Abstract
Seasonal variations in Clostridium difficile-associated diarrhea (CDAD), with a higher incidence occurring during winter months, have been reported. Although winter epidemics of respiratory viruses may be temporally associated with an increase in CDAD morbidity, we hypothesized that this association is mainly due to increased antibiotic use for respiratory infections. The objective of this study was to evaluate the effect of the two most frequent respiratory viruses (influenza virus and respiratory syncytial virus [RSV]) and antibiotics prescribed for respiratory infections (fluoroquinolones and macrolides) on the CDAD incidence in hospitals in the province of Québec, Canada. A multivariable Box-Jenkins transfer function model was built to relate monthly CDAD incidence to the monthly percentage of positive tests for influenza virus and RSV and monthly fluoroquinolone and macrolide prescriptions over a 4-year period (January 2005 to December 2008). Analysis showed that temporal variations in CDAD incidence followed temporal variations for influenza virus (P = 0.043), RSV (P = 0.004), and macrolide prescription (P = 0.05) time series with an average delay of 1 month and fluoroquinolone prescription time series with an average delay of 2 months (P = 0.01). We conclude that influenza virus and RSV circulation is independently associated with CDAD incidence after controlling for fluoroquinolone and macrolide use. This association was observed at an aggregated level and may be indicative of other phenomena occurring during wintertime.
INTRODUCTION
The incidence and severity of Clostridium difficile-associated diarrhea (CDAD) are increasing in North America and Europe (5, 26, 28, 30). The rising CDAD rates have largely been attributed to the emergence of an epidemic hypervirulent strain known as restriction enzyme analysis (REA) type BI, North American pulsed-field gel electrophoresis type 1 (NAP1), and type ribotype 027 (toxinotype III) (30). This strain is more resistant to fluoroquinolones (FQs) than historical isolates (30) and exhibits increased sporulation capacity compared to other strains (16). A large number of C. difficile isolates now show an alarming pattern of resistance to the majority of FQs currently used in hospitals and outpatient settings (39). In addition to FQs, an increased prevalence of resistance to macrolide-lincosamide-streptogramin B (MLSB) and its association with FQ resistance have been reported (1).
Seasonal variations in CDAD with a higher incidence occurring during winter months, have been described (4, 18, 22). The mechanisms implicated in this seasonality remain poorly understood. A recent study found an association between influenza virus activity and CDAD incidence derived from an administrative database (35). This association may be due to increased antibiotic use or to cocirculating respiratory viruses during winter months. Originally considered a pediatric pathogen, respiratory syncytial virus (RSV) has been shown to also be a significant cause of respiratory illness among adults. RSV is the second leading cause of winter morbidity in the elderly, after influenza virus (15). Moreover, influenza virus and RSV are frequent contributors to acute exacerbations of chronic obstructive pulmonary disease (COPD), for which the elderly are treated with antibiotics and hospitalized, especially during the winter season (13, 25, 31). Advanced age and comorbidities are also established risk factors for CDAD.
The province of Québec, Canada, experienced a province-wide CDAD epidemic from 2002 to 2005 which was driven by the spread of the NAP1 strain. Increased resistance to FQs and macrolides (MLs) was observed among the strains from Québec hospitals (9, 21). FQs and MLs are increasingly prescribed for respiratory infections occurring mainly during winter months. Two components are essential for CDAD to occur: first, exposure to antimicrobials and, second, exposure to toxigenic C. difficile (23). While exposure to C. difficile occurs most frequently in the hospital, exposure to antimicrobials as long as 6 months before the start of a CDAD episode has been associated with an increased risk of developing symptomatic CDAD (12). Indeed, almost half of elderly patients admitted to hospitals with community-acquired CDAD in Québec received antibiotics 3 months prior to their admission (14). In a recent cohort study in more than 4,000 patients admitted to Québec hospitals, an association between antibiotic use in the 8 weeks prior to hospitalization and increased risk of CDAD and C. difficile colonization was detected (27). As such, patients receiving antibiotics prior to hospital admission are at increased risk of developing a symptomatic CDAD disease when exposed to the hospital environment.
Therefore, we hypothesized that seasonal variations in antibiotic prescription in the community may be associated with the seasonality of CDAD in hospitalized patients.
