Abstract
This study investigated “creep” in vancomycin and daptomycin MICs among methicillin-resistant Staphylococcus aureus (MRSA) isolates from blood cultures over a 5-year period in a hospital in the United Kingdom, using different susceptibility testing methods. Trends in vancomycin and daptomycin susceptibility were evaluated by using Etest performed prospectively on isolates in routine clinical practice from December 2007 to December 2010 (n = 102). Comparison was made to results from prospective testing of subcultures at the Scottish MRSA Reference Laboratory, using an automated system (Vitek 2) and retrospective testing (Etest and CLSI reference broth microdilution [BMD] method) of stored isolates from 2006 to 2010 (n = 208). Spearman's rank correlations revealed a significant increase in vancomycin MIC (P = 0.012) and a significant decrease in daptomycin MIC (P = 0.03) by year of study for Etest results from the time of isolation. However, neither trend was replicated in MICs from automated or retrospective testing. The Friedman test revealed a significant difference between vancomycin MICs obtained from the same samples by different testing methods (χ2 [3 degrees of freedom] = 97; P < 0.001). MICs from automated testing and Etest analysis of stored isolates were significantly lower than those from Etest analysis at the time of isolation for both antibiotics (P < 0.001). Effects of storage on the MIC appeared within the first 6 months of storage. Inconsistent evidence on vancomycin MIC creep and the relevance of the MIC to clinical outcome may arise from differences in susceptibility testing methods, including storage of isolates. There is a need to standardize and streamline susceptibility testing of vancomycin against MRSA.
INTRODUCTION
Methicillin-resistant Staphylococcus aureus (MRSA) is an important community and hospital pathogen which, when isolated from blood, carries a high mortality rate despite appropriate first-line antimicrobial therapy (4, 24).
Resistance to vancomycin in MRSA (MIC of >2 mg/liter) remains infrequent, but there is growing evidence in the literature that vancomycin may be ineffective against an increasing proportion of isolates with MICs between 1 and 2 mg/liter (5, 18, 28). This has been demonstrated by the emergence of strains of intermediate resistance, i.e., hetero-VISA (vancomycin-intermediate S. aureus) and VISA strains, and of increasing proportions of MRSA isolates with high MICs within the susceptible range, the so-called vancomycin MIC “creep” (13, 17, 28, 30). The creep phenomenon has not been reported consistently (7, 13, 28), but it is concerning because vancomycin still remains the cornerstone of therapy for many serious MRSA infections (7, 14).
Vancomycin use against methicillin-susceptible S. aureus (MSSA) is associated with worse outcomes than beta-lactam therapy, but with limited superior alternative treatment options for MRSA, it has been postulated that the vancomycin MIC may have a role in the clinician's decision to continue to use this drug as therapy (5, 15, 16). Studies evaluating whether an in vitro vancomycin MIC can be used to predict clinical success or failure have not been able to give definitive answers (7, 15, 26). Equally, since outcomes often remain poor despite a vancomycin MIC in the susceptible range (MIC of ≤2 mg/liter), there has been a suggestion of lowering the clinical breakpoints further (5), and a shift in MIC population distributions may have important implications for the incidence of treatment failure beyond individual risk assessment.
However, the interpretation of literature on vancomycin MIC creep is complicated by inconsistencies in and limitations of susceptibility testing methods. In many of the reported studies looking at in vitro susceptibility data, MIC testing was performed retrospectively on isolates retrieved from storage, not at the time of isolation of the organism, and often this is not made explicitly clear (1, 7, 8, 10, 12, 13, 15, 16, 18, 19, 25, 27, 28, 30, 31, 32, 34, 35). It has been demonstrated that certain organisms can become more susceptible when stored (3, 22), and it is possible that this phenomenon, together with differing methodologies, may explain some of the conflicting results in the literature (9).
(This paper was presented in part at the 21st European Congress of Clinical Microbiology and Infectious Diseases, 7 to 10 May 2011, Milan, Italy [3a].)
MATERIALS AND METHODS
Aims and study design.
This study aimed to assess the possibility of vancomycin MIC creep among MRSA isolates from blood cultures obtained over a 5-year period in a teaching hospital in the United Kingdom. We also evaluated trends in daptomycin susceptibility. Second, we sought to identify trends and differences in MICs for both antibiotics by using different susceptibility testing methods.
We evaluated vancomycin susceptibility by Etest performed prospectively on isolates as part of routine clinical practice starting from mid-December 2007. Results were compared firstly with those from prospective testing of nonfrozen subcultures at the Scottish MRSA Reference Laboratory (SMRSARL) by use of an automated system (Vitek 2), available beginning November 2008. Second, results from Etest at the time of isolation were compared with those obtained retrospectively by two methods (Etest and CLSI reference broth microdilution [BMD] method), using stored isolates from the period 2006–2010. Trends in daptomycin susceptibility were also evaluated by Etest at the time of isolation (from mid-December 2007) and on stored isolates.
Study population and data collection.
The Aberdeen Royal Infirmary (ARI) is a tertiary referral center and teaching hospital in North East Scotland, serving a population of 500,000. All MRSA blood culture isolates from ARI between 1 January 2006 and 31 December 2010 were identified from microbiology laboratory records. Any repeat isolates from a patient within 14 days were excluded, as this would be consistent with a treatment course for a single episode of bacteremia.
Laboratory methods.
Original MIC values, obtained by Etest (AB bioMérieux) at the time of isolation, were recovered from laboratory records. All other tests were performed on isolates recovered from storage in Cryobank storage containers (Mast Diagnostics) at −70°C. All isolates recovered from storage were subcultured twice prior to any method of susceptibility testing.
Susceptibility testing at the SMRSARL, Glasgow, United Kingdom, was performed using a Vitek 2 instrument with commercially available Staphylococcus cards (GPS-538; AB bioMérieux). Isolates were sent away on slants and subcultured once but were not frozen prior to susceptibility testing.
