Abstract
Hematogenous Candida meningoencephalitis (HCME) is a serious infection in premature neonates. Anidulafungin is an echinocandin antifungal agent with potent activity against Candida spp., but its efficacy and optimal regimens for human neonates with HCME are not known. A well-validated rabbit model of HCME was used to define pharmacokinetic-pharmacodynamic (PK-PD) relationships of anidulafungin. A mathematical model was fitted to the entire data set. The experimental data were bridged to humans. A population PK model was fitted to the data from human neonates receiving anidulafungin receiving a loading dose of 3 mg/kg, followed by 1.5 mg/kg/day. Monte Carlo simulations were performed to identify candidate anidulafungin regimens for humans. All untreated rabbits succumbed by ≤96 h postinoculation. The PK of anidulafungin was linear with dose-dependent penetration into the cerebrum. Anidulafungin exerted a rapid antifungal effect that was apparent in the first dosing interval. Near-maximal antifungal activity was observed with dosages of 10 to 20 mg/kg/day. The bridging studies suggested that the current regimen of first 3 mg/kg, followed by 1.5 mg/kg/day, is suboptimal. Higher dosages were associated with progressively greater antifungal effect. Anidulafungin is effective for the treatment of experimental HCME. Higher dosages than those currently used for clinical care are required for maximal antifungal effect.
INTRODUCTION
Hematogenous Candida meningoencephalitis (HCME) is an infection that affects premature neonates (3). HCME is associated with significant short-term mortality and devastating longer-term neurodevelopmental abnormalities (3). Diagnosis remains difficult. The neurotropism of Candida in premature neonates means that infection within the central nervous system (CNS) should be assumed. There are relatively few therapeutic options. Newer agents are urgently required and information regarding optimal regimens of existing compounds would facilitate the optimal treatment of HCME.
Anidulafungin is an echinocandin antifungal agent with a number of favorable attributes that include rapid fungicidal activity, a favorable toxicity profile, and a paucity of drug-drug interactions (7). Anidulafungin is an effective compound for the treatment of invasive candidiasis in adults (17). The pharmacokinetics (PK) have been studied in children and neonates (2, 5). In the latter, a loading dose of 3 mg/kg, followed by 1.5 mg/kg/day, results in areas under the concentration-time curve (AUCs) that are comparable to those of a standard adult regimen (5). The use of anidulafungin for neonates requires that the efficacy and potential candidate regimens for HCME must be explicitly established.
We use a well-validated experimental model of HCME and a translational pharmacological approach to identify candidate regimens for anidulafungin for use in premature neonates with HCME. We show that relatively high dosages are required to achieve near maximal antifungal activity in the brain of rabbits. Correspondingly, neonatal regimens that are likely to be associated with near maximal antifungal activity are higher than those that have been recently studied or approved for clini-cal use.
MATERIALS AND METHODS
Experimental model of HCME and challenge strain of Candida albicans.
All experiments were conducted under UK Home Office project license PPL40/3101 and approved by The University of Manchester's ethics committee. A previously described and well-validated non-neutropenic rabbit model of HCME was used with several modifications (9, 12). Briefly, male New Zealand White rabbits weighing 1.7–3.18 kg at the time of surgery were used. Central venous access was established under general anesthesia. (Harlan Laboratories UK, Ltd.). A subcutaneous intravenous (i.v.) port (Titanium Soloports; Linton Instrumentation, Norfolk, United Kingdom) was used for inoculation, the administration of anidulafungin, and the procurement of plasma samples. The line was flushed with 1 ml of saline, followed by 0.5 ml of wash solution (0.1 ml of sodium heparin [5,000 U/ml] plus 9.9 ml of saline), and then locked with 0.1 ml of lock solution (9 ml of 5% dextrose with 1 ml of sodium heparin [5,000 U/ml]) immediately after use.
The challenge strain was ATCC MYA-1237 (NIH 8621), as has been used previously in this model (9, 12). The MIC of anidulafungin was determined using both Clinical and Laboratory Standards Institute (CLSI) (4) and European Committee for Antimicrobial Susceptibility Testing (EUCAST) (1) methodologies in three separate independently conducted experiments.