The objective of this study was to examine temporal relationships between the two most frequent respiratory viruses (influenza virus and RSV), the antibiotics prescribed for respiratory infection (FQs and MLs) in Québec's community pharmacies, and CDAD incidence in Québec hospitals by using multivariable time series analysis. We hypothesized that (i) both influenza virus and RSV are temporally associated with CDAD incidence and (ii) this association is explained by seasonal variations in antibiotic prescriptions. To circumvent two problems of traditional regression models, i.e., (i) the assumption that consecutive respiratory virus counts, antibiotic use data, or hospitalizations are independent and (ii) their inability to detect time-delayed associations, which often exist between antibiotic use/increasing respiratory virus activity and hospitalization, a Box-Jenkins or autoregressive integrated moving average (ARIMA) transfer function model has been used.
MATERIALS AND METHODS
Provincial CDAD-specific surveillance.
Québec's provincial surveillance of CDAD was implemented in August 2004. Hospitals representing 99% of all provincial admissions provide notification of hospitalized CDAD cases according to a standardized definition and the origin of acquisition (health care facility acquired, community acquired, other), number of admissions (excluding psychiatry, nursery, and neonatology), and number of patient days aggregated by 4-week fiscal periods.
Data from January 2005 through December 2008 were included in the analysis and aggregated according to the month of the year, in order to fit the available data on antibiotic prescriptions. On average, 85% of reported hospitalized cases were health care associated, 10% were community associated, and 5% were of unknown origin. We calculated the overall CDAD incidence to be the number of all reported hospitalized CDAD cases per 1,000 discharges, regardless of the origin of acquisition.
Antibiotic prescriptions.
Prescription data for outpatients were obtained from the IMS Brogan's Canadian CompuScript database. Information on prescriptions for outpatients is available for 80% of Québec pharmacies. Prescription data for January 2005 to December 2008 were calculated according to the World Health Organization's (WHO's) Anatomical Therapeutic Chemical (ATC) classification system (http://www.whocc.no) and recommended defined daily dose (DDD). A DDD is the assumed average maintenance dose per day for a drug used for its main indication in an adult. Prescription numbers are presented as DDD per 100,000 Québec inhabitants per month. Antibiotic use per month was calculated for FQs or MLs as a class, as well as for some of the most used specific drugs. Since gatifloxacin was discontinued in 2006 and its mechanism of action and clinical indications are similar to those of moxifloxacin, the combined series gatifloxacin-moxifloxacin was created for the analysis. Denominators for population rates were obtained from census data provided by the Institut de la Statistique du Québec.
Provincial viral surveillance data.
In the province of Québec, laboratory-based surveillance for respiratory viruses is conducted by the Laboratoire de Santé Publique du Québec (LSPQ) throughout the year. This surveillance provides the weekly number of positive tests and number of tests performed for influenza virus and RSV from over 30 laboratories across the province. The monthly percentage of influenza virus- and RSV-positive tests was used for the analysis.
Statistical analysis.
Correlations between CDAD incidence series, respiratory virus series, and antibiotic prescription series were estimated by using the nonparametric Spearman coefficient.
A Box-Jenkins transfer function model was used to assess the potentially delayed impact of antibiotic prescriptions and of influenza virus and RSV (explanatory series) on the CDAD incidence (explained series). These models have been used to estimate the impact of respiratory viruses (10, 17, 19) and of antibiotic use (3, 29, 32, 40) on health care utilization.
ARIMA models were fit for every series using the standard approach of identification, estimation, and checking (19, 20). Stationarity was assessed using the autocorrelation function and the augmented Dickey-Fuller test. The autocorrelation, partial autocorrelation, and inverse autocorrelation functions were assessed for model parameter appropriateness. The relationship between CDAD incidence and influenza virus, RSV, and antibiotic prescriptions was determined by use of the cross-correlation function (CCF), which measures the correlation between two time series as a function of the lag. A transfer function was estimated, and an ARIMA model was fit to the remaining noise. In bivariate analysis, transfer function models showing relationships between each of the explanatory time series and CDAD incidence were assessed. In multivariable analysis, the independent effect of influenza virus, RSV, FQ, and ML time series on CDAD incidence time series was assessed. The effect of specific drugs was not assessed in multivariate models because of the high correlation between individual time series due to similar patterns of variations. Akaike's information criterion was used to identify the appropriate model by minimizing both the residual variance and the number of parameters. The final transfer function models were chosen after repeated identification, estimation, and checking.
Since extreme values of CDAD incidence observed during the epidemic at the beginning of the study period may have influenced some of the observed results, we performed sensitivity analyses by removing values for the period from January 2005 through August 2005.
SAS software (version 9.2; SAS Institute, Inc., Cary, NC) was used for all analyses. A P value of <0.05 was considered statistically significant.