For retrospective testing, Etest was performed per the manufacturer's guidelines, using Mueller-Hinton agar (Oxoid), and tests were read blind in duplicate. For prospective testing, Etest was performed as described above as part of the routine laboratory diagnostic service, so results were read only by the biomedical scientist on duty that day. The BMD method was performed with Mueller-Hinton broth (Fluka Analytical, Sigma-Aldrich) and 11 incremental dilutions of vancomycin, from 0.25 to 8.0 mg/liter (0.25, 0.375, 0.5, 0.75, 1.0, 1.5, 2.0, 3.0, 4.0, 6.0, and 8.0 mg/liter [as in the Etest]). The CLSI-recommended reference strain S. aureus ATCC 29213 was used as the control with every set of tests. MICs derived from the Etest or BMD method were also converted to doubling dilutions (i.e., dilutions of 0.0625, 0.125, 0.25, 0.5, 1.0, and 2.0 mg/liter) by rounding up intermediate dilutions.
Strain data were obtained from the SMRSARL. Isolates were typed by the methods routinely used by the reference laboratory at the time they were received. Methods varied slightly, but nearly all isolates had extended antibiogram and urease testing and either pulsed-field gel electrophoresis (PFGE) or spa typing. Provisionally, most isolates could be assigned phenotypically to a known group, and this was confirmed by the PFGE pattern having fewer than three band differences from a known isolate of the group or the spa type being linked to a known isolate by BURP (based upon repeat pattern) analysis using Ridom software. A subset of isolates, including representatives of all groups and all isolates with unusual phenotypes or PFGE patterns, were subjected to multilocus sequence typing (MLST) to assign the groups to MLST clonal complexes (CCs).
Statistical analysis. (i) MIC creep.
The mean MIC, MIC range, MIC50, MIC90, and percentage of isolates with an MIC above the median MIC in the baseline year (2006 for BMD and Etest analysis of frozen samples and 2007 [December only] for contemporaneous Etest analysis) were calculated by year. Temporal trends in vancomycin and daptomycin MICs were explored by graphing MIC population distributions for each year by each testing method. Trends were assessed formally by nonparametric (Spearman's ρ) correlation (MIC versus year of isolate) and Mantel-Haenszel χ2 tests of linear association (% of MIC above the median MIC in the baseline year versus year of isolate).
(ii) Temporal trends in MRSA strain distribution.
To investigate the possibility of clonal expansion explaining patterns of susceptibility, isolates were summarized by year and MLST clonal complex. Antibiogram data were also summarized as percentages of isolates resistant to beta-lactam and non-beta-lactam antibiotics by year. Temporal trends were assessed by Mantel-Haenszel χ2 tests of linear association. Given the number of isolates available, it was not possible to formally model the effects of temporal trends in strain distribution on MIC creep. However, differences in vancomycin or daptomycin MIC distribution by strain for each testing method were assessed by Kruskal-Wallis tests, with post hoc comparisons of individual strains by Mann-Whitney U tests.
(iii) Comparison of MICs by method.
MICs obtained by different testing methods (contemporaneous Etest, automated testing by Vitek 2, Etest analysis of frozen samples, and BMD analysis of frozen samples) were compared for periods when Etest analysis at the time of isolation and automated testing were routine. Differences in MIC results returned by the four different methods were initially explored by graphing the percentages of MICs from one method against differences in vancomycin increments (per Etest strip or doubling dilution) for the MICs returned by an alternative method. A difference of zero indicated no difference. The Friedman test was then used to compare MICs from the same isolates obtained by different testing methods (α = 0.05). Post hoc analysis to explore comparisons between methods was done by Wilcoxon signed rank tests, with a Bonferroni adjustment for multiple comparisons (α = 0.0083). Differences in daptomycin MICs obtained by Etest at the time of isolation or on stored samples were explored by the Wilcoxon signed rank test.
All analyses were repeated using data converted to doubling dilutions. Results were very similar and conclusions were unchanged, so the results are reported (unless stated otherwise) from the analysis of data in their original form (with intermediate dilutions per Etest increments). All data were entered and analyzed in SPSS 19.0.
RESULTS
In the 5-year study period, a total of 208 MRSA blood culture isolates were identified. It was possible to retrieve 99% of the original isolates from storage for further susceptibility testing. MICs from Etest analyses performed at the time of isolation were available for isolates from December 2007 (n = 102 [49%] for vancomycin and n = 93 [45%] for daptomycin). MICs from automated testing (Vitek 2) were available for 50 isolates (24%).
MIC creep. (i) Vancomycin.
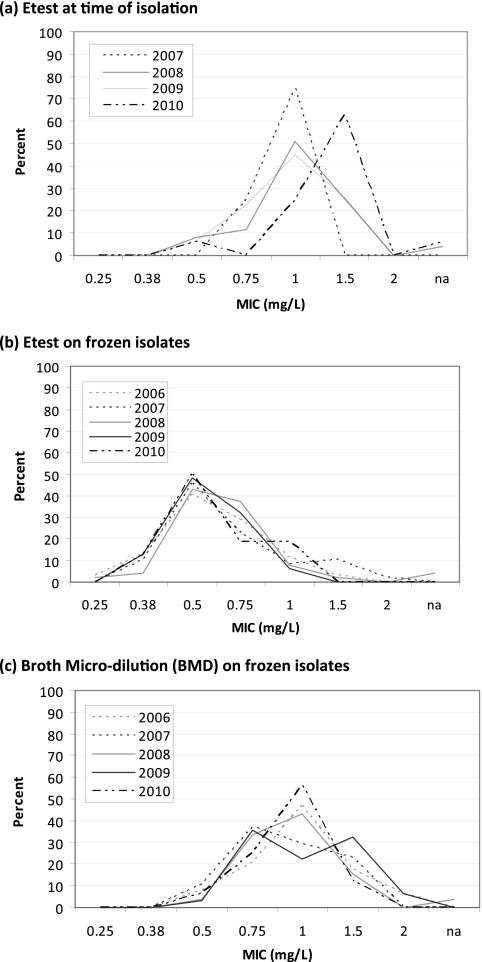
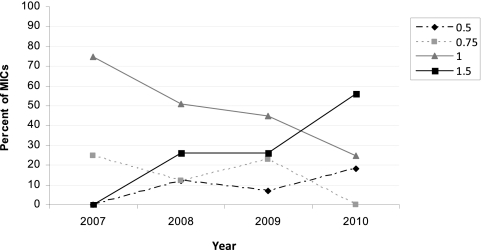
Graphs of MIC distributions from Etest results at the time of isolation by year suggested a shift (creep) toward higher MICs between 2007 and 2010 (Fig. 1a). In contrast, annual MIC distributions from Etest or BMD analysis of stored isolates appeared to be stable. Spearman's rank correlations confirmed a significant increase in MIC by year of study for Etest performed at the time of isolation (P = 0.012) but not in MICs obtained by automated testing (Vitek 2; P = 0.93) or by Etest or BMD analysis of stored isolates (P > 0.80). Between December 2007 and December 2010 (inclusive), the mean MIC increased 1.25-fold for Etest analysis at the time of isolation. This shift is explained largely by an increase in MICs higher than the median MIC in 2008 (1.0 mg/liter), from 25% in 2008 to 56% in 2010 (P = 0.036) (Fig. 2).