C. albicans was retrieved from beads stored at −80°C and plated to Sabouraud agar (Oxoid, United Kingdom) and then incubated at 37°C for 24 h. Several colonies were then placed in Sabouraud broth. The desired inoculum of 106 organisms per rabbit was determined using a hemocytometer. The inoculum was administered intravenously as a bolus in a 1-ml volume of phosphate-buffered saline (PBS). Food and water were provided ad libitum. Anidulafungin was administered at 48 h postinoculation. The clinical formulation of anidulafungin was reconstituted in sterile water and further diluted to obtain the desired concentration. Drug was injected i.v. over 1 min via the indwelling catheter. Dosages of 5, 10, and 20 mg/kg were used following preliminary dose finding studies. Rabbits received a maximum of two dosages at 48 and 72 h postinoculation. The model was not continued beyond 96 h because all controls had died by that time. Rabbits were sacrificed throughout the experimental period at either predefined time points or if neurological signs prevented ready access to food and water. Terminal pharmacokinetic and pharmacodynamic samples were collected from each animal. Rabbits were sacrificed with a lethal dose of i.v. pentobarbital (80 mg/kg; Animalcare, Ltd., York, United Kingdom), administered via the indwelling intravenous line.
Pharmacokinetics and pharmacodynamic studies.
Plasma was collected from each rabbit via the indwelling catheter in the first and second dosing interval at 0, 0.5, 1, 2, 6, and 24 h after administration of the drug. Blood samples were placed on ice and immediately centrifuged at 12,500 rpm for 5 min. Plasma was stored at −80°C until analysis. Immediately after sacrifice, the cerebrum and cerebellum were removed. Representative portions of cerebrum were placed in Whirl-Pak bags (Nasco, CA) and frozen at −80°C for measurement of anidulafungin concentrations. Representative sections of cerebrum were placed in 2 ml of PBS and homogenized. Serial 10-fold dilutions were prepared, and 100 μl was immediately plated to Sabouraud agar. The plates were incubated at 37°C for up to 48 h, after which the colony counts in the brain were enumerated.
Measurement of anidulafungin in rabbit plasma and cerebrum.
Anidulafungin concentrations in rabbit plasma and cerebrum were measured using high-performance liquid chromatography with a Shimadzu Prominence (Shimadzu, Milton Keynes, United Kingdom). A Kinetex 2.6u C18 New Column (75 by 4.6 mm) was used (Phenomenex, Macclesfield, United Kingdom). The injection volume was 5 μl. A standard curve encompassing 0.05 to 10 mg/liter in plasma and 0.006 to 10 mg/liter in the brain was constructed from stock solutions of anidulafungin at 1,000 mg/liter in dimethyl sulfoxide by progressive dilution in methanol (Fisher Scientific, Loughborough, United Kingdom). The internal standard was micafungin. The mobile phase was 65% 0.1% (vol/vol) trifluoroacetic acid (TFA) in water and 35% acetonitrile with 0.1% (vol/vol) TFA with a gradient profile changing to 30 and 70% respectively over 4 min. The overall run time was 6.25 min, and the flow rate was 1 ml/min. Anidulafungin was detected using fluorescence with an excitation wavelength of 273 nm and an emission wavelength of 464 nm. Anidulafungin and the internal standard eluted after 4.5 and 3.4 min, respectively. For plasma, the percent coefficient of variation (%CV) was <2.4% over the concentration range 0.05 to 10 mg/liter. The limit of detection was 0.05 mg/liter. The intra- and interday variation was <2.4%. For cerebrum, the %CV was <4.7% over the concentration range from 0.006 to 10 mg/liter. The limit of detection was 0.006 mg/liter. The intra- and interday %CV were both <5%.
Mathematical modeling.
A four-compartment mathematical model was fitted to the pharmacokinetic-pharmacodynamic (PK-PD) data using a population methodology and with the use of the BIG version of the nonparametric adaptive grid program (BIG NPAG) (15). The structural mathematical model used for these analyses consisted of the following four ordinary differential equations:
| (1) |
| (2) |
| (3) |
| (4) |
| (4a) |
| (4b) |
where X1, X2, and X3 are the amounts of anidulafungin (in milligrams) in the central compartment, brain, and peripheral compartment, respectively. R (1) is the total amount of drug administered i.v. (in milligrams) over the course of the 1-min infusion. SCL is the clearance. Vc and Vbrain represent the volumes of the central compartment and brain, respectively. kcp, kpc, kck, and kkc are the first-order rate constants that connect the respective compartments. N is the density (organisms/gram of brain) of C. albicans. Kgmax is the maximal rate of growth. POPMAX is the theoretical maximal density within the brain. Hg is the slope function for the suppression of growth. C50g is the concentration of anidulafungin in the brain that induces half-maximal suppression of growth. Kkmax is the maximal rate of killing. Hk is the slope functions for the fungal kill. Finally, C50k is the concentration of anidulafungin in the brain where fungal kill is half-maximal. Equation 1 describes the rate of change of anidulafungin in the central compartment (plasma). Equation 2 describes the rate of change of anidulafungin in the brain. Equation 3 describes the rate of change of anidulafungin in the peripheral compartment (i.e., everything other than the blood and the brain). Equation 4 describes the rate of change of fungal burden in the brain that contains terms describing the capacity-limited growth of Candida (equation 4a), the drug-associated suppression of growth (equation 4b), and the drug-associated fungal killing (equation 4c).
