Abstract
Escherichia coli producing the highly virulent, multidrug-resistant, CTX-M-15 extended-spectrum β-lactamase (ESBL), sequence type 131 (ST131), has emerged on three continents since the late 2000s. We described the molecular epidemiology, clinical features, and outcome of ESBL-producing E. coli bacteremia in Taiwan from 2005 to 2010. This study aims to determine whether the risk factors, clinical features, and outcomes of the ST131 isolate differ from those of non-ST131 isolates. From 2005 to 2010, we collected 122 nonduplicated, consecutive, ESBL-producing E. coli isolates from bloodstream infections in a 1,200-bed hospital in Taiwan. Isolates were characterized using multilocus sequence typing. Demographic data, clinical features, and outcomes were collected from medical chart records. Thirty-six (29.5%) patients with bacteremia with ESBL-producing E. coli ST131 were identified. Patients with clone ST131 were more likely to have secondary bacteremia and noncatheterized urinary tract infections (P < 0.05). Secondary bacteremia (odds ratio [OR], 5.05; 95% confidence interval [CI], 1.08 to 23.56) and urinary catheter nonuse (OR, 3.77; 95% CI, 1.17 to 12.18) were independent risk factors for the ST131 clone after adjustment. Mortality rates at day 28 were similar in ST131 and non-ST131 populations. Independent risk factors predicting mortality at day 28 included malignancy, shock, and hospital-acquired bacteremia. In ESBL-producing E. coli bloodstream infections, the ST131 clone was not associated with health-care-associated risk factors, such as urinary catheter use or antibiotic exposure. Although highly virulent and multidrug resistant, the ST131 clone was not associated with higher mortality than non-ST131 clones.
INTRODUCTION
Between 2000 and 2006, Escherichia coli clone O25:H4-ST131, which produces CTX-M-15 extended-spectrum β-lactamase (ESBL), was identified on three continents and later found to be widespread in Europe, Australia, and the United States of America (6, 11, 13, 17, 19, 22, 24). The sequence type 131 (ST131) strain was associated with high, homogenous, extraintestinal virulence and multidrug resistance (6, 11, 13, 17, 19, 22, 24). Virulence gene analysis indicated that clone ST131 strains were more likely than non-ST131 strains to possess traT, iutA, iha, ompT, fimH, malX, fyuA, kpsM, or sat genes (6, 11, 13, 17, 19, 22, 24). Pulsotype analysis revealed that CTX-M-15 ST131 clones were usually approximately 85% similar (6, 11, 13, 17, 19, 22, 24).
According to the published literature, most ST131 clones have been identified as a result of urine culture surveillance, and a few have been isolated from individuals with bacteremia. ST131 clones have been observed in the community, hospitals, and nursing homes (6, 11, 13, 17, 19, 22, 24) as well as in companion animals and poultry (23). In a French study, ST131 isolates were found in 7% of fecal E. coli isolates from independent healthy subjects and were unaccompanied by the CTX-M enzyme (14). However, several case reports have shown that ST131 clones can present in cases of severe infection, such as pyomyositis, emphysematous pyelonephritis, and other life-threatening infections (10, 18, 28). Only two cohorts with ST131 ESBL E. coli bacteremia have been reported in the literature. In these two ESBL E. coli bacteremia series, ST131 was found in 34% of isolates in the Netherlands (27) and 31.3% of isolates in Calgary, Canada (21). In Calgary's ESBL E. coli bacteremia cohort (21), ST131 isolates producing CTX-M-15 became predominant from 2005 to 2007 and were associated with community-onset infection and urosepsis. However, the detailed demographic features, clinical syndromes, and outcomes in these two cohorts of patients with ESBL E. coli bacteremia in clone ST131 are lacking. In previous risk factor analyses, ESBL E. coli infection was more closely associated with antibiotic use, comorbidity, and urinary catheter use than non-ESBL E. coli infection (2, 9, 15). We did not know whether ST131 clones were associated with the same risk factors as previous traditional ESBL E. coli strains. In the meantime, we did not know if ST131 clones were associated with different clinical syndromes or higher mortality rates in the context of bloodstream infection with ESBL E. coli than non-ST131 clones.
In this study, we collected demographic data, including previous antibiotic use history and clinical syndromes, for patients with ESBL E. coli bacteremia and performed a molecular epidemiological study in all ESBL E. coli isolates. We also compared the demographic risk factors, clinical features, and outcomes between ST131 and non-ST131 clones.