Fig 1.
Vancomycin MIC population distribution for each year of available isolates for Etest at the time of isolation (a), Etest on frozen isolates (b), and BMD analysis of frozen isolates (c).
Fig 2.
Vancomycin MIC trends (2007 to 2010) obtained by Etest at the time of isolation.
(ii) Daptomycin.
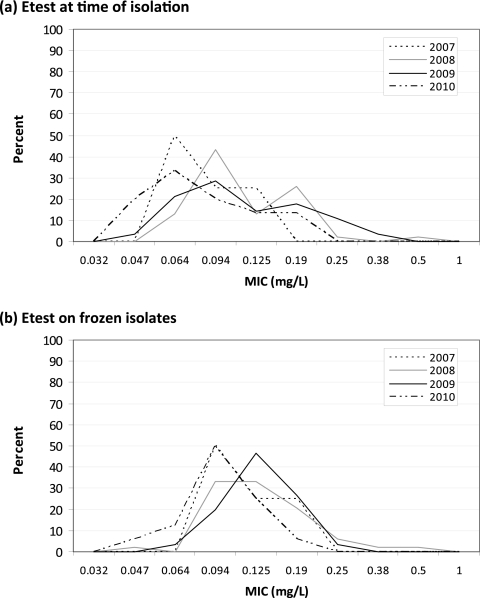
Although the proportion of daptomycin MICs higher than the median MIC in the baseline year fell from 2008 to 2010 for both Etest at the time of isolation and Etest on frozen isolates, the changes were not significant (Table 1 and Fig. 3). However, nonparametric linear correlations suggested a significant downward trend in MICs obtained by Etest at the time of isolation (P = 0.03) that was not present for Etest performed on frozen isolates (P = 0.09).
Table 1.
MRSA MIC (mg/liter) statistics for vancomycin and daptomycin, 2006 to 2010
| Drug and test | Year | No. of isolates | Mean MIC (95% CI) | Modal MIC | MIC range | No. (%) of isolates with MIC > baseline year median MICc | MIC50 | MIC90 |
|---|---|---|---|---|---|---|---|---|
| Vancomycin | ||||||||
| Etest at time of isolation | Total | 99 | 1.08 (1.01–1.14) | 1 | 0.50–1.5 | 30 (29) | 1 | 1.5 |
| 2006a | ||||||||
| 2007a | 4 | 0.94 (0.74–1.14) | 1 | 0.75–1.0 | 1 | 1 | ||
| 2008 | 49 | 1.06 (0.97–1.15) | 1 | 0.50–1.5 | 13 (25) | 1 | 1 | |
| 2009 | 31 | 1.04 (0.93–1.05) | 1 | 0.50–1.5 | 8 (26) | 1 | 1 | |
| 2010 | 15 | 1.23 (1.03–1.44) | 1.5 | 0.50–1.5 | 9 (56) | 1 | 1.5 | |
| Vitek automated testing at time of isolation | Total | 50 | 0.56 (0.51–0.61) | 0.5 | 0.5–1.0 | 6 (12) | 0.5 | 1.5 |
| 2006b | ||||||||
| 2007b | ||||||||
| 2008b | 5 | 0.70 (0.36–1.04) | 0.5 | 0.5–1.0 | 0.5 | 1 | ||
| 2009 | 30 | 0.52 (0.48–0.55) | 0.5 | 0.5–1.0 | 1 (3) | 0.5 | 0.5 | |
| 2010 | 15 | 0.60 (0.49–0.71) | 0.5 | 0.5–1.0 | 3 (20) | 0.5 | 1 | |
| Etest on frozen isolates | Total | 206 | 0.65 (0.62–0.69) | 0.5 | 0.25–2.0 | 90 (43) | 0.5 | 1 |
| 2006 | 62 | 0.64 (0.57–0.70) | 0.5 | 0.25–1.5 | 27 (44) | 0.5 | 1 | |
| 2007 | 48 | 0.72 (0.61–0.83) | 0.5 | 0.38–2.0 | 21 (44) | 0.5 | 1.5 | |
| 2008 | 49 | 0.65 (0.59–0.71) | 0.5 | 0.25–1.5 | 24 (48) | 0.5 | 1 | |
| 2009 | 31 | 0.60 (0.53–0.66) | 0.5 | 0.38–1.0 | 12 (39) | 0.5 | 0.75 | |
| 2010 | 16 | 0.63 (0.51–0.74) | 0.5 | 0.38–1.0 | 6 (38) | 0.5 | 1 | |
| BMD analysis of frozen isolates | Total | 206 | 1.02 (0.97–1.07) | 1 | 0.5–2.0 | 48 (23) | 1 | 1.5 |
| 2006 | 62 | 1.06 (0.97–1.16) | 1 | 0.5–2.0 | 15 (24) | 1 | 1.5 | |
| 2007 | 48 | 0.97 (0.87–1.06) | 0.75 | 0.5–1.5 | 11 (23) | 1 | 1.5 | |
| 2008 | 49 | 0.97 (0.90–1.05) | 1 | 0.5–1.5 | 8 (16) | 1 | 1.5 | |
| 2009 | 31 | 1.12 (0.97–1.27) | 0.75 | 0.5–2.0 | 12 (39) | 1 | 1.5 | |
| 2010 | 16 | 0.97 (0.84–1.11) | 1 | 0.5–1.5 | 2 (13) | 1 | 1.5 | |
| Daptomycin | ||||||||
| Etest at time of isolation | Total | 93 | 0.18 (0.15–0.20) | 0.125 | 0.06–0.75 | 26 (25) | 0.125 | 0.25 |
| 2006a | ||||||||
| 2007a | 4 | 0.19 (0.11–0.27) | 0.19 | 0.13–0.25 | 0.19 | 0.25 | ||
| 2008 | 46 | 0.18 (0.15–0.21) | 0.125 | 0.09–0.75 | 20 (43) | 0.125 | 0.25 | |
| 2009 | 28 | 0.19 (0.15–0.23) | 0.125 | 0.06–0.5 | 13 (46) | 0.125 | 0.38 | |