The weighting functions for the PK-PD data were obtained by initially fitting the same structural mathematical model to the PK and PD data in the program ADAPT 5 (6) using the maximum-likelihood estimator, as previously described (18). Both mean and median parameter estimates were explored and discriminated according to the observed-predicted values for both the PK and the PD data.
Population pharmacokinetics of anidulafungin in neonates.
The previously described data for 15 neonates and infants receiving 3 mg/kg on day 1, followed by 1.5 mg/kg/day infused i.v. over 1 h, were obtained (5). One infant who died and for whom there was some uncertainty about the timing of samples was excluded from the analysis. The population also contained two neonates that were receiving extra corporal membrane oxygenation (ECMO), and these patients were similarly excluded from the analysis (because of the potential for binding of anidulafungin to the ECMO circuit). A previously described allometric pharmacokinetic model (13, 14) was fitted to the PK data from the remaining 12 patients. This structural mathematical model consisted of two ordinary differential equations, which were as follows:
| (1) |
| (2) |
where X1 and X2 represent the amount of anidulafungin (milligrams) in the central (c) and peripheral (p) compartments, respectively. R(1) represents the infusion of drug into the central compartment. SCLstd and Vstd represent the normalized estimates for clearance and volume for a 70-kg individual. kcp and kpc are the first-order intercompartmental rate constants. Clearance was estimated using a 0.75 scaling exponent, and clearance and volume were both normalized to a 70-kg adult patient. The fit of the model to the data was assessed in the same manner as that described above for rabbits.
Bridging from rabbits to humans to identify candidate regimens.
The experimental data were bridged to humans to provide an insight into potential candidate regimens for the use of anidulafungin for neonates with HCME. Monte Carlo simulations were performed using the program ADAPT 5 (6), and the mean parameter values and their variances from the allometric population PK model fitted to the human neonatal data set. We used two different, but complementary in vivo-to-human bridging methods.
In the first instance, the AUCs were estimated for 5,000 simulated human neonates receiving anidulafungin 1.5, 3, 4.5, 6, 7.5, and 9 mg/kg/day i.v. The AUCs from the simulated human neonates for each regimen were collated into groups and organized using a histogram. The linked PK-PD mathematical model was used to estimate the fungal burden in the cerebrum at the end of the experiment (96 h postinoculation) as a function of the AUCs for each of the groups in the histogram. The predicted fungal burden for each AUC was multiplied by the proportion of the simulated population with that particular AUC. An expectation for the predicted fungal burden for the simulated population was then calculated by summation of these products. This method has the advantage of enabling an estimate of the impact of variability in the PK of anidulafungin in neonates on the ultimate antifungal effect but also the disadvantage of not readily enabling the effect of loading dosages to be discerned (because a question arises as to which AUC is most relevant).
To provide an additional perspective on candidate antifungal regimens that incorporate loading dosages, we “humanized” the rabbit pharmacokinetics in silico and used the mathematical model to obtain a pharmacodynamic readout of this human-like regimen. The human population PK model was used to define the 5th and 95th centile of drug concentrations for a neonatal population receiving a variety of candidate anidulafungin regimens. Anidulafungin was then administered to rabbits (in silico) so that the concentration-time profile in rabbits was completely contained within the concentration profiles of the 5th and 95th centile of the simulated human population. The rabbit mathematical model was then used to obtain a pharmacodynamic readout for these humanized regimens. An advantage of this approach is the ability to explore the potential antifungal effect of a loading dose.
RESULTS
The MIC for the challenge strain was 0.0039 mg/liter using both CLSI and EUCAST methodologies. After inoculation there was progressive logarithmic growth in the cerebrum throughout the experimental period. Without antifungal therapy, no rabbit survived beyond 96 h. Rabbits that did not reach predefined study time points were sacrificed for signs that were consistent with focal neurological events (e.g., paralysis of a limb or inability to hop), which meant animals were unable to move to food and water. We occasionally observed fitting but did not see signs that were consistent with uncontrolled sepsis. All animals appeared mildly symptomatic (e.g., subdued behavior) prior to the initiation of antifungal therapy 48 h postinoculation. Taken collectively, this model of HCME is severe and therefore is a rigorous test of any antifungal agent.