MATERIALS AND METHODS
The study population consisted of all patients age 16 years or older admitted to the E-Da Hospital with at least one positive blood culture of ESBL-producing E. coli during a 6-year period from 1 January 2005 to 31 December 2010. E-Da Hospital is a 1,200-bed major teaching hospital in southern Taiwan that provides both primary and tertiary medical care. Only strains from the first bacteremic episode were included in the analysis. This study was approved by the institutional review board at E-Da Hospital (no. EMRP-098-006).
Data collection.
All patients were evaluated using a structured recording form. Each clinical course of infection was evaluated and recorded according to information supplied by primary care physicians and medical records. The diagnosis of infection focus of bacteremia was based on clinical, bacteriological, and radiological investigations. If no infection focus could be identified, the bacteremia was classified as primary bacteremia.
The following items were recorded for each patient: age; sex; underlying illness; severity of illness (classified with a Charlson comorbidity score) (3); history of hospitalization or outpatient department involvement; antibiotic use history of more than 7 days before the bacteremic episode; operation history within the previous 3 months; existence of a nasogastric tube, central venous catheter, or urinary catheter; initial empirical antimicrobial agents; and outcome. If an in vitro active antimicrobial agent was administered before the final result of blood culture, it was considered adequate empirical therapy.
Microbiological laboratory procedures: bacteriology and antimicrobial susceptibility testing.
ESBL production was determined according to the Clinical and Laboratory Standards Institute (CLSI) standards (5). The ESBL status of each isolate was determined by phenotypic analysis. Isolates that were identified as potential ESBL-producing E. coli underwent a confirmatory double-disk diffusion test according to CLSI guidelines.
Clonal relationships were established by pulsed-field gel electrophoresis (PFGE) of XbaI-digested genomic DNA. The GelCompar software package (version 6.0; Applied Maths, Bionumerics) was used to compare the banding patterns of aggregated data. Strains exhibiting >80% similarity in the banding pattern were considered to have similar or identical electrokaryotypes. All E. coli isolates were characterized by multilocus sequence typing (MLST) using the 7 standard housekeeping genes (adk, fumC, gyrB, icd, mdh, purA, and recA) according to the protocol and primers specified at the E. coli MLST website (http://mlst.ucc.ie/mlst/dbs/Ecoli) (26). The blaCTX-M gene groups 1, 2, and 9 in ESBL-producing E. coli isolates were detected by multiplex PCR using specific primers as previously reported. A specific PCR for CTX-M-14 and CTX-M-15 was also performed. DNA sequence analysis of the amplicons was performed to confirm the identity of the β-lactamase genes detected in the multiplex PCR assays (4, 7, 25).
The demographic distributions and clinical features of patients with ST131 or non-ST131 ESBL E. coli bacteremia were compared. Means and standard deviations were calculated for continuous variables. Percentages were used for categorical variables. The associations between potential risk factors and ST131 clone and non-ST131 clone ESBL E. coli bacteremia were investigated using univariate and multivariate logistical regressions. Crude and adjusted odds ratios (ORs) and the corresponding 95% confidence intervals (CIs) were calculated. The potential factors associated with day 28 survival of ESBL E. coli bacteremia were examined by logistic regression. Data were analyzed with SPSS software for Windows (release 10.0; SPSS, Chicago, IL).
RESULTS
ESBL-producing E. coli was isolated from the blood of 122 patients during the 6-year study period. Sixty-four patients (52.5%) were males, 57 (46.7%) were classified as having hospital-acquired infections, and 65 (53.3%) were classified as having community-onset infections.
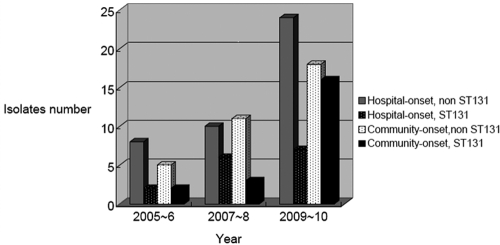
MLST analysis identified 36 different sequence types among the 122 ESBL-producing E. coli isolates. The most common ST was ST131 (29.5%, n = 36), followed by ST38 (9.0%, n = 11) and ST405 (6.2%, n = 8). The majority of clones (58.3% of ST131 clones and 51.2% of non-ST131 clones) were collected in the emergency room (i.e., community onset). The case numbers of ST131 and non-ST131 clones classified with hospital-onset or community-onset infection during the 6-year study period are shown in Fig. 1. The number of community-onset ST131 cases was greater in 2009-2010 (n = 16) than in 2005-2006 (n = 2) or 2007-2008 (n = 3) (Fig. 1).
