Abstract
Women of childbearing age commonly receive azithromycin for the treatment of community-acquired infections, including during pregnancy. This study determined azithromycin pharmacokinetics in pregnant and nonpregnant women and identified covariates contributing to pharmacokinetic variability. Plasma samples were collected by using a sparse-sampling strategy from pregnant women at a gestational age of 12 to 40 weeks and from nonpregnant women of childbearing age receiving oral azithromycin for the treatment of an infection. Pharmacokinetic data from extensive sampling conducted on 12 healthy women were also included. Plasma samples were assayed for azithromycin by high-performance liquid chromatography. Population data were analyzed by nonlinear mixed-effects modeling. The population analysis included 53 pregnant and 25 nonpregnant women. A three-compartment model with first-order absorption and a lag time provided the best fit of the data. Lean body weight, pregnancy, ethnicity, and the coadministration of oral contraceptives were covariates identified as significantly influencing the oral clearance of azithromycin and, except for oral contraceptive use, intercompartmental clearance between the central and second peripheral compartments. No other covariate relationships were identified. Compared to nonpregnant women not receiving oral contraceptives, a 21% to 42% higher dose-adjusted azithromycin area under the plasma concentration-time curve (AUC) occurred in non-African American women who were pregnant or receiving oral contraceptives. Conversely, azithromycin AUCs were similar between pregnant African American women and nonpregnant women not receiving oral contraceptives. Although higher levels of maternal and fetal azithromycin exposure suggest that lower doses be administered to non-African American women during pregnancy, the consideration of azithromycin pharmacodynamics during pregnancy should guide any dose adjustments.
INTRODUCTION
Drug therapy in pregnant women must take into account the physiological changes accompanying pregnancy. These physiological changes can impact drug disposition by altering plasma protein binding, hepatic clearance, and renal excretion (6, 24). Depending on the type and extent of any alterations, an adjustment of the dose may be needed to ensure that the desired outcome is achieved and that the mother or fetus is not exposed to excess drug. For agents cleared by drug-metabolizing enzymes or transport proteins, an insufficient understanding of how pregnancy affects the activities of these pathways often hampers the devising of appropriate dosing strategies.
Azithromycin is among the drugs most commonly prescribed to pregnant women (7). Its frequent use in pregnancy reflects its established safety and efficacy in nonpregnant women and men for the outpatient treatment of respiratory (23, 39, 44), skin (30), and gynecological (45) infections as well as the lack of an association between the maternal administration of azithromycin and an increased occurrence of major congenital malformations (14, 50). Pregnant women receive the dose of azithromycin determined to be safe and effective for nonpregnant women and men (45, 50). This extrapolation of dose requirements assumes that the clinical consequences of any pregnancy-related changes in azithromycin pharmacokinetics are negligible. It also ignores the impact that functional changes in the immune system during pregnancy may have on antibiotic responsiveness and dose requirements (25).
Azithromycin exhibits several distinct pharmacokinetic characteristics. It is incompletely absorbed following oral administration (43), extensively distributed into tissues (9), and eliminated primarily by hepatobiliary excretion (34, 43). Not surprisingly, dose-adjusted azithromycin exposures vary widely among individuals (33). Limited data are available regarding the influence of pregnancy on azithromycin pharmacokinetics. An interpretation of data from two studies which have examined azithromycin pharmacokinetics in pregnancy is confounded by their conflicting results and unique populations (46, 49). Women undergoing caesarean section were evaluated in one study (46), and women in Papua New Guinea receiving antimalarial treatment were evaluated in the other (49). Intrinsic or environmental differences between these subjects and pregnant women receiving azithromycin for community-acquired infections in the United States hinder the generalization of their findings.
This study investigated the population pharmacokinetics of azithromycin in women receiving treatment for an infection during the second and third trimesters of pregnancy. Women of childbearing age who were not pregnant were included for comparison. To ensure a representative population, subjects were recruited from four university-based obstetrical practices. The interindividual variability of the pharmacokinetic parameters was determined, and factors contributing to this variability were identified.
MATERIALS AND METHODS
Performance sites and subjects.
This research was conducted at Brigham & Women's Hospital (Boston, MA), Meriter Hospital (Madison, WI), the University of Illinois at Chicago (Chicago, IL), and the University of Michigan (Ann Arbor, MI). The study consisted of two components, an initial pilot trial with 12 healthy women to establish the structural model and baseline pharmacokinetic parameter estimates and a population pharmacokinetic analysis with pregnant and nonpregnant women receiving azithromycin for the treatment of an infection. Institutional Review Board approval was obtained from each of the institutions mentioned above as well as from the University of Wisconsin—Madison and the U.S. Food and Drug Administration's Research Involving Human Subjects Committee. Subjects provided written informed consent prior to participation in the study.
The pilot trial was performed solely at the University of Illinois at Chicago. Women of childbearing age based on menstrual history, at least 18 years of age, not pregnant or breastfeeding, and within 25% of their acceptable range of weight as referenced by the Table of Desirable Body Weights and Heights (35a) were recruited. Subjects were judged to be healthy by medical history, physical examination, and screening laboratory testing (complete blood count, serum chemistries, and urine pregnancy test). Women were required to use either a barrier or hormonal form of contraception throughout the study. Exclusion criteria included a history of tobacco use or alcohol or drug abuse in the last 12 months and the administration of any medication known to interact with azithromycin within 28 days before the start of the study. Subjects were required to be free of all medications, except oral contraceptives, within 1 week and free of alcohol within 48 h prior to the start of the study and continuing until 96 h after the last dose of azithromycin.
Participants in the population study included women with childbearing potential (based on menstrual history) greater than 18 years of age who were receiving azithromycin for the treatment of an infection and were either (i) at a gestational age of at least 12 weeks or (ii) nonpregnant and, if previously pregnant, at least 3 months postpartum.
Study design for the pilot trial.
The pilot trial utilized a single-period, open-label, multiple-dose design. Eligible women received 500 mg oral azithromycin on day 1 and 250 mg daily on days 2 to 5. Azithromycin, as 250-mg tablets (Pfizer, New York, NY), was dispensed into bottles and caps equipped for the electronic recording of administration times (MEMS; Aardex Ltd., Union City, CA). Participants were admitted to the University of Illinois at Chicago Clinical Research Center on the evening of day 4. Following an overnight fast, subjects received the last dose of oral azithromycin on the morning of day 5. Standardized meals were provided at 4 and 10 h after the final dose. Approximately 5 ml of blood was collected through an indwelling catheter into a heparinized evacuated tube prior to and 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 12, 24, 48, 72, and 96 h following the last azithromycin dose. Participants were discharged from the Clinical Research Center after the 12-h sample was taken and subsequently returned each morning for the next 4 days.
Study design for the population pharmacokinetic analysis.