The pharmacokinetics of anidulafungin were linear. The administration of 5 to 20 mg/kg resulted in high peak concentrations, with rapid distribution and a longer elimination phase (Fig. 1 and 2). Anidulafungin was well tolerated, with no objective evidence of drug related toxicity at the time of injection or thereafter. Anidulafungin penetrated the brain, with quantifiable concentrations in the first dosing interval using all dosages in the present study. The concentration-time profile of anidulafungin in the brain was much flatter than that observed in plasma. The AUCcerebrum/AUCplasma ratio was 15%. There was no evidence of hysteresis with delayed trafficking of anidulafungin into and out of the brain. The extent of the disparity in drug exposure between these compartments can also be appreciated from the data and the mathematical model shown in Fig. 2.
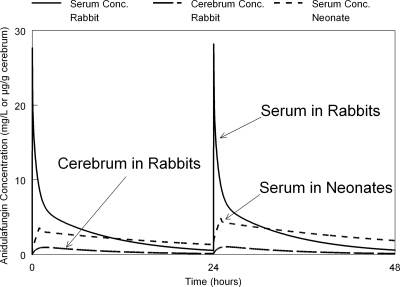
Fig 1.
Simulated concentration-time profiles of anidulafungin in rabbits and neonates both receiving 5 mg/kg. The concentrations in rabbit serum and the cerebrum are shown after a 1-min i.v. push and are compared to concentrations in the serum of neonates receiving anidulafungin 5 mg/kg infused over a 1-h period.
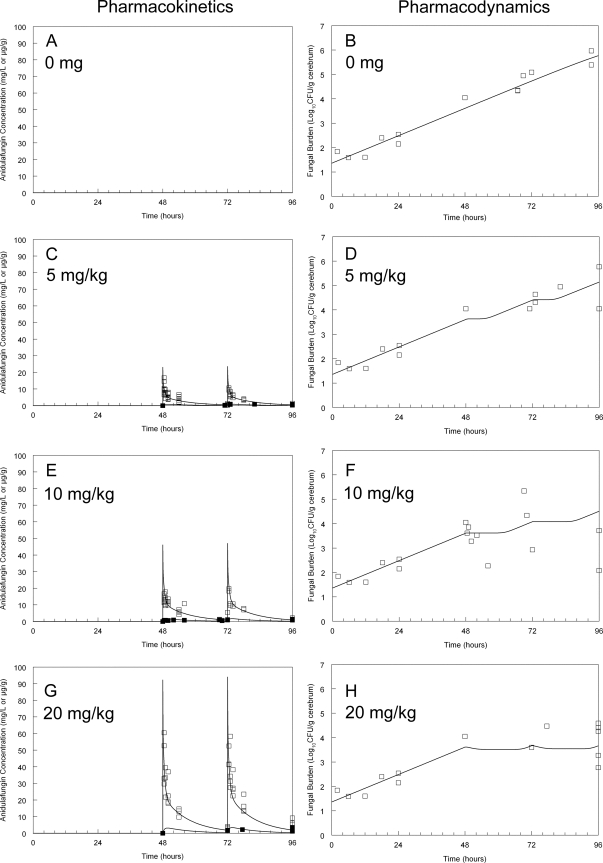
Fig 2.
The pharmacokinetics (A, C, G, and E) and pharmacodynamics (B, D, F, and H) of anidulafungin in rabbits against C. albicans in the cerebrum. Raw plasma and cerebral pharmacokinetic data are depicted by open and solid squares, respectively, in panels A, C, G, and E. The data from rabbits have been pooled. The raw pharmacodynamic data in panels B, D, F, and H are depicted by open squares. For the pharmacodynamics, each data point is derived from a single rabbit. The solid line is the fit of the population mathematical model to the pharmacokinetic and pharmacodynamic data.
Anidulafungin induced an unequivocal antifungal effect that was apparent in the first dosing interval following the administration of 10 and 20 mg/kg. The antifungal effect following the administration of 5 mg/kg was small, with evidence that this dosage only marginally retarded fungal growth in the brain. The fit of the mathematical model to the data was acceptable (Table 1). Although both the mean and median parameter values could have been used, the medians performed slightly better in accounting for the observed data and were used in the subsequent simulations. The mathematical model suggested that doses of 5 and 10 mg/kg resulted in a submaximal antifungal effect. The administration of 20 mg/kg had a profound antifungal effect in that progressive fungal growth was prevented.