Fig 1.
The numbers of ST131 and non-ST131 isolates in both community-onset and hospital-onset ESBL E. coli bacteremia from 2005 to 2010.
The demographic data from all ST131 and non-ST131 ESBL E. coli bacteremic patients are summarized in Table 1. Age and sex distributions were similar between patients with ST131 clones and non-ST131 clones. Although patients with ST131 clones were less likely to have chronic hepatitis or liver cirrhosis, the data did not reach statistical significance (13.9% versus 30.2%, P = 0.065). Patients with ST131 clones were less likely to have a urinary catheter in use during the onset of bacteremia (11.1% versus 27.9%, P = 0.044). A greater percentage of patients with ST131 clones had a history that was negative for antibiotic exposure within the 3 months prior to bacteremia (41.7% versus 24.4%, P = 0.057), although these data did not reach statistical significance. When other risk factors for health-care-associated infection in the prior 3 months (such as recent operation, nasogastric tube/central line use, nursing home residence, or hospitalization) were compared, ST131 and non-ST131 clones were similar (Table 1).
Table 1.
Univariate analyses of ST131 and non-ST131 clones in patients with ESBL E. coli bacteremia
| Characteristic | No. (%) of patientsa |
OR (95% CI) | Univariate P value | |
|---|---|---|---|---|
| ST131 (n = 36) | Non-ST131 (n = 86) | |||
| Mean patient age (in yrs) ± SD (range) | 64 ± 15.2 (27–86) | 64.4 ± 16.8 (23–91) | 1.00 (0.98–1.02) | 0.905 |
| Males | 21 (58.3) | 43 (50.0) | 1.40 (0.64–3.07) | 0.401 |
| Elderly patients | 20 (55.6) | 50 (58.1) | 0.90 (0.41–1.97) | 0.792 |
| Comorbid conditions | ||||
| Diabetes mellitus | 12 (33.3) | 25 (29.1) | 1.22 (0.53–2.81) | 0.640 |
| Cancer | 10 (27.8) | 24 (27.9) | 0.99 (0.42–2.37) | 0.988 |
| Chronic hepatitis/cirrhosis | 5 (13.9) | 29 (30.2) | 0.37 (0.13–1.06) | 0.065 |
| Congestive heart failure | 19 (52.8) | 41 (47.7) | 1.23 (0.56–2.67) | 0.607 |
| Cardiovascular disease | 10 (27.8) | 23 (26.7) | 1.05 (0.44–2.52) | 0.907 |
| Dementia | 1 (2.8) | 7 (8.1) | 0.32 (0.04–2.72) | 0.298 |
| Chronic obstructive pulmonary disease | 4 (11.1) | 12 (14) | 0.77 (0.23–2.57) | 0.672 |
| End-stage renal disease | 2 (5.6) | 9 (10.5) | 0.50 (0.10–2.45) | 0.396 |
| Charlson comorbidity score of <3 | 6 (16.7) | 10 (11.6) | 1.52 (0.51–4.55) | 0.454 |
| Primary bacteremia | 2 (5.6) | 19 (22.1) | 0.21 (0.05–0.94) | 0.042 |
| Secondary bacteremia | ||||
| Pneumonia | 1 (2.8) | 5 (5.8) | 0.46 (0.05–4.11) | 0.489 |
| Skin and soft tissue infection | 1 (2.8) | 3 (3.5) | 0.79 (0.08–7.86) | 0.841 |
| Surgical site infection | 2 (5.6) | 1 (1.2) | 5.00 (0.44–56.98) | 0.195 |
| Central-line-associated infection | 3 (8.3) | 3 (3.5) | 2.52 (0.48–23.10) | 0.273 |
| Biliary tract infection | 5 (13.9) | 11 (12.8) | 1.10 (0.35–3.43) | 0.870 |
| Intraabdominal infection | 1 (2.8) | 5 (5.8) | 0.46 (0.05–4.11) | 0.489 |
| Urinary tract infection, not catheter associated | 20 (55.6) | 27 (31.4) | 2.73 (1.23–6.08) | 0.014 |
| Health care-associated risk in prior 3 months | ||||
| Community onset | 21 (58.3) | 44 (51.2) | 1.34 (0.61–2.93) | 0.470 |
| No antibiotic use | 15 (41.7) | 21 (24.4) | 2.21 (0.97–5.05) | 0.060 |
| Fluoroquinolone use | 10 (27.8) | 19 (22.1) | 1.36 (0.56–3.30) | 0.502 |
| Cephalosporin use | 19 (52.8) | 55 (64) | 0.59 (0.27–1.27) | 0.177 |
| Operation | 10 (27.8) | 24 (27.9) | 0.99 (0.42–2.37) | 0.988 |
| Hospitalization | 21 (58.3) | 41 (47.7) | 1.54 (0.70–3.37) | 0.284 |
| Nasogastric tube use | 9 (25) | 25 (29.1) | 0.813 (0.34–1.97) | 0.648 |
| Central catheter use | 6 (16.7) | 13 (15.1) | 1.12 (0.39–3.23) | 0.830 |
| Urinary catheter use | 4 (11.1) | 24 (27.9) | 0.32 (0.10–1.01) | 0.052 |
Values are the numbers and percentages of patients unless otherwise noted.