| 2010 | 15 | 0.13 (0.09–0.16) | 0.094 | 0.06–0.25 | 4 (27) | 0.094 | 0.25 | |
| Etest on frozen isolates | Total | 98 | 0.14 (0.12–0.15) | 0.125 | 0.047–0.5 | 61 (60) | 0.125 | 0.19 |
| 2006a | ||||||||
| 2007a | 4 | 0.13 (0.05–0.20) | 0.094 | 0.094–0.19 | 0.110 | 0.19 | ||
| 2008 | 48 | 0.15 (0.12–0.17) | 0.125 | 0.047–0.5 | 15 (31) | 0.125 | 0.25 | |
| 2009 | 30 | 0.14 (0.12–0.15) | 0.125 | 0.064–0.25 | 9 (29) | 0.125 | 0.19 | |
| 2010 | 16 | 0.10 (0.08–0.12) | 0.094 | 0.05–0.19 | 1 (6) | 0.094 | 0.125 |
Etest analysis was not routine before December 2007.
Vitek automated testing was not routine before November 2008.
The baseline year was taken to be the first full year of data (2008 for Etest at time of isolation and Etest on frozen isolates for daptomycin MIC and 2009 for Vitek 2 automated testing).
Fig 3.
Daptomycin MIC population distribution for each year of available isolates for Etest at the time of isolation (a) and Etest on frozen isolates (b).
Trends in MRSA strain distribution and alternative antibiotic resistance.
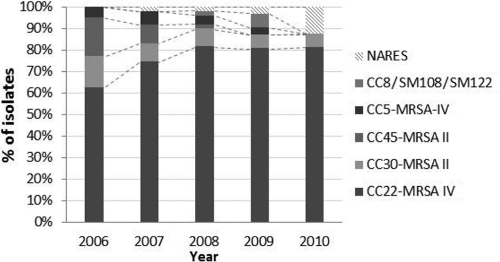
PFGE typing identified the majority of isolates as belonging to MLST clonal complexes CC22-MRSA-IV (73%) and CC30-MRSA-II (10%). There was a significant shift in overall strain distribution by year (χ2 [df], 26.3 [16]; P = 0.029) (Fig. 4 and Table 2). This was mostly explained by a significant increase in isolates of the CC22-MRSA-IV group (62% in 2006 to 81% in 2011; P = 0.019) and a decline in CC45-MRSA-II strains (18% to 0%; P = 0.001).
Fig 4.
Trends in MRSA strain distribution, given as % of isolates per year. Assignment of isolates to MLST clonal complexes was done as described in Materials and Methods.
Table 2.
Trends in MRSA strain distribution by yeara
| Strain/clonal complex | No. (%) of isolates |
P valueb | |||||
|---|---|---|---|---|---|---|---|
| Total | 2006 | 2007 | 2008 | 2009 | 2010 | ||
| All strains | 0.029 | ||||||
| CC22-MRSA IV | 151 (73) | 38 (62) | 35 (74) | 40 (82) | 25 (81) | 13 (81) | 0.019 |
| CC30-MRSA II | 21 (10) | 9 (15) | 4 (9) | 4 (8) | 2 (6) | 1 (6) | 0.175 |
| CC45-MRSA II | 16 (8) | 11 (18) | 4 (9) | 1 (2) | 0 (0) | 0 (0) | <0.001 |
| CC5-MRSA IV | 9 (4) | 3 (5) | 3 (6) | 2 (4) | 1 (3) | 0 (0) | 0.428 |
| CC8/SM108/SM122 | 3 (1) | 0 (0) | 0 (0) | 1 (2) | 2 (6) | 0 (0) | 0.113 |
| NARES (not a recognized strain) | 0 (0) | 0 (0) | 1 (2) | 1 (2) | 1 (3) | 2(13) | 0.036 |
Assignment of isolates to MLST clonal complexes was done as described in Materials and Methods.
By Mantel-Haenszel test of linear association.
Irrespective of the testing method, there were significant differences in vancomycin and daptomycin MIC distributions between strains (Kruskal-Wallis test; P < 0.05). Vancomycin and daptomycin MICs for CC45, CC5, and CC8 strains were typically higher than those for CC22 or CC30 strains (Tables 3 and 4). Considering these two groups, there was a notable decline in those strains with higher MICs between 2006 and 2010 (from 23% to 0%; P < 0.001) and a relative increase in those with lower MICs (77% to 87%) (Tables 3 and 4).
Table 3.