Table 1.
Parameter means, medians, and standard deviations from the mathematical model fitted to rabbit PK and PC data
| Parametera | Mean | Median | SD |
|---|---|---|---|
| SCL (liters/h) | 0.163 | 0.208 | 0.079 |
| Vc (liters) | 0.503 | 0.509 | 0.006 |
| kcb (h−1) | 1.192 | 1.284 | 0.172 |
| kbc (h−1) | 0.469 | 0.621 | 0.234 |
| kcp (h−1) | 7.948 | 6.572 | 4.195 |
| kpc (h−1) | 13.045 | 16.308 | 7.638 |
| Vbrain (liters) | 7.891 | 7.049 | 6.785 |
| Kgmax (log10 CFU/g/h) | 0.137 | 0.108 | 0.040 |
| Hg | 8.245 | 9.772 | 3.227 |
| C50g (mg/liter) | 0.462 | 0.457 | 0.108 |
| POPMAX (CFU/g) | 29,474,300 | 3,022,935 | 43,887,300 |
| Kkmax (log10 CFU/g/h) | 0.188 | 0.077 | 0.207 |
| Hk | 5.263 | 7.130 | 2.777 |
| C50k (mg/liter) | 2.569 | 2.690 | 0.388 |
| Initial condition (CFU/g) | 18.83 | 22.87 | 8.02 |
SCL is the clearance. Vc and Vbrain are the volumes of the central compartment and brain, respectively. kcp, kpc, kck, and kkc are the first-order rate constants that connect the respective compartments. Kgmax is the maximal rate of growth. POPMAX is the theoretical maximal density within the brain. Hg is the slope function for the suppression of growth. C50g is the amount of drug in the brain where there is half-maximal suppression of growth. Kkmax is the maximal rate of kill. Hk is the slope functions for the fungal kill. C50k is the concentration of anidulafungin in the brain at which fungal killing is half-maximal. The initial condition is the fungal density in the brain immediately postinoculation.
The fit of the allometric population pharmacokinetic model to the data from 12 neonates and infants was acceptable. The parameter means, medians, and standard deviations are summarized in Table 2. These estimates are consistent with those described previously (5). The normalized values for clearance and volume are comparable to healthy adult volunteers receiving 100 mg of anidulafungin. The predicted AUCs (mean ± the standard deviation) for human neonates receiving various regimens of anidulafungin are shown in Fig. 3.
Table 2.
Parameter means, medians, and standard deviations for the allometric model fitted to the data from 12 neonates and infants receiving anidulafungin
| Parametera | Mean | Median | SD |
|---|---|---|---|
| SCLstd (liters/h/70 kg) | 0.489 | 0.493 | 0.180 |
| Vstd (L/70 kg) | 11.514 | 9.868 | 4.710 |
| kcp (h−1) | 7.650 | 3.387 | 9.829 |
| kpc (h−1) | 3.962 | 0.675 | 7.461 |
| Wt (kg) | 3.27 | 2.80 | 2.82 |
SCLstd is the clearance normalized to a 70-kg adult. Vstd is the volume normalized to a 70-kg adult, and kcp and kpc are the first-order inter compartmental rate constants.
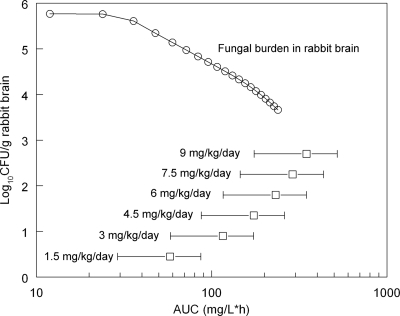
Fig 3.
Relationship between the area under the plasma concentration-time curve (AUC) and the decline in fungal density in the cerebrum in rabbits (open circles) as predicted from the mathematical model. The human neonatal AUCs resulting from the administration of 1.5 to 9 mg/kg/day are also shown (data are simulated population AUC means ± the standard deviation in the second dosing interval as determined from the allometric population pharmacokinetic model fitted to the human neonatal data).