Regarding clinical syndromes, patients with ST131 isolates were more likely than patients with non-ST131 isolates to have noncatheterized urinary tract infections (55.6% versus 31.4%, P = 0.012). Patients with ST131 isolates were also less likely to be classified as having primary bacteremia (5.6% versus 22.1%, P = 0.027). Secondary bacteremia without an indwelling urinary catheter is still an independent risk factor of ST131 isolates in multivariate analysis after adjustment using logistic regression (Table 2).
Table 2.
Multivariate analysis of risk factors of ST131 clones in cases of ESBL E. coli bacteremia using logistic regression by the backward Wald method
| Variable | OR (95% CI) | P value |
|---|---|---|
| Secondary bacteremia | 5.05 (1.08–23.56) | 0.040 |
| Without urinary catheter | 3.77 (1.17–12.18) | 0.026 |
| Chronic hepatitis/liver cirrhosis | 0.34 (0.07–0.88) | 0.053 |
Outcomes, including mortality on day 14, mortality on day 28, and recurrent bacteremia after 7 days of antibiotic use, are depicted in Table 3. There were no differences between patients with ST131 and non-ST131 isolates. Univariate analysis indicated that malignancy, a high Charlson comorbidity index, chronic liver disease/liver cirrhosis, and presentation with shock were predictors of mortality after 28 days (P < 0.05) (Table 4). Community-onset infection and urinary tract infection were negative predictors of mortality after 28 days (P < 0.05) (Table 4). Multivariate analysis with logistic regression indicated that underlying malignancy and presentation with shock were independent predictors of mortality after 28 days (P < 0.05) (Table 4). Multivariate analysis indicated that community-onset infection was still a negative predictor of mortality after 28 days. Neither univariate nor multivariate analysis revealed any difference in mortality rates between patients with ST131 and non-ST131 isolates.
Table 3.
Univariate analysis of outcome in cases of ESBL E. coli bacteremia
| Treatment and outcome | No. (%) of isolates |
P value | |
|---|---|---|---|
| ST131 (n = 36) | Non-ST131 (n = 86) | ||
| Initial appropriate empirical antibiotics | 8 (22.2) | 19 (22.1) | 0.987 |
| Recurrent bacteremia | 3(8.3) | 8 (9.4) | 1.000 |
| Day 14 mortality | 8 (22.2) | 19 (22.1) | 0.987 |
| Day 28 mortality | 9 (25.0) | 21 (24.4) | 0.946 |
Table 4.
Univariate and multivariate analyses of variables associated with day 28 mortality of ESBL E. coli bacteremia
| Characteristic | OR (95% CI)a |
|
|---|---|---|
| Univariate | Multivariate | |
| Age | 0.98 (0.96–1.01) | NS |
| Male sex | 1.05 (0.46–2.39) | NS |
| Charlson score | 1.13 (1.01–1.25) | NS |
| Cancer | 2.59 (1.08–6.18) | 2.81 (1.03–7.66) |
| Chronic hepatitis/cirrhosis | 3.86 (1.59–9.40) | NS |
| ICU onset | 2.04 (0.79–5.26) | NS |
| Urinary tract infection | 0.32 (0.14–0.77) | NS |
| Community-onset | 0.28 (0.11–0.67) | 0.29 (0.11–0.77) |
| Shock | 5.77 (2.36–14.1) | 6.75 (2.52–18.0) |
| Initial appropriate empirical antibiotic | 0.46 (0.15–1.46) | NS |
| ST131 clone | 1.03 (0.42–2.54) | NS |
NS, not significant.
The antibiotic susceptibilities of ST131 and non-ST131 strains were similar in both groups (Table 5). ST131 had a trend of greater nonsusceptibility to ciprofloxacin and gentamicin, but these data did not reach statistical significance. Among all antibiotics, ertapenem and amikacin had the best susceptibility profiles (Table 5).