The population pharmacokinetic analysis phase of the study was conducted as a prospective, open-label, multicenter trial. A sparse-sampling scheme guided data collection. Participants received the dose regimen of azithromycin prescribed by their treating physician. Blood samples of approximately 5 ml were collected from participants within each of 4 sampling windows: predose (if not the first dose), 10 min to 1.5 h after any dose, 2 to 5 h after any dose, and 24 to 96 h after the last dose. A single sample was obtained within each sampling window, except the 24- to 96-h window, where 2 samples were collected at least 2 h apart. Also, if sampling occurred with the first dose, no predose sample was obtained, and two samples were collected at least 1 h apart during the 2- to 5-h window. Sampling windows were constructed from the D-optimal sample times computed for two- and three-compartment models with first-order absorption and elimination. Calculations were performed by using estimates of azithromycin pharmacokinetics for nonpregnant women and men (4, 9, 48) and ADAPT II software (16, 17). An additional 2 to 3 ml of blood was collected at a single sampling time for determinations of serum creatinine levels. Demographic and clinical characteristics were recorded for each participant, along with the azithromycin oral formulation and dosing, meal, and sampling times. Medication compliance was assessed from patient interviews and drug administration records. Women having at least one quantifiable azithromycin plasma concentration and documented dosing and sampling times and who were judged to be compliant were included in the pharmacokinetic data set.
Laboratory analysis.
Following collection, blood samples were centrifuged, and plasma was separated and stored at −70°C until it was shipped to the University of Illinois at Chicago on dry ice for analysis.
Azithromycin plasma concentrations were assayed by a high-performance liquid chromatographic procedure with electrochemical detection derived from methods described previously by Shepard et al. (51) and Patel et al. (41). Briefly, an internal standard, clarithromycin, and a 0.1 M sodium carbonate solution were added to each plasma sample. Samples were then extracted with tert-methyl-butyl ether, and the ether layer was evaporated and reconstituted in the mobile phase. The reconstituted sample was washed with hexane, and 50 μl was injected onto a Waters XTerra C18 5-μm, 4.6- by 150-mm column. Samples were eluted with a mobile phase consisting of 0.01 M ammonium acetate at pH 10 and 50% (vol/vol) acetonitrile at a flow rate of 1 ml/min. The analytes were detected with a Coulochem II electrochemical detector (ESA Inc., Bedford, MA) with applied potentials set at +600 and +850 mV.
The assay was linear in the range of 10.1 to 505 ng/ml. For plasma concentrations above the upper limit, samples were diluted with blank plasma to fall within the range of the standard curve. The mean accuracy ranged from 97.1% to 104.8% of the theoretical concentration, and the precision (relative standard deviation) was less than 4% for back-calculated calibration standards (n = 5 assay runs). The between-run accuracy and precision for quality control samples were 98.2% and 3.2% at 302 ng/ml, 101.4% and 3.8% at 101 ng/ml, 102.7% and 5.8% at 30.2 ng/ml, and 103.1% and 13.1%, respectively, at the lower limit of quantitation. Owing to logistical problems at the University of Illinois at Chicago laboratory, 12 plasma samples were analyzed by a proprietary high-performance liquid chromatographic assay at the Pharmacokinetics Laboratory, National Jewish Health (Denver, CO). Cross-validation of 15 patient samples showed a reasonable correspondence between laboratories, with average relative deviations of +3.9% for concentrations in the mid-to-upper range (>150 ng/ml) of the standard curve and −4.7% for concentrations in the lower range.
Pharmacokinetic analysis.
Data from both parts of the study were analyzed by using NONMEM (nonlinear mixed-effects modeling) software (version VI 2.0; Icon Development Solutions, Ellicott City, MD) with a Compaq Visual Fortran 6.6 compiler.
The plasma concentration-time data from the pilot study of healthy women were fit separately for each individual using the first-order method in NONMEM. Several alternative models were assessed, including two and three compartments; first-, zero-, or mixed first-zero-order absorption; and the inclusion of a lag time (tlag) prior to the onset of absorption. Models were parameterized as clearances (CL) and distribution volumes (V). As data were collected only following oral administration, clearance and volume parameters were expressed as apparent values, i.e., uncorrected for bioavailability. A proportional residual error was incorporated. Model selection criteria included the visual inspection of diagnostic plots, the standard error of the parameter estimates, and the minimum value of the objective function (OFV). The OFV is a NONMEM goodness-of-fit criterion and provides a statistical test for comparing competing models. The difference in OFV (ΔOFV) between hierarchical models is approximately χ2 distributed with degrees of freedom (df) equal to the number of additional model parameters.
The data from the population study were analyzed by using the first-order conditional estimation method of NONMEM. The azithromycin plasma concentrations of the healthy women were pooled with those of the patients in the population database. Prior to pooling, the plasma concentration data for the healthy women were condensed (from 17 to 5 samples) to emulate the data sets from the patients by randomly selecting plasma concentrations within the sampling windows using the RAND function of Microsoft Excel. This approach allowed the performance of the sparse-sampling design to be evaluated, with the goal of informing the design of future pharmacokinetic studies for pregnancy. The impact of the condensing of the data set was assessed by reanalyzing the data with the inclusion of the full-profile data from the healthy volunteers.
The structural model selected from the fitting of the individual data served as the starting point for the development of the population pharmacokinetic model. The appropriateness of the structural (base) model was verified by evaluating the fitting criteria as described above and comparing the fit with alternative models. The parameters from the individual data provided the initial estimates for the population fitting. A log-normal distribution was assumed for the pharmacokinetic parameters, and the interindividual variability (IIV) was modeled as an exponential error. The IIV was initially determined for all pharmacokinetic parameters and was retained for a parameter in the final model only if its inclusion produced a significant decrease in the OFV (ΔOFV > 3.84; P < 0.05 by χ2 test). Covariance between parameters was also explored by estimating the full variance-covariance matrix. Residual variability was described as a proportional error. The drug analysis laboratory was evaluated as an independent factor influencing the residual error.
After the structural and error models were defined, covariates explaining the interindividual variability in pharmacokinetic parameter estimates were identified. In addition to the above-described criteria, the covariate analysis was guided by the reduction in the IIV and the biological plausibility of any covariate relationship. First, relationships between body size measures and the CL and V terms were separately examined as linear, power, and proportional functions. The body size measures included total body weight, lean body weight (26), body surface area (18), and body mass index (29). The body size measure producing the greatest reduction in the OFV for each parameter, providing that the minimum drop was at least 6.6 (P < 0.01 by χ2 test; df = 1), was included in the model. Next, other variables were evaluated, including age, pregnancy status, gestational age of the fetus (confirmed by ultrasound), estimated creatinine clearance (13), ethnicity, concurrent medications, significant hepatic or renal impairment, healthy volunteer or patient status, type of infection, azithromycin dose, administration of drug fasting (>3 h after a meal) or with a meal, and study site. Concurrent medication was analyzed as the presence or absence of any drug, drugs suspected to interact with azithromycin, and specific agents received by 7 or more patients. Based on their similar pharmacokinetic behaviors during the graphical analysis, the Asian, Caucasian, Hispanic, and Pacific Islander ethnic groups were combined, and ethnicity was reexpressed as a categorical variable, indicating whether or not the subject was African American.