The bridging studies provided an insight into human neonatal anidulafungin regimens that are likely to be associated with near-maximal antifungal activity. The relationship between the AUC in rabbits and the decline in fungal density in the brain (as predicted from the mathematical model) is shown in Fig. 3. The estimated AUCs (means ± the standard deviations) in human neonates receiving 1.5, 3, 4.5, 6, 7.5, and 9 mg/kg/day in the second dosing interval were 57.93 ± 28.86, 115.87 ± 57.71, 173.80 ± 86.57, 231.74 ± 115.43, 289.67 ± 144.28, and 347.60 ± 173.14 mg·h/liter, respectively. These estimates are displayed with the relationship between residual fungal burden versus AUC in rabbits to provide an indication as to the likely effect associated with these regimens. An expectation for the residual fungal density for human neonates receiving 1.5, 3, 4.5, 6, 7.5, and 9 mg/kg/day were 5.29, 4.69, 4.30, 4.08, 3.95, and 3.88 log10 CFU/g, respectively (the residual fungal burden in untreated rabbits was 5.77 log10 CFU/g).
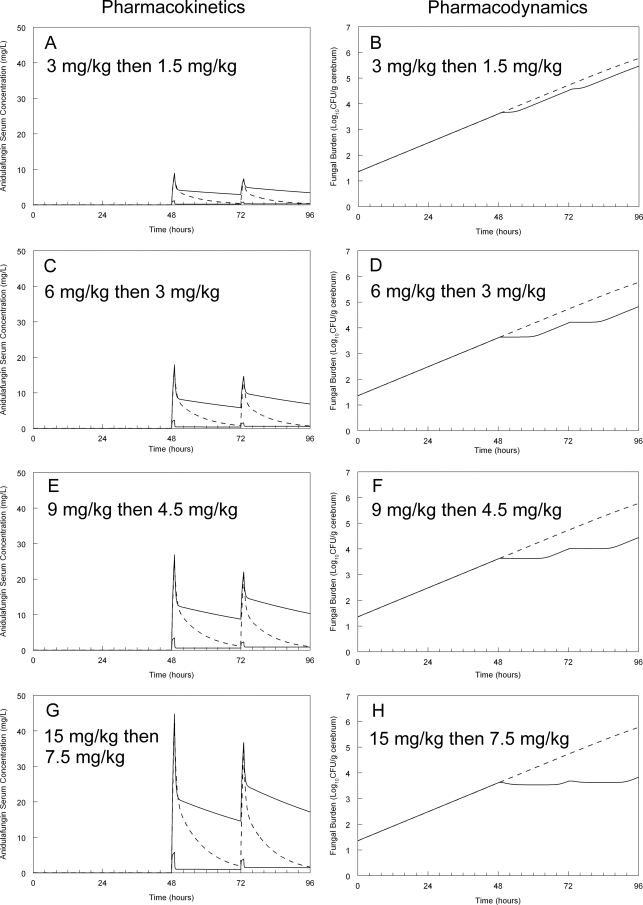
The bridging studies were subsequently extended by “humanizing” the pharmacokinetics in silico. This was done to provide an insight into the use of a loading dose, as is currently recommended in adults. These humanized regimens are depicted in Fig. 4. Of note, the current regimen of a loading dose of 3 mg/kg, followed by 1.5 mg/kg/day, is predicted to result in progressive fungal growth in the brain of neonates with HCME. The use of a higher dosage results in a progressive antifungal effect, although since concentrations in the brain fell to those approximating the lower 95% centile of the neonatal population in the latter parts of the dosing interval, a degree of regrowth was observed.
Fig 4.
Predicted antifungal effect resulting from various humanized anidulafungin regimens. The solid lines in the pharmacokinetic panels (A, C, G, and E) represent the lower 5th and upper 95th centiles for human neonates receiving anidulafungin. The dotted line in these panels is the concentration-time profile of a rabbit that has been administered anidulafungin (in silico) so that the peak touches the 95th human centile and then falls to the lower the lower 5th centile before redosing. The pharmacodynamic panels (B, D, F, and H) represent the predicted response to the humanized rabbit concentration-time profile. In these panels, the dotted line represents the increase in fungal density in untreated animals.
DISCUSSION
HCME is a potentially lethal syndrome of premature neonates that is difficult to diagnose and treat (3). There are few therapeutic options. There are two challenges when attempting to identify candidate regimens for new treatments of HCME: (i) a neonatal dosage that results in comparable drug exposure to those observed in adults and (ii) an antifungal regimen that is effective in the central nervous system (CNS). The latter is almost impossible to predict a priori. The use of a model that is a faithful mimic of human pathogenesis with bridging of the results to the clinic using PK-PD modeling tools represents a highly efficient way to identify candidate regimens suitable for further study in clinic trials (11).