Table 5.
Nonsusceptibility rate of ST131 and non-ST131 isolates in ESBL E. coli bacteremia
| Drug | No. (%) of nonsusceptible isolatesa |
P value | |
|---|---|---|---|
| ST131 | Non-ST131 | ||
| Gentamicin | 29 (80.6) | 57 (66.3) | 0.115 |
| Amoxicillin-clavulanate | 28 (77.8) | 68 (79.1) | 1.000 |
| Ciprofloxacin | 27 (75.0) | 53 (61.6) | 0.156 |
| Trimethoprim-sulfamethoxazole | 27 (75.0) | 68 (79.1) | 0.621 |
| Cefmetazole | 16 (44.4) | 35 (40.7) | 0.702 |
| Flomoxef | 14 (41.2) | 26 (31.0) | 0.288 |
| Amikacin | 4 (11.0) | 9 (10.5) | 1.000 |
| Ertapenem | 1 (4.2) | 4 (6) | 1.000 |
Nonsusceptibility (% resistant + % intermediate).
Nine of 36 ST131 isolates produced ESBL with CTX-M-15. The remaining 27 ST131 isolates were producers of CTX-M-14 (n = 13) or CTX-M-3 (n = 4) or did not produce CTX-M (n = 10). The XbaI PFGE dendrogram (Fig. 2) for the 36 isolates included 18 PFGE groups, as defined based on >80% similarity of the PFGE profiles. The largest PFGE group consists of 8 isolates (Fig. 2, black square), which were all CTX-M-15 positive. Except for CTX-M-15, all of the other ST131 isolates (CTX-M-14 producers, CTX-M-3 producers, and CTX-M nonproducers) were in different pulsotypes.
Fig 2.
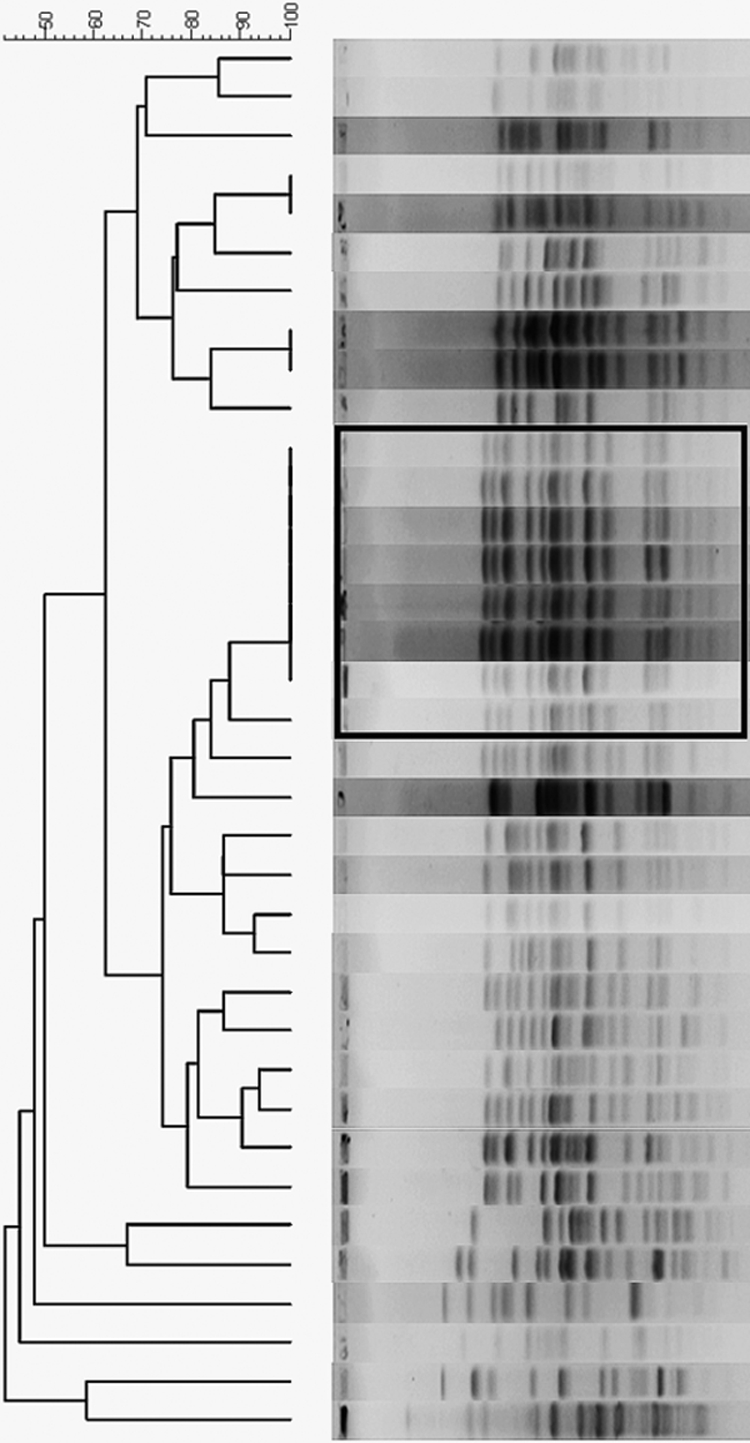
The XbaI pulsed-field gel electrophoresis (PFGE) dendrogram for the 36 ST131 isolates included 18 PFGE groups, as defined based on >80% similarity of PFGE profiles.