Individual empirical Bayesian estimates of the pharmacokinetic parameters were obtained from the base pharmacokinetic model with any body size covariates included. Relationships between the Bayesian estimates and covariates were screened by graphical and generalized additive modeling procedures (S-Plus, version 6.1; Insightful Corporation, Seattle, WA). Covariates identified in the screening analysis were first added alone to expressions of the pharmacokinetic parameters in the base model. Those producing a decrease in the OFV of >3.84 (P < 0.05 by χ2 test; df = 1) were entered in a stepwise fashion into an intermediate model and retained if their addition decreased the OFV by >3.84. A backward elimination step followed, with covariates entered during the forward addition step being individually eliminated and retained only if their removal increased the OFV by >6.6 (P < 0.01 by χ2 test; df = 1). Continuous covariates were normalized to an accepted standard (e.g., 70 kg for total body weight and 50 kg for lean body weight) or population median (e.g., gestational age of 29 weeks) and modeled as linear or power functions of the pharmacokinetic parameter. Categorical covariates were input as indicator variables with a value of 1 if the trait was present and a value of 0 otherwise.
Model validation.
The validity of the final population pharmacokinetic model was evaluated by bootstrap analysis using Wings for NONMEM (http://wfn.sourceforge.net) (40). Resampling with replacement from the data set was used to construct 1,000 bootstrap data sets. Each data set was fit to the final population model, and the median and 2.5th and 97.5th percentiles were determined for the fixed- and random-effect parameters. The performance of the final population model was also evaluated by a visual predictive check (54). Briefly, 250 data sets were simulated for an oral azithromycin dosage regimen of 500 mg on day 1 and 250 mg daily on days 2 through 5. The simulations employed covariate values from the patient data set and the final population estimates for the fixed- and random-effect parameters. The median and 80% prediction intervals for the simulated azithromycin plasma concentrations partitioned by ethnicity, pregnancy, and oral contraceptive use were plotted against the observed values. To adjust for the various azithromycin dosage regimens among patients, observed values were normalized to reflect the simulated dose prior to plotting. The assumption of a linear relationship between the azithromycin dose and the plasma concentration is supported by data reported previously by others (15, 20, 33).
Statistical analysis.
Based on the results of the population analysis, subjects were categorized into the following groups: (i) pregnant African American women, (ii) pregnant women of non-African American (i.e., Asian, Caucasian but not Hispanic, Hispanic, or Pacific Islander) ethnicity, (iii) nonpregnant women of any ethnicity who were not receiving oral contraceptives, and (iv) nonpregnant and non-African American women who were receiving oral contraceptives. Empirical Bayesian estimates of the pharmacokinetic parameters for each woman were derived from NONMEM and used to estimate the area under the plasma concentration-time curve (AUC) from time zero to infinity for an oral azithromycin dosage regimen of 500 mg on day 1 and 250 mg daily on days 2 through 5. One-way analysis of variance (ANOVA) was used to compare the individual estimates of azithromycin AUCs among groups. Differences between groups were identified by Tukey's honestly significant difference (HSD) method.
RESULTS
Seventeen healthy women (pilot study) and 72 pregnant or nonpregnant women receiving azithromycin for the treatment of an infection were enrolled. Five healthy volunteers did not meet the eligibility criteria and thus did not continue to the drug administration phase. Six subjects in phase 2 were excluded from the pharmacokinetic data set as a result of incomplete dose administration information for three patients, no evaluable azithromycin plasma concentrations for two patients, and withdrawal from the study for one patient. Accordingly, the population pharmacokinetic database consisted of 344 azithromycin plasma concentrations collected from 78 women. Azithromycin plasma concentrations ranged from 10.3 ng/ml to 1,270 ng/ml. For healthy volunteers, only five randomly selected concentrations per subject were integrated into the population database. Isolated plasma samples from 11 patients were excluded from the data set for being below the quantifiable limit of the assay.
The demographic and clinical characteristics of the participants included in the population analysis are summarized in Table 1. The 3 groups displayed similar ages, lean body weights, heights, and azithromycin dosage regimens. An imbalance in ethnicity occurred among groups, with only two African Americans being found among the nonpregnant patients and none being found among the healthy volunteers. As expected, total body weights, creatinine clearances, infection types, and concomitant medications differed between the pregnant women and the other two groups. No subjects with clinically significant renal (creatinine clearance < 30 ml/min/1.73 m2) or hepatic disease were enrolled. Fifteen women reported azithromycin-related adverse effects. The adverse effects were mild to moderate in intensity and included nausea, vomiting, diarrhea, and abdominal cramping.
Table 1.
Characteristics of patients and healthy volunteers
| Parametera | Value for group |
||
|---|---|---|---|
| Pregnant patients | Nonpregnant patients | Healthy women | |
| No. of patients | 53 | 13 | 12 |
| Median age (yr) (range) | 28 (18–41) | 33 (28–49) | 24 (21–32) |
| Median gestational age of fetus (wk) (range) | 29.1 (11.9–39) | ||
| Median total body wt (kg) (range) | 76 (47–178) | 67 (47–112) | 61 (45–84) |
| Median lean body wt (kg) (range) | 45 (32–68) | 43 (33–57) | 40 (30–51) |
| Median ht (cm) (range) | 163 (138–175) | 160 (155–173) | 160 (150–176) |
| Median creatinine clearance (ml/min) (range) | 127 (45–229) | 83 (37–115) | 91 (69–109) |
| No. of patients of ethnicity | |||
| African American | 17 | 2 | 0 |
| Asian | 3 | 1 | 1 |
| Caucasian (non-Hispanic) | 28 | 6 | 8 |
| Hispanic | 5 | 3 | 1 |
| Pacific Islander | 0 | 1 | 2 |
| No. of patients with infection | |||
| Upper or lower RTI | 30 | 11 | |
| PROMs | 14 | 0 | |
| Chlamydia | 8 | 0 | |
| Skin | 1 | 2 | |
| No. of patients on azithromycin oral regimen | |||
| 500 or 1,000 mg on day 1 and 250 mg daily for 4 days | 34 | 11 | 12 |
| 1,000-mg single dose | 8 | 0 | 0 |
| Other | 11 | 2 | 0 |
| No. of patients on concurrent medicationb | |||
| Albuterol | 3 | 1 | 0 |
| Amoxicillin | 14 | 0 | 0 |
| Ampicillin | 13 | 0 | 0 |
| Betamethasone | 6 | 0 | 0 |
| Ceftriaxone | 7 | 0 | 0 |
| Fluoxetine | 3 | 0 | 0 |
| Fluticasone | 3 | 0 | 0 |
| Gabapentin | 0 | 2 | 0 |
| Insulin | 4 | 1 | 0 |
| Oral contraceptives | 0 | 4 | 6 |
| Prednisone | 2 | 1 | 0 |
Abbreviations: PROMS, premature rupture of membranes; RTI, respiratory tract infection.