The echinocandins are consistently touted as agents that do not have activity in the CNS. The experimental PK and PD data presented here and elsewhere (10) suggest that this view is inaccurate. Anidulafungin does penetrate the CNS and achieves concentrations that have a rapid and profound antifungal effect. The dosages required for an effect in the CNS are higher than have been reported for other organs. This likely reflects the concentration gradient that is required to drive this relatively large water-soluble compound across the blood-brain barrier even if disrupted by infection. In the present study we estimated anidulafungin concentrations in both infected and uninfected portions of the cerebrum (i.e., we submitted whole portions of brain weighing ∼1 g) and are therefore likely to have underestimated the true drug concentrations at the site of infection, which is typified by focal inflammatory areas throughout the parenchyma. A more precise estimate would have required an alternative sampling approach such as microdialysis or microdissection of affected areas. Despite this potential dilutional effect, anidulafungin concentrations were quantifiable throughout the dosing interval for all drug dosages used in the present study. Anidulafungin exerted an immediate antifungal effect that was most obvious for rabbits receiving 20 mg/kg. This dosage results in retardation of logarithmic fungal growth that if unchecked results in immediate neurological impairment and imminent death. More prolonged dosing would likely induce a progressive decline in fungal burden. In the preliminary phase of the present study, we conducted several experiments wherein anidulafungin dosing was continued for 5 days (i.e., 7 days postinoculation). A progressive decline in fungal burden was observed. These data were not used because all of the controls had died by 4 days postinoculation.
The bridging study is notable for the relatively high human anidulafungin dosages that are predicted to have a significant antifungal effect in the brain. The currently licensed dosage for treatment of candidemia and invasive candidiasis in adults is a single loading dose of 200 mg, followed by 100 mg/day (17). The clearance of anidulafungin in healthy volunteers is ∼1 liter/h, and the AUC with currently licensed adult dosages (for which clinical efficacy has been established) is approximately 100 to 120 mg·h/liter (8). Our experimental data suggest that this magnitude of drug exposure is associated with a submaximal antifungal effect in the CNS (see Fig. 2). In fact, a systemic AUC of 200 to 240 achieves complete suppression of fungal growth in the brain, which highlights important pharmacodynamic differences between adults (who rarely have involvement of the CNS) and neonates (who frequently have involvement of the CNS). Although it is impossible to know what decline in fungal burden in the CNS of a rabbit is associated with a high probability of clinical recovery without neurological sequelae (there are no human autopsy data or prospective clinical pharmacodynamic trials), the most conservative position is to design regimens that are associated with the least residual fungal density in the greatest number of individuals. The bridging studies suggest that a human loading dosage of at least 9 mg/kg, followed by 4.5 mg/kg/day, prevents inexorable fungal growth in the brain. The use of higher dosages may be possible, but they begin to encroach on drug exposures for which there is no safety or toxicological data.
A potential limitation of our study is the use of a single strain of C. albicans. There may be strain-to-strain variability in the pharmacodynamics of anidulafungin for HCME. A major assumption that underpins the present study is that the rate and extent of trafficking of anidulafungin from plasma into and out of the rabbit and neonatal brain are comparable. There is little that can be done to prospectively test this hypothesis because serial sampling from the neonatal brain is not possible. There is, however, indirect evidence that anidulafungin does penetrate the CNS (10, 16). The initial studies of anidulafungin (LY303366) in a persistently neutropenic rabbit model of subacute disseminated candidiasis demonstrated antifungal activity in the brain at dosages of ≥0.5 mg/kg/day (16). The bridging study and Monte Carlo simulations suggest that higher human dosages than those that have currently been studied may be required to achieve near-maximal antifungal activity. Clearly, therefore, further PK and safety studies are required to confirm that the PK are linear and that anidulafungin is safe and well tolerated at these higher dosages.
In summary, anidulafungin is effective in an experimental model of HCME and is potentially a valuable alternative agent for the treatment of this syndrome. Higher dosages than those equivalent for the effective for the treatment of invasive candidiasis in adults are required. Further clinical studies are now required to examine the pharmacokinetics and safety of these higher dosages. These studies, along with this bridging study, can then be used to plan definitive phase III clinical trials for this vulnerable population.
ACKNOWLEDGMENTS
The laboratory animal study reported here was supported by Pfizer. W.W.H. was supported by a National Institute of Health Research (NIHR) Clinician Scientist Fellowship. T.W.F. was supported by an Medical Research Council Training Fellowship in Clinical Pharmacology. M.C.-W. received support from NICHD 1K23HD064814-01, the Thrasher Research Fund, and Pfizer, Inc., for neonatal and pediatric drug development. D.K.B. received support from the U.S. Government for his work in pediatric and neonatal clinical pharmacology (1R01HD057956-02, 1R01FD003519-01, 1U10-HD45962-06, 1K24HD058735-01, and government contract HHSN275201000002I), from the nonprofit organization Thrasher Research Foundation for his work in neonatal candidiasis (http://www.thrasherresearch.org), and from industry for neonatal and pediatric drug development (http://www.dcri.duke.edu/research/coi.jsp).