We performed subgroup analysis of 36 patients with ST131 isolates. Regarding onset year, CTX-M-15-producing ST131 clones were more likely than non-CTX-M-15-producing ST131 clones to be found in 2010 (88.9% versus 37.0%, P = 0.018). The mean ages in patients with CTX-M-15-producing isolates and non-CTX-M-15-producing isolates were similar (mean age, 66.1 ± 13.9 versus 63.3 ± 15.7). Patients with CTX-M-15-producing ST131 clones were more likely to have urinary tract infection (88.9% versus 55.3%, P = 0.219) and history of fluoroquinolone exposure (55.6% versus 18.9, P = 0.079), although these data did not reach statistical significance. When underlying diseases, other risk factors for health-care-associated infection, clinical syndromes, and outcomes were compared, CTX-M-15-producing clones and non-CTX-M-15-producing clones were similar (data not shown).
DISCUSSION
To our knowledge, this is the first large series of ESBL E. coli bacteremia in which to compare detailed demographic data and infection syndromes in ST131 and non-ST131 isolates. The study cohort showed that ST131 isolates have been found in both community-onset and hospital-onset infections. Similar to the global spread, ST131 has emerged as the most predominant isolate in our cohort of ESBL E. coli bloodstream infections.
ESBL E. coli bacteremia caused by ST131 isolates had a broad spectrum of infection syndromes, including urinary tract infection, pulmonary infection, skin and soft tissue infection, intraabdominal infection, and primary bloodstream infection. Our study results agree with the findings from a previous study suggesting that ST131 had a trend toward involving urinary tract infection (21). Our study further clarifies that this urinary tract infection is especially likely to occur in patients without a urinary catheter. This association with ST131 may be explained by the virulence factor usp or other virulence genes (1, 6, 11, 13, 17, 19, 22, 24). Previous studies including our institute suggest that the usp virulence gene was more likely to be observed in ST131 (6, 11, 13, 17, 19, 22, 24).
The findings of this study regarding demographic data also have implications for the possible source of ST131. The risk factors for ST131 appear to differ from those of traditional ESBL E. coli infection (2, 9, 15). In our study, ST131 isolates were less associated with more comorbidity, antibiotic use, or health care exposure than non-ST131 isolates. In our analysis of health-care-associated factors, ST131 was significant of non-urinary catheter use and had a trend of less antibiotic exposure than non-ST131 isolates. The true origin or transmission route of ST131 in the community remains unknown. Companion animal carriers (23), intrafamilial transmission (10, 18), or environmental contamination (8) may explain the emergence of ST131 isolates. Further investigation of food animal antibiotic consumption or environmental surveillance may help clarify the origin and transmission of ST131.
ST131 and non-ST131 isolates were associated with similar outcomes. Our study demonstrated that underlying cancer, hospital-onset infection, and shock were independent risk factors for day 28 mortality. This result was similar to a previous study with mortality analysis (12). Although more virulence factors and antibiotic resistance were observed in the ST131 isolate, patients with the ST131 isolate did not have worse outcomes than patients with non-ST131 isolates.
PFGE analysis indicates that CTX-M-15 is likely to be 80% similar to other CTX-M types. As in Canada and Spain (16, 20), both CTX-M-14 and CTX-M-15 ST131 circulated in the community in Taiwan. CTX-M-15-positive strains had a more homogenous pattern in PFGE. Rapid emergence of CTX-M-15 in 2010 in Taiwan needs to be carefully monitored. Except for carbapenem and amikacin, the antibiotic susceptibility of ST131 ESBL E. coli was poor. As circulation of the ST131 isolate grows and resulting community-onset and hospital-onset infection increase, more and more carbapenem use may be unavoidable for patients presenting with urosepsis.
