PFGE types (10% with Dice similarities > 0.79) or different PFGE types (14% with Dice similarities < 0.64) was greater than the United Kingdom study, we would suggest this as a minimum level of diversity among human cases of campylobacteriosis in our study area. In this study, a maximum of just five isolates were tested from each patient, and for 31 of the 49 cases just two isolates were typed. Genotyping of more isolates from each patient would undoubtedly have recovered more genotypes. The true level of diversity undoubtedly changes with different sources of infection. It is also likely that the diversity of genotypes causing campylobacteriosis will be underestimated, due to the selective nature of media used to recover isolates.
This report does not purport to definitively identify the carriage rate of multiple genotypes of Campylobacter. It does, however, provide support for the clustering of similar isolates and confirm that in cases where isolates from the suspected source and the patients have different genotypes, caution should be exercised before a putative link between the isolates is dismissed. Where epidemiological evidence connects cases, clustering two or more different genotypes together may be valid. Furthermore, where a suspected source is identified, analysis of multiple isolates from both source and patient may be warranted.
ACKNOWLEDGMENTS
We thank the staff of Medlab South and Southern Community Laboratories for the provision of isolates. We acknowledge the financial support for this study from the Health Research Council of New Zealand.
Footnotes
Published ahead of print 23 November 2011
REFERENCES
- 1. Anonymous. 2011. New Zealand Public Health Surveillance Report, vol. 9, p 1–8 http://www.surv.esr.cri.nz/PDF_surveillance/NZPHSR/2011/NZPHSR2011March.pdf
- 2. Chaban B, Musil KM, Himsworth CG, Hill JE. 2009. Development of cpn60-based real-time quantitative PCR assays for the detection of 14 Campylobacter species and application to screening of canine fecal samples. Appl. Environ. Microbiol. 75:3055–3061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Clark CG, et al. 2003. Characterization of waterborne outbreak-associated Campylobacter jejuni, Walkerton, Ontario. Emerg. Infect. Dis. 9:1232–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dice LR. 1945. Measures of the amount of ecologic association between species. Ecology 26:297–302 [Google Scholar]
- 5. Forbes KJ, et al. 2009. Campylobacter immunity and coinfection following a large outbreak in a farming community. J. Clin. Microbiol. 47:111–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gilpin B, et al. 2006. Application of pulsed-field gel electrophoresis to identify potential outbreaks of campylobacteriosis in New Zealand. J. Clin. Microbiol. 44:406–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gilpin BJ, Scholes P, Robson B, Savill MG. 2008. The transmission of thermotolerant Campylobacter spp. to people living or working on dairy farms in New Zealand. Zoonoses Public Health 55:352–360 [DOI] [PubMed] [Google Scholar]
- 8. Gilpin BJ, et al. 2008. Comparison of Campylobacter jejuni genotypes from dairy cattle and human sources from the Matamata-Piako District of New Zealand. J. Appl. Microbiol. 105:1354–1360 [DOI] [PubMed] [Google Scholar]
- 9. Hanel I, et al. 2009. Genomic and phenotypic changes of Campylobacter jejuni strains after passage of the chicken gut. Vet. Microbiol. 136:121–129 [DOI] [PubMed] [Google Scholar]
- 10. Hanninen ML, Hakkinen M, Rautelin H. 1999. Stability of related human and chicken Campylobacter jejuni genotypes after passage through chick intestine studied by pulsed-field gel electrophoresis. Appl. Environ. Microbiol. 65:2272–2275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mullner P, et al. 2010. Molecular and spatial epidemiology of human campylobacteriosis: source association and genotype-related risk factors. Epidemiol. Infect. 138:1372–1383 [DOI] [PubMed] [Google Scholar]
- 12. Mullner P, et al. 2009. Assigning the source of human campylobacteriosis in New Zealand: a comparative genetic and epidemiological approach. Infect. Genet. Evol. 9:1311–1319 [DOI] [PubMed] [Google Scholar]
- 13. Richardson JF, et al. 2001. Coinfection with Campylobacter species: an epidemiological problem? J. Appl. Microbiol. 91:206–211 [DOI] [PubMed] [Google Scholar]
- 14. Schouls L, et al. 2003. Comparative genotyping of Campylobacter jejuni by amplified fragment length polymorphism, multilocus sequence typing, and short repeat sequencing: strain diversity, host range, and recombination. J. Clin. Microbiol. 41:15–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thakur S, Gebreyes WA. 2010. Phenotypic and genotypic heterogeneity of Campylobacter coli within individual pigs at farm and slaughter in the US. Zoonoses Public Health 57(Suppl. 1):100–106 [DOI] [PubMed] [Google Scholar]
- 16. Wong T, et al. 2004. Validation of a PCR method for Campylobacter detection on poultry packs. Br. Food J. 106:642–650 [Google Scholar]