Comparison of vancomycin MICs by strain and testing methoda
| Strain/clonal complex | No. (%) of isolates | Median (IQR) vancomycin MIC (mg/liter)b |
||
|---|---|---|---|---|
| Etest at time of isolation | Etest on frozen isolates | BMD analysis of frozen isolates | ||
| CC22-MRSA IV | 151 (73) | 1.0 (1.0–1.5) | 0.5 (0.5–0.75) | 1.0 (0.5–1.0) |
| CC30-MRSA II | 21 (10) | 0.75 (0.75–1.0) | 0.5 (0.5–0.75) | 0.75 (0.5–1.0) |
| CC45-MRSA II | 16 (8) | 1.5 (—) | 1.0 (0.75–1.0) | 1.5 (1.5–2.0) |
| CC5-MRSA IV | 9 (4) | 1.5 (—) | 1.0 (1.0–1.5) | 1.5 (1.0–1.5) |
| CC8/SM108/SM122 | 3 (1) | 1.5 (—) | 1.0 (—) | 1.5 (—) |
All strains were compared by the Kruskal-Wallis test, and the P values were 0.001, <0.001, and <0.001 for Etest at time of isolation, Etest on frozen isolates, and BMD analysis of frozen isolates, respectively. CC22/CC30 and CC45/CC5/CC8 strains were compared by the Mann-Whitney U test, and the P values were <0.001 and 0.007 for Etest on frozen isolates and BMD analysis of frozen isolates, respectively.
IQR, interquartile range. —, numbers were too small for valid calculation.
Table 4.
Comparison of daptomycin MICs by strain and testing methoda
| Strain/clonal complex | No. (%) of isolates | Median (IQR) daptomycin MIC (mg/liter)b |
|
|---|---|---|---|
| Etest at time of isolation | Etest on frozen isolates | ||
| CC22-MRSA IV | 151 (73) | 0.125 (0.094–0.19) | 0.125 (0.094–0.125) |
| CC30 (ST36)-MRSA II | 21 (10) | 0.25 (0.125–0.25) | 0.125 (0.094–0.19) |
| Weighted avg (CC22 and CC30) | 0.125 (0.094–0.19) | 0.125 (0.094–0.19) | |
| CC45 (ST45)-MRSA II | 16 (8) | — | — |
| CC5-MRSA IV | 9 (4) | 0.250 (—) | 0.19 (0.125–0.19) |
| CC8/SM108/SM122 | 3 (1) | 0.250 (—) | 0.19 (0.19–0.25) |
| Weighted avg (CC45, CC5, and CC8) | 0.250 (0.125–0.250) | 0.19 (0.125–0.19) | |
All strains were compared by the Kruskal-Wallis test, and the P values were 0.047 and 0.012 for Etest at time of isolation and Etest on frozen isolates, respectively. CC22/CC30 and CC45/CC5/CC8 strains were compared by the Mann-Whitney U test, and the P values were 0.004 and 0.003 for Etest at time of isolation and Etest on frozen isolates, respectively.
IQR, interquartile range. —, numbers were too small for valid calculation.
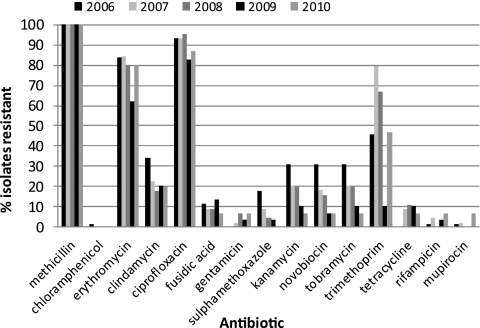
Antibiogram data revealed significant reductions in resistance to sulfamethoxazole, kanamycin, novobiocin, and tobramycin (P < 0.05), which have previously been attributed to regional shifts in predominant strains from CC30-MRSA-II to CC22-MRSA-IV (11) (Fig. 5). Patterns of resistance were otherwise stable.
Fig 5.
Percentages of isolates resistant to antibiotics by year and antibiotic.
MICs obtained by different methods.
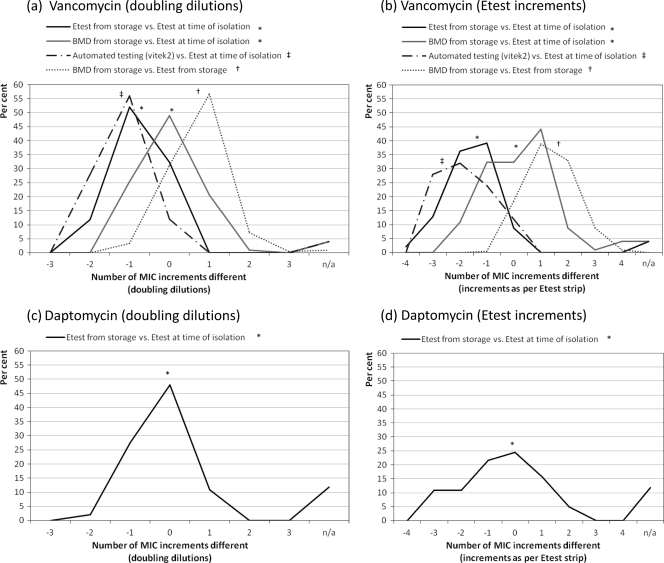
We found a significant difference in vancomycin MICs obtained for the same isolates by four different testing methods (Friedman test χ2 [3 degrees of freedom] = 97; P < 0.001) (Fig. 6a and b). Post hoc testing revealed that vancomycin MICs from Etest analysis of stored isolates were significantly lower than those from Etest analysis at the time of isolation (Z = −8.37; P < 0.001). In contrast, there was no significant difference in the MICs obtained by BMD analysis of stored isolates and those obtained by Etest at the time of isolation (Z = −1.19; P = 0.235). Vancomycin MICs from automated testing (Vitek 2) were also significantly lower than those found by Etest at the time of isolation (Z = −5.75; P < 0.001).
Fig 6.
Comparison of MICs by method of testing, given as percentages within MIC increments of alternative method. †, January 2006 to December 2010 (n = 206 isolates); ∗, December 2007 to December 2010 (n = 102); ‡, November 2008 to December 2010 (n = 50).