A limitation of this study is that those data were obtained from a single center in Taiwan. ST131 isolates producing CTX-M-14 were predominant in Taiwan, which may differ somewhat from other parts of the world, in which ST131 usually produces CTX-M-15. In addition, because our study was limited to patients with ESBL-producing E. coli, we do not know whether ST131 would have similar risk factors and presentation in non-ESBL-producing E. coli infections. Additional surveillance of both ESBL and non-ESBL E. coli infection may be needed; such surveillance should include more detailed epidemiological data, such as animal contact, poultry consumption, and risk of environmental exposure.
In conclusion, as in other parts of the world, ST131 has emerged in ESBL E. coli bloodstream infections in Taiwan. Clone ST131 seems be a new strain that differs from other ESBL E. coli strains and is not related to more health-care-associated risk factors, such as Foley catheter use. Although more virulence factor has been detected in ST131, patients with the ST131 clone in ESBL E. coli bacteremia did not exhibit a higher mortality rate.
ACKNOWLEDGMENTS
We have no conflicts of interest to report.
This work was supported by research grants (EDAHP98012 and EDAHI98001) from E-Da Hospital.
Footnotes
Published ahead of print 28 November 2011
REFERENCES
- 1. Bauer RJ, et al. 2002. Molecular epidemiology of 3 putative virulence genes for Escherichia coli urinary tract infection—usp, iha, and iroN(E. coli). J. Infect. Dis. 185: 1521–1524 [DOI] [PubMed] [Google Scholar]
- 2. Calbo E, et al. 2006. Risk factors for community-onset urinary tract infections due to Escherichia coli harbouring extended-spectrum beta-lactamases. J. Antimicrob. Chemother. 57: 780–783 [DOI] [PubMed] [Google Scholar]
- 3. Charlson ME, Pompei P, Ales KL, MacKenzie CR. 1987. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 40: 373–383 [DOI] [PubMed] [Google Scholar]
- 4. Chia JH, et al. 2005. Development of a multiplex PCR and SHV melting-curve mutation detection system for detection of some SHV and CTX-M beta-lactamases of Escherichia coli, Klebsiella pneumoniae, and Enterobacter cloacae in Taiwan. J. Clin. Microbiol. 43: 4486–4491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clinical and Laboratory Standards Institute 2008. Performance standards for antimicrobial susceptibility testing: eighteenth informational supplement. CLSI document M100-S18. CLSI, Wayne, PA [Google Scholar]
- 6. Coque TM, et al. 2008. Dissemination of clonally related Escherichia coli strains expressing extended-spectrum beta-lactamase CTX-M-15. Emerg. Infect. Dis. 14: 195–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dallenne C, Da Costa A, Decre D, Favier C, Arlet G. 2010. Development of a set of multiplex PCR assays for the detection of genes encoding important beta-lactamases in Enterobacteriaceae. J. Antimicrob. Chemother. 65: 490–495 [DOI] [PubMed] [Google Scholar]
- 8. Dhanji H, et al. 2011. Isolation of fluoroquinolone-resistant O25b:H4-ST131 Escherichia coli with CTX-M-14 extended-spectrum beta-lactamase from UK river water. J. Antimicrob. Chemother. 66: 512–516 [DOI] [PubMed] [Google Scholar]
- 9. Ena J, Arjona F, Martinez-Peinado C, Lopez-Perezagua Mdel M, Amador C. 2006. Epidemiology of urinary tract infections caused by extended-spectrum beta-lactamase-producing Escherichia coli. Urology 68: 1169–1174 [DOI] [PubMed] [Google Scholar]
- 10. Ender PT, et al. 2009. Transmission of an extended-spectrum-beta-lactamase-producing Escherichia coli (sequence type ST131) strain between a father and daughter resulting in septic shock and emphysematous pyelonephritis. J. Clin. Microbiol. 47: 3780–3782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Johnson JR, Johnston B, Clabots C, Kuskowski MA, Castanheira M. 2010. Escherichia coli sequence type ST131 as the major cause of serious multidrug-resistant E. coli infections in the United States. Clin. Infect. Dis. 51: 286–294 [DOI] [PubMed] [Google Scholar]
- 12. Kang CI, et al. 2010. Risk factors and treatment outcomes of community-onset bacteraemia caused by extended-spectrum beta-lactamase-producing Escherichia coli. Int. J. Antimicrob. Agents. 36: 284–287 [DOI] [PubMed] [Google Scholar]