Excludes vitamins, oral iron, and medications received by 2 or fewer participants.
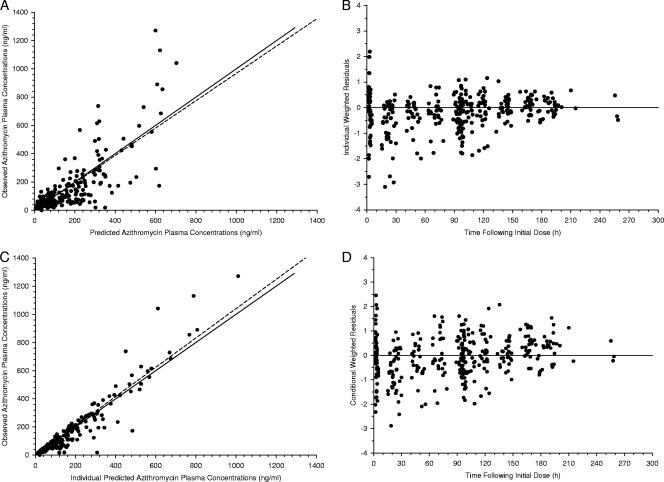
A triexponential decline in azithromycin plasma concentrations with a lag time preceding absorption was consistently observed following the oral administration of azithromycin in the pilot study. Based on these observations, a three-compartment model with elimination from the central compartment, first-order absorption, and a lag time was selected for the population pharmacokinetic (base) model. The fit with the three-compartment model provided a statistically significant improvement compared to a two-compartment model (ΔOFV = −113; P < 0.001 by χ2 test; df = 2). The suitability of the three-compartment model is further supported by the diagnostic plots in Fig. 1.
Fig 1.
Goodness-of-fit plots for azithromycin plasma concentrations fitted to a three-compartment model with first-order absorption and a lag time. (A) Observed versus model-predicted azithromycin plasma concentrations. (B) Individual weighted residuals versus time following the initial dose. (C) Observed versus individual predicted azithromycin plasma concentrations. (D) Conditional weighted residuals versus time following the initial dose. The solid lines in panels A and C represent the line of identity, and the dotted lines are the linear regression line. The solid lines in panels B and D represent the zero-intercept line.
The population parameters for the base model were a lag time (tlag) of 1.3 h, an oral clearance (CL/F) of 94 liters/h, an apparent intercompartmental clearance from the central to the first peripheral compartment (CLD-P1/F) of 485 liters/h, an apparent intercompartmental clearance from the central to second peripheral compartment (CLD-P2/F) of 63 liters/h, an apparent volume of distribution of the central compartment (Vc/F) of 415 liters, an apparent volume of distribution of the first peripheral compartment (VP1/F) of 1,900 liters, and an apparent volume of distribution of the second peripheral compartment (VP2/F) of 13,800 liters. Data were insufficient to allow an estimation of the absorption rate constant (ka). Consequently, a fixed value of 0.8 h−1 was selected based on the median value from the pilot study and those reported in the literature (9, 36, 49). The insensitivity of the parameter estimates to this fixed value was verified by varying the ka between 0.2 h−1 and 8 h−1. Estimates of the IIV were available for CL/F, CLD-P2/F, Vc/F, and VP1/F. The model was unable to accommodate IIV terms for tlag, CLD-P1/F, and VP2/F. The use of a full variance-covariance matrix did not improve the model fit, and therefore, a diagonal matrix was employed. A proportional error best described the residual variability. The drug assay laboratory was not found to influence the residual error.
Several body size descriptors, including total body weight, lean body weight, body surface area, and body mass index, were evaluated as potential covariates. A significant decrease in the OFV was observed following the incorporation of lean body weight (ΔOFV = 13; P < 0.001 by χ2 test; df = 1) in the model as a covariate of CL/F. A direct proportional relationship between lean body weight and CL/F provided a fit comparable to that of a linear function and a better fit than a power function. After incorporating the proportional relationship between lean body weight and CL/F, the IIV decreased by 15%. No significant relationships were identified between descriptors of body size and other pharmacokinetic parameters.
The screening analysis identified (i) clinical site, gestational age, oral contraceptive use, pregnancy, ethnicity, and ethnicity-pregnancy interaction as potential covariates for weight-adjusted CL/F; (ii) pregnancy, race, and ethnicity-pregnancy interaction as potential covariates for CLD-P2/F; and (iii) gestational age and pregnancy as potential covariates for Vc/F. Following the forward inclusion and backward elimination processes, the model retained only pregnancy in non-African American women (ΔOFV = 21.4; P < 0.001 by χ2 test; df = 1) and oral contraceptive use (ΔOFV = 6.9; P < 0.01 by χ2 test; df = 1) as covariates for CL/F and pregnancy in non-African American women (ΔOFV = 29.9) as a covariate for CLD-P2/F. The coadministration of oral contraceptives occurred only in nonpregnant women (Table 1). The covariates for CL/F modestly reduced the IIV from 41% to 36% and reduced the residual error from 40% to 32%. The IIV for CLD-P2/F decreased from 101% to 86% with the inclusion of the covariate. Other covariates did not produce a statistically significant change in the OFV and were not included in the final model.
By reason of the similar magnitudes of the coefficients for the two covariates of CL/F and the potential mediation of both effects through the actions of female sex hormones, the assignment of a single coefficient to describe the impact of each covariate on CL/F was evaluated. Following the substitution of a single coefficient to describe the effect of pregnancy in non-African American women and oral contraceptive use on CL/F, no deterioration in the fit was observed. Also, the coefficient, OFV, and IIV remained unchanged despite the loss of a parameter.
The more parsimonious approach was adopted for the final model, with CL/F expressed as CL/F = (θ1 + race × Preg × θ2 + OC × θ2) × (LBW/50), where θ1 represents CL/F in nonpregnant women not receiving oral contraceptives, race equals 0 for African American women and 1 for non-African American women, Preg equals 0 if not pregnant and 1 if pregnant, OC equals 1 for women receiving oral contraceptives and 0 for women not receiving oral contraceptives, θ2 is the change in CL/F for pregnancy in non-African Americans or the use of oral contraceptives, and LBW is lean body weight. The CLD-P2/F was expressed as CLD-P2/F = θ3 + race × Preg × θ4, where θ3 represents CLD-P2/F in nonpregnant women, race and Preg are defined above, and θ4 equals the change in CLD-P2/F for pregnancy in non-African Americans.
Parameter estimates for the final model are presented in Table 2. The typical value for the azithromycin CL/F in a 50-kg lean-body-weight woman of any race who was not pregnant and not receiving oral contraceptives was 134 liters/h. Pregnancy in non-African American women or the coadministration of oral contraceptives lowered the CL/F of azithromycin by approximately 38%. For the azithromycin CLD-P2/F, an approximately 65% decrease occurred during pregnancy in non-African American women. Pregnancy in African American women had no effect on either the CL/F or CLD-P2/F. Even after the incorporation of the covariates, a high degree of IIV remained for the CL and V terms.