Daptomycin MICs obtained by repeat Etest analysis of frozen isolates were significantly lower than those obtained by Etest at the time of isolation (Wilcoxon signed rank test; Z = −4.808; P < 0.001) (Fig. 6b).
There were no significant associations between the duration of storage of isolates (months from initial isolation) and the difference between MICs from Etest on frozen samples and MICs from Etest at the time of isolation for either vancomycin (Spearman's ρ = 0.089; P = 0.372) or daptomycin (ρ = −0.158; P = 0.138).
All of the above analyses were repeated on MIC data converted to doubling dilutions. Results for both analysis of creep and intermethod comparisons were very similar to those derived from data for intermediate dilutions, and all conclusions were unchanged.
DISCUSSION
This study from North East Scotland found limited evidence of vancomycin MIC creep in susceptibility testing performed at the time of isolation. However, vancomycin MIC creep appears to be an unstable phenomenon that is not necessarily reproducible in stored MRSA isolates or even between different testing methods at the time of isolation. In particular, evidence of creep was absent from results from automated testing (Vitek 2) at the time of isolation and both BMD and Etest methods used on previously frozen isolates. Similarly, a declining trend in daptomycin MICs from Etest at the time of isolation was not seen for frozen isolates. The results revealed significant intermethod variation in MICs obtained from the same isolates, irrespective of antibiotic. We noted significant changes in MRSA strain during the study period, which may have affected trends in susceptibility.
In the methodology of similar studies assessing vancomycin MIC creep, isolates have been frozen previously (8, 13, 25, 32) or this has not been made explicitly clear (1, 7, 12, 27, 28), though there appears to be no correlation between known previously frozen isolates and the presence or absence of creep. As far as we are aware, there has been only one study where MIC trends have been evaluated by BMD analysis of isolates at the time of clinical isolation, and this study showed MIC creep (34). Our results show that storage significantly affects the MIC, which in turn obfuscates susceptibility trends. This effect was demonstrated by two antibiotics with divergent trends in antibiotic susceptibility: an increase in vancomycin MICs and a decrease in daptomycin MICs over time. The reliance on data from retrospective cohort studies may explain some of the inconsistency in reporting of MIC creep for vancomycin, and this certainly highlights the need for prospective data collection (5).
We can draw no conclusions about whether the MIC at the time of isolation is the most accurate reflection of an isolate's susceptibility to vancomycin, although it would seem reasonable to assume that this MIC is the most clinically relevant. We know that storage affects the susceptibility of MRSA; in particular, in studying VISA and hetero-VISA, it can be necessary to use media containing vancomycin to reinduce resistance after storage (2). Our data suggest that changes in susceptibility occur early in storage (within the first 6 months), as we found no association between the duration of storage and the disparity in MICs beyond that reported here. Further work is ongoing to determine a potential cutoff duration for storage, before which isolates could safely be assumed to have the same susceptibility as that at the time of isolation.
This series suggests that BMD might be a more accurate way of assessing frozen isolates, as it might be more representative of the virulence of the original isolates. Unusually, this series showed a BMD MIC higher than that obtained by Etest, which is in conflict with most, but not all, of the literature (5, 21, 29, 33). It is possible that this may be explained by the use of different suppliers for Mueller-Hinton broth and agar. Since all controls were satisfactory for each set of tests, we can offer no further explanation for this discordant result. In our data, the BMD MIC obtained from frozen isolates was closer to the original MIC obtained by Etest at the time of isolation than the Etest MIC obtained from frozen isolates.
Contention remains regarding the appropriate vancomycin breakpoint for S. aureus, and the clear differences in MIC values highlighted here, not only by methodology but also for isolates from storage versus those at the time of isolation, may cloud this issue further (5). For many laboratories, automated systems are in routine use for assessing the antibiotic susceptibility of MRSA, possibly with very few centers additionally employing either Etest or BMD as part of routine benchwork. These issues need to be addressed in order for both accurate and reproducible assessments to be made. Differences in methodology are well known to affect the MIC. In this setting, even small changes could have significant consequences because of the narrow therapeutic window of vancomycin. If we used the proposed breakpoint of 1 mg/liter by Etest on our prospectively collected data, the percentage of resistant isolates would increase from 25% to 56% over the period represented by the study (5). Automated systems return vancomycin MICs below the breakpoint (MICs of ≤2 mg/liter) that are known to be unreliable predictors of clinical response (5, 29, 33). Consistent with this, our study showed large disparities between results obtained by a Vitek 2 instrument and those obtained by Etest at the time of isolation.
As yet, only Spanish guidelines have addressed the potential role of vancomycin MIC in the treatment of MRSA, but this may change as increasing evidence of clinical failure associated with MRSA strains that have elevated MICs within the susceptible range is gathered (6). Notably, the Infectious Diseases Society of America (IDSA) guidelines currently suggest that the MIC is irrelevant in determining the continued use of vancomycin for any infections caused by MRSA when the MIC is within the susceptible range (15). Any change in therapy should be the clinician's decision, based on the clinical response to treatment. Unfortunately, the IDSA guidelines do not detail which methods should be employed for susceptibility testing. We recommend that if any guidelines incorporate a requirement for MIC testing for vancomycin treatment, these would need to detail specific methods, not merely breakpoints.
Recent articles evaluating the necessary pharmacokinetic/pharmacodynamic (PK/PD) targets also suggest that the probability of attaining the appropriate area under the concentration-time curve (AUC)/MIC ratio of ≥400 is the best predictor of outcome with vancomycin (14, 23). The likelihood of attaining this is considerably decreased as the MIC approaches 2 mg/liter, with the associated nephrotoxicity one would expect from the larger doses required (14). As more data become available, the AUC/MIC ratio may become incorporated into routine practice. However, our study suggests that interpretation in clinical practice and research should take into account the MIC testing method used.