- 13. Lau SH, et al. 2008. UK epidemic Escherichia coli strains A-E, with CTX-M-15 beta-lactamase, all belong to the international O25:H4-ST131 clone. J. Antimicrob. Chemother. 62: 1241–1244 [DOI] [PubMed] [Google Scholar]
- 14. Leflon-Guibout V, et al. 2008. Absence of CTX-M enzymes but high prevalence of clones, including clone ST131, among fecal Escherichia coli isolates from healthy subjects living in the area of Paris, France. J. Clin. Microbiol. 46: 3900–3905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moor CT, et al. 2008. Extended-spectrum beta-lactamase (ESBL)-producing enterobacteria: factors associated with infection in the community setting, Auckland, New Zealand. J. Hosp. Infect. 68: 355–362 [DOI] [PubMed] [Google Scholar]
- 16. Mora A, et al. 2011. Emergence of clonal groups O1:HNM-D-ST59, O15:H1-D-ST393, O20:H34/HNM-D-ST354, O25b:H4-B2-ST131 and ONT:H21,42-B1-ST101 among CTX-M-14-producing Escherichia coli clinical isolates in Galicia, northwest Spain. Int. J. Antimicrob. Agents. 37: 16–21 [DOI] [PubMed] [Google Scholar]
- 17. Nicolas-Chanoine MH, et al. 2008. Intercontinental emergence of Escherichia coli clone O25:H4-ST131 producing CTX-M-15. J. Antimicrob. Chemother. 61: 273–281 [DOI] [PubMed] [Google Scholar]
- 18. Owens RC, Jr, et al. 2011. Community transmission in the United States of a CTX-M-15-producing sequence type ST131 Escherichia coli strain resulting in death. J. Clin. Microbiol. 49: 3406–3408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peirano G, Pitout JD. 2010. Molecular epidemiology of Escherichia coli producing CTX-M beta-lactamases: the worldwide emergence of clone ST131 O25:H4. Int. J. Antimicrob. Agents 35: 316–321 [DOI] [PubMed] [Google Scholar]
- 20. Peirano G, et al. 2010. High prevalence of ST131 isolates producing CTX-M-15 and CTX-M-14 among extended-spectrum-beta-lactamase-producing Escherichia coli isolates from Canada. Antimicrob. Agents Chemother. 54: 1327–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pitout JD, Gregson DB, Campbell L, Laupland KB. 2009. Molecular characteristics of extended-spectrum-beta-lactamase-producing Escherichia coli isolates causing bacteremia in the Calgary Health Region from 2000 to 2007: emergence of clone ST131 as a cause of community-acquired infections. Antimicrob. Agents Chemother. 53: 2846–2851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Platell JL, et al. 2011. Commonality among fluoroquinolone-resistant sequence type ST131 extraintestinal Escherichia coli isolates from humans and companion animals in Australia. Antimicrob. Agents Chemother. 55: 3782–3787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Platell JL, Johnson JR, Cobbold RN, Trott DJ. 2011. Multidrug-resistant extraintestinal pathogenic Escherichia coli of sequence type ST131 in animals and foods. Vet. Microbiol. 153: 99–108 [DOI] [PubMed] [Google Scholar]
- 24. Rogers BA, Sidjabat HE, Paterson DL. 2011. Escherichia coli O25b-ST131: a pandemic, multiresistant, community-associated strain. J. Antimicrob. Chemother. 66: 1–14 [DOI] [PubMed] [Google Scholar]
- 25. Sidjabat HE, et al. 2009. Molecular epidemiology of CTX-M-producing Escherichia coli isolates at a tertiary medical center in western Pennsylvania. Antimicrob. Agents Chemother. 53: 4733–4739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tartof SY, Solberg OD, Manges AR, Riley LW. 2005. Analysis of a uropathogenic Escherichia coli clonal group by multilocus sequence typing. J. Clin. Microbiol. 43: 5860–5864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van der Bij AK, et al. 2011. Clinical and molecular characteristics of extended-spectrum-beta-lactamase-producing Escherichia coli causing bacteremia in the Rotterdam Area, Netherlands. Antimicrob. Agents Chemother. 55: 3576–3578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vigil KJ, et al. 2010. Escherichia coli pyomyositis: an emerging infectious disease among patients with hematologic malignancies. Clin. Infect. Dis. 50: 374–380 [DOI] [PubMed] [Google Scholar]