W.W.H. has given talks, received research grants, and served as a consultant to Pfizer, Inc.
Footnotes
Published ahead of print 28 November 2011
REFERENCES
- 1. Arendrup MC, et al. EUCAST technical note on anidulafungin. Clin. Microbiol. Infect. 17:E18–E20 [DOI] [PubMed] [Google Scholar]
- 2. Benjamin DK, Jr, et al. 2006. Safety and pharmacokinetics of intravenous anidulafungin in children with neutropenia at high risk for invasive fungal infections. Antimicrob. Agents Chemother. 50:632–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benjamin DK, Jr, et al. 2006. Neonatal candidiasis among extremely low birth weight infants: risk factors, mortality rates, and neurodevelopmental outcomes at 18 to 22 months. Pediatrics 117:84–92 [DOI] [PubMed] [Google Scholar]
- 4. Clinical and Laboratory Standards Institute 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts, 3rd ed Approved standard M27–A3(28) Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 5. Cohen-Wolkowiez M, et al. Safety and pharmacokinetics of multiple-dose anidulafungin in infants and neonates. Clin. Pharmacol. Ther. 89:702–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. D'Argenio DZ, Schumitzky A, Wang X. 2009. ADAPT 5 user's guide: pharmacokinetic/pharmacodynamic systems analysis software. Biomedical Simulations Resource, Los Angeles, CA [Google Scholar]
- 7. Denning DW. 2003. Echinocandin antifungal drugs. Lancet 362:1142–1151 [DOI] [PubMed] [Google Scholar]
- 8. Dowell JA, et al. 2004. Population pharmacokinetic analysis of anidulafungin, an echinocandin antifungal. J. Clin. Pharmacol. 44:590–598 [DOI] [PubMed] [Google Scholar]
- 9. Groll AH, et al. 2000. Comparative efficacy and distribution of lipid formulations of amphotericin B in experimental Candida albicans infection of the central nervous system. J. Infect. Dis. 182:274–282 [DOI] [PubMed] [Google Scholar]
- 10. Groll AH, et al. 2001. Pharmacokinetic and pharmacodynamic modeling of anidulafungin (LY303366): reappraisal of its efficacy in neutropenic animal models of opportunistic mycoses using optimal plasma sampling. Antimicrob. Agents Chemother. 45:2845–2855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hope WW, Drusano GL. 2009. Antifungal pharmacokinetics and pharmacodynamics: bridging from the bench to bedside. Clin. Microbiol. Infect. 15:602–612 [DOI] [PubMed] [Google Scholar]
- 12. Hope WW, et al. 2008. The pharmacokinetics and pharmacodynamics of micafungin in experimental hematogenous Candida meningo-encephalitis: implications for echinocandin therapy in neonates. J. Infect. Dis. 197:163–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hope WW, et al. 2007. Population pharmacokinetics of micafungin in pediatric patients and implications for antifungal dosing. Antimicrob. Agents Chemother. 51:3714–3719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hope WW, et al. 2010. Population pharmacokinetics of micafungin in neonates and young infants. Antimicrob. Agents Chemother. 54:2633–2637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Leary R, Jelliffe R, Schumitzky A, van Guilder M. 2001. An adaptive grid, non-parametric approach to pharmacokinetic and dynamic (PK/PD) models, p 389–394 Proceedings of the 14th IEEE Computer Society Meeting, Bethesda, MD [Google Scholar]
- 16. Petraitiene R, et al. 1999. Antifungal activity of LY303366, a novel echinocandin B, in experimental disseminated candidiasis in rabbits. Antimicrob. Agents Chemother. 43:2148–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reboli AC, et al. 2007. Anidulafungin versus fluconazole for invasive candidiasis. N. Engl. J. Med. 356:2472–2482 [DOI] [PubMed] [Google Scholar]
- 18. Warn PA, et al. 2009. Pharmacokinetics and pharmacodynamics of a novel triazole, isavuconazole: mathematical modeling, importance of tissue concentrations, and impact of immune status on antifungal effect. Antimicrob. Agents Chemother. 53:3453–3461 [DOI] [PMC free article] [PubMed] [Google Scholar]