Table 2.
Population pharmacokinetic parameters and bootstrap results from the final covariate model
| Parametera | Value |
|||
|---|---|---|---|---|
| Final model |
Bootstrap (n = 1,000) |
|||
| Estimate | RSE (%) | Median | 2.5th–97.5th percentiles | |
| ka (h−1) | 0.8 | |||
| tlag (h) | 1.3 | 0.1 | 1.3 | 1.0–1.6 |
| CL/F (liters/h/50-kg LBW) | 134 | 12 | 135 | 85–176 |
| Effect of pregnancy in non-African Americans or effect of coadministration of oral contraceptives | −51 | 28 | −44 | −78–−4 |
| CLD-P1/F (liters/h) | 401 | 14 | 398 | 235-609 |
| CLD-P2/F (liters/h) | 120 | 15 | 115 | 35–208 |
| Effect of pregnancy in non-African Americans | −78 | 31 | −72 | −140–29 |
| Vc/F (liters) | 456 | 11 | 436 | 189–716 |
| VP1/F (liters) | 1,560 | 29 | 1,630 | 925–3,629 |
| VP2/F (liters) | 16,100 | 16 | 17,400 | 6,124–31,837 |
| Interindividual variability (CV [%]) | ||||
| CL/F | 36 | 39 | 34 | 16–49 |
| CLD-P2/F | 86 | 48 | 86 | 3–133 |
| Vc/F | 114 | 29 | 116 | 75–161 |
| VP1/F | 60 | 48 | 60 | 0.5–110 |
| Residual error (CV [%]) | 32 | 34 | 32 | 18–42 |
Abbreviations: RSE, relative standard error; CV, coefficient of variation; ka, absorption rate constant; tlag, lag time; CL/F, oral clearance; CLD-P1/F, apparent intercompartmental clearance from the central compartment to peripheral compartment 1; CLD-P2/F, apparent intercompartmental clearance from the central compartment to peripheral compartment 2; Vc/F, apparent volume of the central compartment; VP1/F, apparent volume of peripheral compartment 1; VP2/F, apparent volume of peripheral compartment 2.
The close agreement, ±15%, between the population parameters from the final model and bootstrap medians supports the stability of the model and the accuracy of the parameter estimates. The 2.5th to 97.5th percentiles from the bootstrap and the relative standard errors from the model fitting indicate that the fixed- and random-effect parameters were estimated with reasonable precision. An exception is the coefficient for the effect of pregnancy in non-African American women on the CLD-P2/F, where the bootstrap confidence interval overlapped zero. However, despite the imprecision, the parameter was retained in the model as a result of the significant improvement in the model fitting following its addition and the relatively narrow asymptotic standard error.
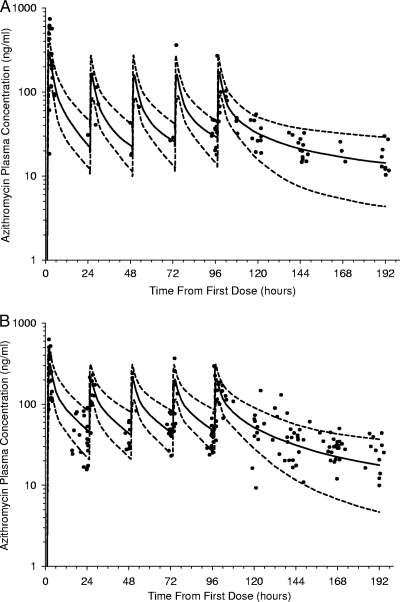
The visual predictive checks adjusted for pregnancy, race, and oral contraceptive use are shown in Fig. 2 and indicate an acceptable predictive performance by the model. The numbers of observed plasma concentrations within the 80% prediction intervals were 84 of 92 (91%) in Fig. 2A and 163 of 189 (86%) in Fig. 2B.
Fig 2.
Visual predictive check plots showing the 10th (dashed line)-, 50th (solid line)-, and 90th (dashed line)-percentile azithromycin plasma concentrations (log scale) versus time simulated from the final covariate model for pregnant African American women and nonpregnant women not receiving oral contraceptives (A) and for pregnant women of other ethnicities (Asian, Caucasian, Hispanic, and Pacific Islander ancestry) and nonpregnant women receiving oral contraceptives (B). The closed circles represent observed concentrations.
The condensing of the full profile data for the healthy women to provide a complete sparse-sampling data set for the population analysis did not affect the parameter estimates or covariate selection. Reanalysis of the population data with the inclusion of the full-profile data produced similar estimates, ±15%, for the fixed- and random-effect parameters compared to those of the sparse data set. The only exceptions were a 37% difference for Vc/F and a 20% difference for CLD-P2/F. Interestingly, the relative standard errors for the fixed- and random-effect parameters averaged 21% lower when the analysis was performed with the sparse data set, indicating a modestly improved precision compared to that of the hybrid dense-sparse sampling data set.
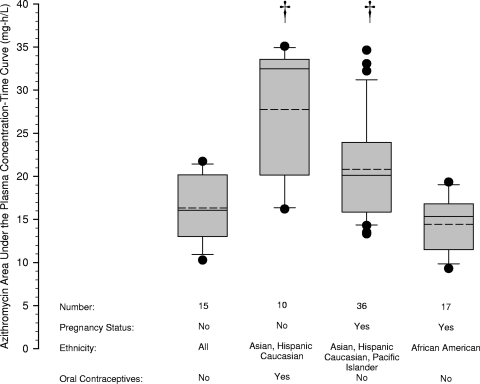
The azithromycin AUC for each individual was derived from the individual Bayesian estimates of the pharmacokinetic parameters. The AUCs are summarized in Fig. 3. Compared to nonpregnant women not receiving oral contraceptives, the AUC was significantly lower during pregnancy in non-African American women (mean difference, 4.5; 95% simultaneous confidence interval, 0.1 to 8.8 mg · h/liter) or with the coadministration of oral contraceptives (mean difference, 11.4; 95% simultaneous confidence interval, 5.7 to 17.2 mg · h/liter). The AUC during pregnancy in non-African American women (mean difference, 6.4; 95% simultaneous confidence interval, 2.3 to 10.5 mg · h/liter) or with the coadministration of oral contraceptives (mean difference, 13.3; 95% simultaneous confidence interval, 7.8 to 18.9 mg · h/liter) was also significantly lower than the AUC for African American women during pregnancy.
Fig 3.
Azithromycin area under the plasma concentration-time curve derived from empirical Bayesian estimates of azithromycin pharmacokinetic parameters for each individual in the population database and grouped by pregnancy status, ethnicity, and the coadministration of oral contraceptives. The limits of the box represent the 25th to 75th percentiles of the distribution, the solid line in the box is the median value, the dashed line is the average, and the whiskers represent the 10th and 90th percentiles of the distribution. Solid circles outside the whiskers are outliers. Daggers indicate a P value of <0.05 compared to nonpregnant women who were not receiving oral contraceptives.