RESULTS
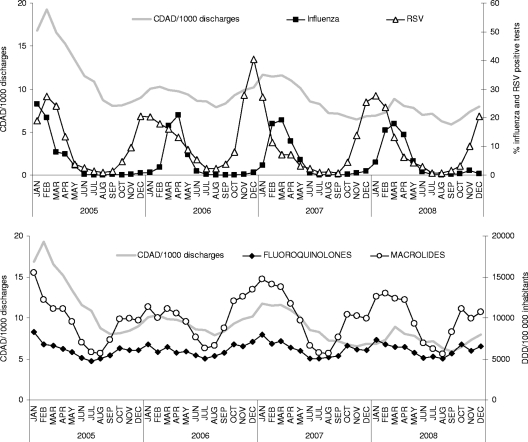
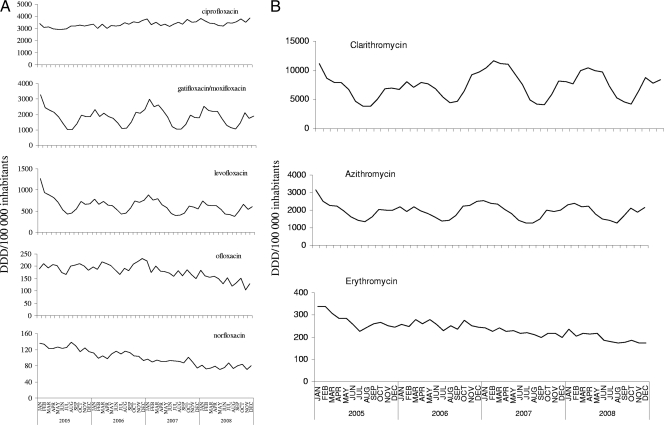
Time series of CDAD incidence, influenza virus and RSV circulation, and antibiotic prescriptions are presented in Fig. 1. CDAD incidence seems to follow closely influenza virus and RSV seasonal activity, as well as FQ and ML prescriptions. Some specific drugs also showed obvious seasonal variations (among FQs, gatifloxacin-moxifloxacin and levofloxacin; among MLs, clarithromycin and azithromycin) (Fig. 2A and B).
Fig 1.
Time series of CDAD incidence, influenza virus, RSV, fluoroquinolone prescriptions, and macrolide prescriptions.
Fig 2.
Time series of fluoroquinolone prescriptions (A) and macrolide prescriptions (B) in outpatients in Québec, Canada.
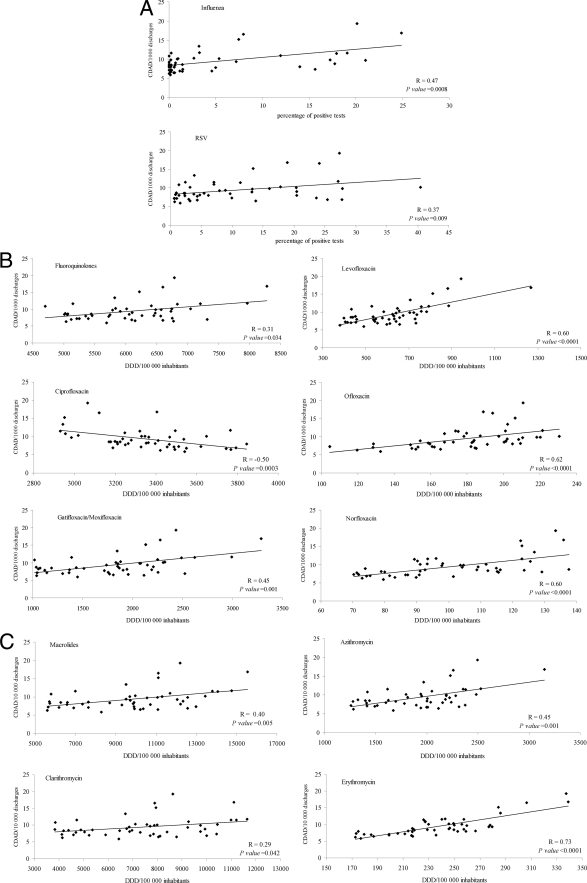
By applying the Spearman coefficient, both respiratory viruses were positively and significantly correlated with CDAD incidence (R = 0.47 for influenza virus; R = 0.37 for RSV) (Fig. 3A). FQs (R = 0.31) and MLs (R = 0.40), as well as all specific drug prescriptions, with the exception of ciprofloxacin, were positively and significantly correlated with CDAD incidence (Fig. 3B and C). Ciprofloxacin was negatively correlated with CDAD incidence (R = −0.50). The magnitude of the correlation was greater for levofloxacin (R = 0.60), ofloxacin (R = 0.62), and norfloxacin (R = 0.60) among the FQs (Fig. 3B) and for erythromycin (R = 0.73) and azithromycin (R = 0.45) among the MLs (Fig. 3C).