We are aware that this study has limitations. A small sample size and limited clinical and therapeutic data did not permit meaningful exploration of correlations between MIC and clinical outcome. Evidence of actual vancomycin use was available for 128 (62%) patients, although it is likely that a proportion of those for whom treatment was not detailed (n = 60 [38%]) may have received vancomycin in line with the hospital's empirical antibiotic guidelines. We did not seek to prove the reproducibility of MIC results for each of the testing methods, which may limit conclusions about intermethod differences in MICs. However, duplicate and independent readings of MIC results, with good agreement, suggested limited interobserver variation (data not shown). Furthermore, tests of difference suggested systematic differences between results returned by different testing methods that were not accounted for by variation in MICs within each testing method.
We were able to obtain only limited data on PFGE typing and characterization of trends in strain distribution. Previous work has shown that North East Scotland saw a relatively rapid change in strain distribution, from a predominance of CC30/E16 to CC22/E15 strains, from 2003 to 2007 (11), which appears to have been sustained. Given the associations between strain and MIC, clonal expansion may have implications for trends in susceptibility. The higher MICs obtained for CC45 are convergent with a large proportion of VISA isolates described for the related USA600 lineage (20). The decline in strains with higher MICs in our study may offer an explanation for increasing susceptibility of MRSA isolates to daptomycin. However, it suggests an opposing pressure to described increases in vancomycin MICs. Nevertheless, without detailed typing throughout the study period, we were unable to rule out the possibility of dissemination of subtypes within strains (e.g., CC22) which may account for vancomycin MIC creep.
This study highlights the need to increase awareness among clinicians of the importance of the vancomycin MIC in treating MRSA infections, as well as the problems in its interpretation. Our findings suggest a need to streamline susceptibility testing, and perhaps to lower vancomycin breakpoints further, to avoid clinical failure and the increased risk of mortality. As the MIC becomes increasingly crucial in treatment decisions, the issue of MIC discrepancies for isolates after storage and for measurements by different methods will benefit from further prospective study.
ACKNOWLEDGMENTS
We acknowledge the invaluable contributions of everyone in the diagnostic laboratory and media departments of ARI and also the SMRSARL for sending missing isolates and providing MIC and typing data.
I. M. Gould has received personal and grant financial support from companies manufacturing diagnostics and therapeutics for MRSA. B. Edwards has received grant financial support from Novartis. All other authors have no conflicts to declare.
We are grateful for financial support from Novartis.
Footnotes
Published ahead of print 30 November 2011
REFERENCES
- 1. Alós J-I, García-Cañas A, García-Hierro P, Rodríguez-Salvanés F. 2008. Vancomycin MICs did not creep in Staphylococcus aureus isolates from 2002 to 2006 in a setting with low vancomycin usage. J. Antimicrob. Chemother. 62: 773–775 [DOI] [PubMed] [Google Scholar]
- 2. Chesneau O, Morvan A, El Solh N. 2000. Retrospective screening for heterogeneous vancomycin resistance in diverse Staphylococcus aureus clones disseminated in French hospitals. J. Antimicrob. Chemother. 45: 887–890 [DOI] [PubMed] [Google Scholar]
- 3. Dan M, Richardson J, Miliotis MD, Koornhof HJ. 1989. Comparison of preservation media and freezing conditions for storage of specimens of faeces. J. Med. Microbiol. 28: 151–154 [DOI] [PubMed] [Google Scholar]
- 3a. Edwards B, Milne K, Lawes T, Cook I, Gould IM. 2011. Vancomycin susceptibility trends in MRSA isolated from blood cultures in Aberdeen Royal Infirmary from 2006–2010. Is vancomycin “creep” method dependent?, abstr. P765. Abstr. 21st Eur. Cong. Clin. Microbiol. Infect. Dis., 7 to 10 May 2011, Milan, Italy [Google Scholar]
- 4. Gomez J, et al. 2007. Predictors of mortality in patients with methicillin-resistant Staphylococcus aureus (MRSA) bacteraemia: the role of empiric antibiotic therapy. Eur. J. Clin. Microbiol. Infect. Dis. 26: 239–245 [DOI] [PubMed] [Google Scholar]
- 5. Gould IM. 2010. Is vancomycin redundant for serious staphylococcal infection? Int. J. Antimicrob. Agents 36(Suppl 2): S55–S57 [DOI] [PubMed] [Google Scholar]
- 6. Gudiol F, et al. 2009. Documento de consenso sobre el tratamiento de la bacteriemia y la endocarditis causada por Staphylococcus aureus resistente a la meticilina. Enferm. Infecc. Microbiol. Clin. 27: 105–115 [DOI] [PubMed] [Google Scholar]
- 7. Hawser SP, Bouchillon SK, Hoban DJ, Dowzicky M, Babinchak T. 2011. Rising incidence of Staphylococcus aureus with reduced susceptibility to vancomycin and susceptibility to antibiotics: a global analysis 2004–2009. Int. J. Antimicrob. Agents 37: 219–224 [DOI] [PubMed] [Google Scholar]
- 8. Holmes RL, Jorgensen JH. 2008. Inhibitory activities of 11 antimicrobial agents and bactericidal activities of vancomycin and daptomycin against invasive methicillin-resistant Staphylococcus aureus isolates obtained from 1999 through 2006. Antimicrob. Agents Chemother. 52: 757–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Howden BP, Davies JK, Johnson PDR, Stinear TP, Grayson ML. 2010. Reduced vancomycin susceptibility in Staphylococcus aureus, including vancomycin-intermediate and heterogeneous vancomycin-intermediate strains: resistance mechanisms, laboratory detection, and clinical implications. Clin. Microbiol. Rev. 23: 99–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hsu DI, et al. 2008. Comparison of method-specific vancomycin minimum inhibitory concentration values and their predictability for treatment outcome of methicillin-resistant Staphylococcus aureus (MRSA) infections. Int. J. Antimicrob. Agents 32: 378–385 [DOI] [PubMed] [Google Scholar]