DISCUSSION
This study represents the first report describing how pregnancy affects the pharmacokinetics of a drug cleared by hepatobiliary excretion in an ethnically diverse population. Pregnancy significantly impacted the pharmacokinetics of azithromycin. Uniquely, the influence of pregnancy on azithromycin pharmacokinetics depended on ethnicity. Compared to women who were not pregnant and were not receiving oral contraceptives, the azithromycin CL/F and CLD-P2/F were significantly lower during pregnancy in women of Asian, Caucasian, Hispanic, and Pacific Islander ethnicities. On the contrary, the CL/F and CLD-P2/F for African American women during pregnancy were nearly identical to the values for nonpregnant women. Unfortunately, the small number of African American women in the nonpregnant group did not provide an adequate sample to definitively determine whether CL/F and CLD-P2/F are the same for nonpregnant women of African American and non-African American ancestries. Bayesian estimates of CL/F and CLD-P2/F for the two nonpregnant African American women in the study, neither of whom were receiving oral contraceptives, fell within the 25th and 75th percentiles of the values seen among all nonpregnant women. Although this limited sample suggests that CL/F and CLD-P2/F are not impacted by ethnicity in nonpregnant women, further data are needed to establish this point.
The coadministration of oral contraceptives in nonpregnant women also influenced the azithromycin CL/F, producing a decrease comparable to that observed during pregnancy in women of non-African American ancestry. The occurrence of analogous alterations in drug clearance from oral contraceptive administration and pregnancy was reported previously for other agents (24, 35, 38, 42). Neither the presence of an infection, the type of infection, renal or hepatic disease, creatinine clearance, concurrent medications other than oral contraceptives, nor dose was found to affect the pharmacokinetics of azithromycin.
The similar effects of oral contraceptives and pregnancy in non-African Americans on the azithromycin CL/F suggest a common estrogen- or progesterone-mediated mechanism. Likely possibilities for the mechanism include an increase in the bioavailability or a reduction in the hepatobiliary excretion of azithromycin (3, 4, 34). The oral absorption and hepatobiliary elimination of azithromycin are mediated in part by the drug efflux transporters, multidrug resistance protein 2 (MRP2) and P-glycoprotein (3, 5, 8, 21, 52). The decreased level of expression of MRP2 on the canalicular membrane of hepatocytes during pregnancy and following the administration of ethinyl estradiol in rats suggests a role for MRP2 in the hormone-mediated changes in the hepatobiliary clearance of azithromycin (11, 12, 32, 53). As the distribution clearance depends on blood flow and the permeation of the drug from the vasculature to the tissues, the changes in CLD-P2/F most likely represent pregnancy-related alterations in tissue binding or intracellular concentrations of azithromycin.
The reduced oral clearance results in an increased systemic exposure to azithromycin with the administration of standard doses in pregnant women of non-African American ethnicity compared to nonpregnant women. While a proportional decrease in the dose would offset the increased maternal and fetal drug exposure in these populations, a limited understanding of the pharmacodynamics of azithromycin during pregnancy hampers the ability to make an informed decision for altering the dose. As the antimicrobial efficacy of azithromycin best relates to the ratio of the AUC over the MIC (2), the administration of a lower dose during pregnancy in non-African American women would not be expected to adversely impact the therapeutic response. A potential factor complicating this inference is the immune system changes reported to accompany pregnancy, generally enhanced humoral and suppressed cell-mediated immunity (25). These changes may alter the bacterial responsiveness to azithromycin and the AUC required for therapeutic effectiveness. Uncertainty on how to alter the target AUC complicates the adjustment of doses. The greater exposure must also be considered from the viewpoint of maternal and fetal safety. The limited passage of azithromycin across the placenta observed by in vitro (22) and in vivo (46) studies and the good safety profile of azithromycin administration in pregnancy from observational reports (14, 45, 50) suggest that the increased exposure is unlikely to enhance harm to the fetus. However, systematic investigations are needed to confirm safe and effective levels of azithromycin exposure in pregnancy.
Interestingly, our findings for African American women are consistent with those reported previously by Salman et al. (49), where pregnancy was found not to influence either CL/F or CLD-P2/F in an investigation of azithromycin pharmacokinetics in pregnant and age-matched nonpregnant Papua New Guinean women. The only significant relationship identified by Salman et al. in their population pharmacokinetic analysis was between pregnancy and Vc/F (49), with the Vc/F being 86% higher during pregnancy. Ramsey et al. previously reported an elimination half-life of approximately 12 h for azithromycin in 20 women near term and scheduled for a cesarean section (46). However, the estimation of this value from plasma concentrations collected at 6 to 24 h after the dose indicates that the values actually represent the distribution half-lives and are consistent with values reported by Salman et al. (49) for the distribution half-life of azithromycin in nonpregnant and pregnant women.
A secondary aim was to confirm the ability of the sparse-sampling strategy to provide appropriate estimates of pharmacokinetic parameters and identify factors contributing to pharmacokinetic variability in pregnancy. The collection of a small number of samples per individual offers several advantages, including facilitating the implementation of the study in a clinical setting, allowing informative pharmacokinetic data to be obtained in a population representative of the patients typically receiving treatment, and minimizing the impact of the research on the subject (28). However, reliance on sparse data for the population pharmacokinetic analysis may potentially reduce the precision of the parameter estimates and the power of the covariate analysis (10, 17, 27, 47). These drawbacks reflect the influence that the number of samples per individual has on the standard error of the parameter estimates (17, 37), interindividual and residual variability (1, 27), and the shrinkage of individual parameter estimates (10, 27). These issues were not found to have a noticeable impact on the outcome of the current study. Validation by goodness-of-fit plots, bootstrap analysis, visual predictive checks, and reanalysis using a combined dense-sparse data set supported the ability of the limited sampling model to accurately and precisely characterize the population pharmacokinetics of azithromycin and appropriately identify important covariates. Similar to data from other reports, the addition of some subjects with full profiles did not improve the performance (10, 31). In fact, the sparse data set estimated the parameters with greater precision than the hybrid dense-sparse data set. The good performance of our sparse-sampling strategy likely relates to the use of D-optimally constructed sampling windows to guide the sampling times. Others have shown previously that the collection of sparse samples at optimal times compensates for the analytical problems cited above (10, 19).
A limitation of this study is the small number of African American women in the nonpregnant group. Although the values for CL/F in the 2 nonpregnant African American patients provide preliminary evidence of CL/F values similar to those observed for the non-African American patients, the data did not allow us to definitively establish this value. The influence of ethnicity on the action of oral contraceptives was also unable to be assessed due to the lack of African Americans among the women receiving oral contraceptives.
The population pharmacokinetic analysis identified several factors contributing to pharmacokinetic variability in women of childbearing age, including lean body weight, pregnancy, and the coadministration of oral contraceptives. Ethnicity influenced the changes in azithromycin pharmacokinetics seen during pregnancy. The environmental and genetic causes for these ethnically related differences are important considerations for future studies. The pharmacokinetic changes during pregnancy predict increased maternal and fetal exposure to azithromycin when non-African American women receive standard (i.e., those given to nonpregnant women) doses during pregnancy. Potential immunological changes in pregnant women and a limited understanding of safe levels of fetal azithromycin exposure warrant further investigation to determine the clinical implications of these pharmacokinetic changes.
ACKNOWLEDGMENTS
This work was supported by Department of Health and Human Services contract 233-03-0066 from the Office of Women's Health, U.S. Food and Drug Administration, and NIH M01-RR-13987.
We thank Lorene Seman, Karen McCarthy, and Zan Daley for their assistance in overseeing the conduct of the study. We acknowledge the efforts of Jie Lu and Charles Peloquin in performing the drug assays.
Footnotes
Published ahead of print 21 November 2011
REFERENCES
- 1. al-Banna MK, Kelman AW, Whiting B. 1990. Experimental design and efficient parameter estimation in population pharmacokinetics. J. Pharmacokinet. Biopharm. 18:347–360 [DOI] [PubMed] [Google Scholar]
- 2. Ambrose PG, et al. 2007. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it's not just for mice anymore. Clin. Infect. Dis. 44:79–86 [DOI] [PubMed] [Google Scholar]
- 3. Amsden GW, Gregory TB, Michalak CA, Glue P, Knirsch CA. 2007. Pharmacokinetics of azithromycin and the combination of ivermectin and albendazole when administered alone and concurrently in healthy volunteers. Am. J. Trop. Med. Hyg. 76:1153–1157 [PubMed] [Google Scholar]
- 4. Amsden GW, Nafziger AN, Foulds G. 1999. Pharmacokinetics in serum and leukocyte exposures of oral azithromycin, 1,500 milligrams, given over a 3- or 5-day period in healthy subjects. Antimicrob. Agents Chemother. 43:163–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Amsden GW, Nafziger AN, Foulds G, Cabelus LJ. 2000. A study of the pharmacokinetics of azithromycin and nelfinavir when coadministered in healthy volunteers. J. Clin. Pharmacol. 40:1522–1527 [PubMed] [Google Scholar]
- 6. Anderson GD. 2005. Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin. Pharmacokinet. 44:989–1008 [DOI] [PubMed] [Google Scholar]
- 7. Andrade SE, et al. 2004. Prescription drug use in pregnancy. Am. J. Obstet. Gynecol. 191:398–407 [DOI] [PubMed] [Google Scholar]
- 8. Asakura E, et al. 2004. Azithromycin reverses anticancer drug resistance and modifies hepatobiliary excretion of doxorubicin in rats. Eur. J. Pharmacol. 484:333–339 [DOI] [PubMed] [Google Scholar]
- 9. Ballow CH, Amsden GW, Highet VS, Forrest A. 1998. Pharmacokinetics of oral azithromycin in serum, urine, polymorphonuclear leucocytes and inflammatory vs non-inflammatory skin blisters in healthy volunteers. Clin. Drug Invest. 15:159–167 [DOI] [PubMed] [Google Scholar]
- 10. Bertrand J, Comets E, Laffont CM, Chenel M, Mentre F. 2009. Pharmacogenetics and population pharmacokinetics: impact of the design on three tests using the SAEM algorithm. J. Pharmacokinet. Pharmacodyn. 36:317–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cao J, et al. 2001. Differential regulation of hepatic bile salt and organic anion transporters in pregnant and postpartum rats and the role of prolactin. Hepatology 33:140–147 [DOI] [PubMed] [Google Scholar]
- 12. Cao J, Stieger B, Meier PJ, Vore M. 2002. Expression of rat hepatic multidrug resistance-associated proteins and organic anion transporters in pregnancy. Am. J. Physiol. Gastrointest. Liver Physiol. 283:G757–G766 [DOI] [PubMed] [Google Scholar]
- 13. Cockcroft DW, Gault MH. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41 [DOI] [PubMed] [Google Scholar]
- 14. Cooper WO, et al. 2009. Antibiotics potentially used in response to bioterrorism and the risk of major congenital malformations. Paediatr. Perinat. Epidemiol. 23:18–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Danesi R, et al. 2003. Comparative distribution of azithromycin in lung tissue of patients given oral daily doses of 500 and 1000 mg. J. Antimicrob. Chemother. 51:939–945 [DOI] [PubMed] [Google Scholar]
- 16. D'Argenio D, Schumitzky A. 1997. ADAPT II user's guide. Pharmacokinetic/Pharmacodynamic Systems Analysis Software. Biomedical Simulations Resource, Los Angeles, CA [Google Scholar]
- 17. D'Argenio DZ. 1981. Optimal sampling times for pharmacokinetic experiments. J. Pharmacokinet. Biopharm. 9:739–756 [DOI] [PubMed] [Google Scholar]
- 18. DuBois D, DuBois EF. 1916. Clinical calorimetry. Tenth paper. A formula to estimate the approximate surface area if height and weight be known. Arch. Intern. Med. 17:863–871 [Google Scholar]
- 19. Duffull S, Waterhouse T, Eccleston J. 2005. Some considerations on the design of population pharmacokinetic studies. J. Pharmacokinet. Pharmacodyn. 32:441–457 [DOI] [PubMed] [Google Scholar]
- 20. Ehnhage A, Rautiainen M, Fang AF, Sanchez SP. 2008. Pharmacokinetics of azithromycin in serum and sinus fluid after administration of extended-release and immediate-release formulations in patients with acute bacterial sinusitis. Int. J. Antimicrob. Agents 31:561–566 [DOI] [PubMed] [Google Scholar]
- 21. He XJ, Zhao LM, Qiu F, Sun YX, Li-Ling J. 2009. Influence of ABCB1 gene polymorphisms on the pharmacokinetics of azithromycin among healthy Chinese Han ethnic subjects. Pharmacol. Rep. 61:843–850 [DOI] [PubMed] [Google Scholar]
- 22. Heikkinen T, Laine K, Neuvonen PJ, Ekblad U. 2000. The transplacental transfer of the macrolide antibiotics erythromycin, roxithromycin and azithromycin. BJOG 107:770–775 [DOI] [PubMed] [Google Scholar]
- 23. Henry DC, Riffer E, Sokol WN, Chaudry NI, Swanson RN. 2003. Randomized double-blind study comparing 3- and 6-day regimens of azithromycin with a 10-day amoxicillin-clavulanate regimen for treatment of acute bacterial sinusitis. Antimicrob. Agents Chemother. 47:2770–2774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hodge LS, Tracy TS. 2007. Alterations in drug disposition during pregnancy: implications for drug therapy. Expert Opin. Drug Metab. Toxicol. 3:557–571 [DOI] [PubMed] [Google Scholar]