- 11. Hunt AC, et al. 2011. Methicillin-resistant Staphylococcus aureus in northeastern Scotland in 2003 to 2007: evolving strain distribution and resistance patterns. J. Clin. Microbiol. 49: 1975–1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jones RN. 2006. Microbiological features of vancomycin in the 21st century: minimum inhibitory concentration creep, bactericidal/static activity, and applied breakpoints to predict clinical outcomes or detect resistant strains. Clin. Infect. Dis. 42(Suppl 1): S13–S24 [DOI] [PubMed] [Google Scholar]
- 13. Kehrmann J, et al. 2011. Vancomycin MIC creep in MRSA blood culture isolates from Germany: a regional problem? Eur. J. Clin. Microbiol. Infect. Dis. 30: 677–683 [DOI] [PubMed] [Google Scholar]
- 14. Kullar R, Davis SL, Levine DP, Rybak MJ. 2011. Impact of vancomycin exposure on outcomes in patients with methicillin-resistant Staphylococcus aureus bacteraemia: support for consensus guidelines suggested targets. Clin. Infect. Dis. 52: 975–981 [DOI] [PubMed] [Google Scholar]
- 15. Liu C, et al. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 52: 285–292 [DOI] [PubMed] [Google Scholar]
- 16. Lodise TP, et al. 2008. Relationship between vancomycin MIC and failure among patients with methicillin-resistant Staphylococcus aureus bacteremia treated with vancomycin. Antimicrob. Agents Chemother. 52: 3315–3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lubin AS, Snydman DR, Ruthazer R, Bide P, Golan Y. 2011. Predicting high vancomycin minimum inhibitory concentration in methicillin-resistant Staphylococcus aureus bloodstream infections. Clin. Infect. Dis. 52: 997–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moise PA, North D, Steenberg JN, Sakoulas G. 2009. Susceptibility relationship between vancomycin and daptomycin in Staphylococcus aureus: facts and assumptions. Lancet Infect. Dis. 9: 617–624 [DOI] [PubMed] [Google Scholar]
- 19. Moise PA, Sakoulas G, Forrest A, Schentag JJ. 2007. Vancomycin in vitro bactericidal activity and its relationship to efficacy in clearance of methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob. Agents Chemother. 51: 2582–2586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moore C, et al. 2010. USA600 (ST45) methicillin-resistant Staphylococcus aureus bloodstream infections in urban Detroit. J. Clin. Microbiol. 48: 2307–2310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Musta AC, et al. 2009. Vancomycin MIC plus heteroresistance and outcome of methicillin-resistant Staphylococcus aureus bacteremia: trends over 11 years. J. Clin. Microbiol. 47: 1640–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ohkusa T, Miwa H, Endo S, Okayasu I, Sato N. 2004. Helicobacter pylori is a fragile bacteria when stored at low and ultra-low temperatures. J. Gastroenterol. Hepatol. 19: 200–204 [DOI] [PubMed] [Google Scholar]
- 23. Patel N, et al. 2011. Vancomycin: we can't get there from here. Clin. Infect. Dis. 52: 969–974 [DOI] [PubMed] [Google Scholar]
- 24. Paul M, et al. 2010. Importance of appropriate empirical antibiotic therapy for methicillin-resistant Staphylococcus aureus bacteraemia. J. Antimicrob. Chemother. 65: 2658–2665 [DOI] [PubMed] [Google Scholar]
- 25. Robert J, Bismuth R, Jarlier V. 2006. Decreased susceptibility to glycopeptides in methicillin-resistant Staphylococcus aureus: a 20 year study in a large French teaching hospital, 1983–2002. J. Antimicrob. Chemother. 57: 506–510 [DOI] [PubMed] [Google Scholar]
- 26. Rojas L, et al. 2010. Impact of vancomycin MIC and vancomycin serum levels on the outcome of methicillin-resistant Staphylococcus aureus (MRSA) bloodstream infection (BSI), abstr K-337. Abstr. Intersci. Conf. Antimicrob. Agents Chemother., Boston, MA [Google Scholar]
- 27. Rybak MJ, et al. 2008. Characterization of vancomycin-heteroresistant Staphylococcus aureus from the metropolitan area of Detroit, Michigan, over a 22-year period (1986 to 2007). J. Clin. Microbiol. 46: 2950–2954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sader HS, et al. 2009. Evaluation of vancomycin and daptomycin potency trends (MIC creep) against methicillin-resistant Staphylococcus aureus isolates collected in nine U.S. medical centers from 2002 to 2006. Antimicrob. Agents Chemother. 10: 4127–4132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sader HS, Rhomberg PR, Jones RN. 2009. Nine-hospital study comparing broth microdilution and Etest method results for vancomycin and daptomycin against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 53: 3162–3165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sakoulas G, et al. 2004. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J. Clin. Microbiol. 42: 2398–2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Soriano A, et al. 2008. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin. Infect. Dis. 46: 193–200 [DOI] [PubMed] [Google Scholar]
- 32. Steinkraus G, White R, Friedrich L. 2007. Vancomycin MIC creep in non-vancomycin-intermediate Staphylococcus aureus (VISA), vancomycin-susceptible clinical methicillin-resistant S. aureus (MRSA) blood isolates from 2001–05. J. Antimicrob. Chemother. 60: 788–794 [DOI] [PubMed] [Google Scholar]
- 33. Tenover FC. 2010. The quest to identify heterogeneously resistant vancomycin-intermediate Staphylococcus aureus strains. Int. J. Antimicrob. Agents 36: 303–306 [DOI] [PubMed] [Google Scholar]
- 34. Wang G, Hindler JF, Ward KW, Bruckner DA. 2006. Increased vancomycin MICs for Staphylococcus aureus clinical isolates from a university hospital during a 5-year period. J. Clin. Microbiol. 44: 3883–3886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yoon YK, Kim JY, Park DW, Sohn JW, Kim MJ. 2010. Predictors of persistent methicillin-resistant Staphylococcus aureus bacteremia in patients treated with vancomycin. J. Antimicrob. Chemother. 65: 1015–1018 [DOI] [PubMed] [Google Scholar]