- 25. Jamieson DJ, Theiler RN, Rasmussen SA. 2006. Emerging infections and pregnancy. Emerg. Infect. Dis. 12:1638–1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Janmahasatian S, et al. 2005. Quantification of lean bodyweight. Clin. Pharmacokinet. 44:1051–1065 [DOI] [PubMed] [Google Scholar]
- 27. Jonsson EN, Wade JR, Karlsson MO. 1996. Comparison of some practical sampling strategies for population pharmacokinetic studies. J. Pharmacokinet. Biopharm. 24:245–263 [DOI] [PubMed] [Google Scholar]
- 28. Kastrissios H, Ratain MJ. 2001. Screening for sources of interindividual pharmacokinetic variability in anticancer drug therapy: utility of population analysis. Cancer Invest. 19:57–64 [DOI] [PubMed] [Google Scholar]
- 29. Keys A, Fidanza F, Karvonen MJ, Kimura N, Taylor HL. 1972. Indices of relative weight and obesity. J. Chronic Dis. 25:329–343 [DOI] [PubMed] [Google Scholar]
- 30. Lassus A. 1990. Comparative studies of azithromycin in skin and soft-tissue infections and sexually transmitted infections by Neisseria and Chlamydia species. J. Antimicrob. Chemother. 25(Suppl. A):115–121 [DOI] [PubMed] [Google Scholar]
- 31. Lee PI. 2001. Design and power of a population pharmacokinetic study. Pharm. Res. 18:75–82 [DOI] [PubMed] [Google Scholar]
- 32. Leslie KK, et al. 2000. Estrogens in intrahepatic cholestasis of pregnancy. Obstet. Gynecol. 95:372–376 [DOI] [PubMed] [Google Scholar]
- 33. Lucchi M, et al. 2008. Pharmacokinetics of azithromycin in serum, bronchial washings, alveolar macrophages and lung tissue following a single oral dose of extended or immediate release formulations of azithromycin. J. Antimicrob. Chemother. 61:884–891 [DOI] [PubMed] [Google Scholar]
- 34. Luke DR, Foulds G. 1997. Disposition of oral azithromycin in humans. Clin. Pharmacol. Ther. 61:641–648 [DOI] [PubMed] [Google Scholar]
- 35. McGready R, et al. 2003. Pregnancy and use of oral contraceptives reduces the biotransformation of proguanil to cycloguanil. Eur. J. Clin. Pharmacol. 59:553–557. [DOI] [PubMed] [Google Scholar]
- 35a. Metropolitan Life Insurance Company 1983. Table of desirable body weights and heights. Metropolitan Life Insurance Company, New York, NY [Google Scholar]
- 36. Muto C, Liu P, Chiba K, Suwa T. 2011. Pharmacokinetic-pharmacodynamic analysis of azithromycin extended release in Japanese patients with common respiratory tract infectious disease. J. Antimicrob. Chemother. 66:165–174 [DOI] [PubMed] [Google Scholar]
- 37. Nedelman JR. 2005. On some “disadvantages” of the population approach. AAPS J. 7:E374–E382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ohman I, Luef G, Tomson T. 2008. Effects of pregnancy and contraception on lamotrigine disposition: new insights through analysis of lamotrigine metabolites. Seizure 17:199–202 [DOI] [PubMed] [Google Scholar]
- 39. Paris R, et al. 2008. Efficacy and safety of azithromycin 1 g once daily for 3 days in the treatment of community-acquired pneumonia: an open-label randomised comparison with amoxicillin-clavulanate 875/125 mg twice daily for 7 days. J. Chemother. 20:77–86 [DOI] [PubMed] [Google Scholar]
- 40. Parke J, Holford NH, Charles BG. 1999. A procedure for generating bootstrap samples for the validation of nonlinear mixed-effects population models. Comput. Methods Programs Biomed. 59:19–29 [DOI] [PubMed] [Google Scholar]
- 41. Patel KB, et al. 1996. Comparison of bronchopulmonary pharmacokinetics of clarithromycin and azithromycin. Antimicrob. Agents Chemother. 40:2375–2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pennell PB, et al. 2004. The impact of pregnancy and childbirth on the metabolism of lamotrigine. Neurology 62:292–295 [DOI] [PubMed] [Google Scholar]
- 43. Pfizer 2010. Zithromax (azithromycin tablets and azithromycin for oral suspension) U.S. prescribing information. Pfizer Laboratories, New York, NY [Google Scholar]
- 44. Piscitelli SC, Danziger LH, Rodvold KA. 1992. Clarithromycin and azithromycin: new macrolide antibiotics. Clin. Pharm. 11:137–152 [PubMed] [Google Scholar]
- 45. Pitsouni E, Iavazzo C, Athanasiou S, Falagas ME. 2007. Single-dose azithromycin versus erythromycin or amoxicillin for Chlamydia trachomatis infection during pregnancy: a meta-analysis of randomised controlled trials. Int. J. Antimicrob. Agents 30:213–221 [DOI] [PubMed] [Google Scholar]
- 46. Ramsey PS, Vaules MB, Vasdev GM, Andrews WW, Ramin KD. 2003. Maternal and transplacental pharmacokinetics of azithromycin. Am. J. Obstet. Gynecol. 188:714–718 [DOI] [PubMed] [Google Scholar]
- 47. Ribbing J, Jonsson EN. 2004. Power, selection bias and predictive performance of the population pharmacokinetic covariate model. J. Pharmacokinet. Pharmacodyn. 31:109–134 [DOI] [PubMed] [Google Scholar]
- 48. Ripa S, Ferrante L, Prenna M. 1996. A linear model for the pharmacokinetics of azithromycin in healthy volunteers. Chemotherapy 42:402–409 [DOI] [PubMed] [Google Scholar]
- 49. Salman S, et al. 2010. Pharmacokinetic properties of azithromycin in pregnancy. Antimicrob. Agents Chemother. 54:360–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sarkar M, Woodland C, Koren G, Einarson AR. 2006. Pregnancy outcome following gestational exposure to azithromycin. BMC Pregnancy Childbirth 6:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shepard RM, Duthu GS, Ferraina RA, Mullins MA. 1991. High-performance liquid chromatographic assay with electrochemical detection for azithromycin in serum and tissues. J. Chromatogr. 565:321–337 [DOI] [PubMed] [Google Scholar]
- 52. Sugie M, et al. 2004. Possible involvement of the drug transporters P glycoprotein and multidrug resistance-associated protein Mrp2 in disposition of azithromycin. Antimicrob. Agents Chemother. 48:809–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Trauner M, et al. 1997. The rat canalicular conjugate export pump (Mrp2) is down-regulated in intrahepatic and obstructive cholestasis. Gastroenterology 113:255–264 [DOI] [PubMed] [Google Scholar]
- 54. Yano Y, Beal SL, Sheiner LB. 2001. Evaluating pharmacokinetic/pharmacodynamic models using the posterior predictive check.J. Pharmacokinet. Pharmacodyn. 28:171–192 [DOI] [PubMed] [Google Scholar]