Fig 3.
CDAD incidence plotted against monthly respiratory virus positive tests (A), monthly fluoroquinolone prescriptions (B), and monthly macrolide prescriptions (C) with least-squares lines, Spearman correlation coefficients, and P values.
For influenza virus and RSV time series, as well as for most antibiotic time series, significant terms of the order of 1 (corresponding to the previous month) and 12 (corresponding to the similar month of the previous year and indicating seasonality) were detected (Table 1). For CDAD incidence time series, an autoregressive term of order 12 presented a trend (P = 0.06).
Table 1.
Univariate models of CDAD incidence, influenza virus, RSV, and antibiotic prescription time series during the period of January 2005 to December 2008, Québec, Canada
| Time series (units) | Monthly value (minimum-maximum) | AR or MAa | Orderb | Parameter (SE) | P value |
|---|---|---|---|---|---|
| CDAD incidence (no. of cases/1,000 discharges) | 9.4 (5.9–19.3) | AR | 1 | 1.305 (0.145) | <0.0001 |
| AR | 2 | −0.318 (0.146) | 0.0342 | ||
| ARc | 12 | 0.308 (0.161) | 0.0621 | ||
| Respiratory viruses (% positive tests) | |||||
| Influenza virus | 5 (0–25) | AR | 1 | 0.960 (0.049) | <0.0001 |
| ARc | 12 | 0.331 (0.164) | 0.049 | ||
| RSV | 11 (1–40) | AR | 1 | 0.972 (0.174) | <0.0001 |
| AR | 2 | −0.485 (0.165) | 0.0052 | ||
| ARc | 12 | 0.421 (0.155) | 0.0096 | ||
| MA | 1 | −0.529 (0.172) | 0.0036 | ||
| Antibiotic prescriptions (DDD/100,000 inhabitants) | |||||
| Fluoroquinolones, all | 6,089 (4,698–8,281) | AR | 1 | 0.923 (0.058) | <0.0001 |
| ARc | 12 | 0.838 (0.1224) | <0.0001 | ||
| Ciprofloxacin | 3,351 (2,930–3,846) | AR | 1 | 0.402 (0.146) | 0.0083 |
| AR | 2 | 0.429 (0.146) | 0.0051 | ||
| ARc | 12 | 0.739 (0.148) | <0.0001 | ||
| Gatifloxacin | 130 (0–811) | NAd | |||
| Moxifloxacin | 1,701 (768–2,987) | ||||
| Gatifloxacin-moxifloxacine | AR | 1 | 0.942 (0.050) | <0.0001 | |
| ARc | 12 | 0.888 (0.113) | <0.0001 | ||
| Levofloxacin | 627 (379–1,267) | AR | 1 | 0.985 (0.031) | <0.0001 |
| MA | 12 | −0.491 (0.141) | 0.0016 | ||
| Ofloxacin | 179 (104–230) | AR | 1 | 0.913 (0.086) | <0.0001 |
| MA | 4 | 0.415 (0.157) | 0.0115 | ||
| Norfloxacin | 100 (70–138) | AR | 1 | 0.976 (0.045) | <0.0001 |
| ARc | 3 | 0.380 (0.152) | 0.0158 | ||
| Gemifloxacin | 1 (0–3) | NAe | |||
| Macrolides, all | 9,850 (5,601–5,539) | AR | 1 | 0.937 (0.052) | <0.0001 |
| ARc | 12 | 0.860 (0.122) | <0.0001 | ||
| Clarithromycin | 7,388 (3,837–1,648) | AR | 1 | 0.934 (0.054) | <0.0001 |
| ARc | 12 | 0.893 (0.121) | <0.0001 | ||
| Azithromycin | 1,932 (1,255–3,152) | AR | 1 | 0.952 (0.046) | <0.0001 |
| ARc | 12 | 0.748 (0.136) | <0.0001 | ||
| Erythromycin | 237 (173–339) | AR | 1 | 0.994 (0.034) | <0.0001 |
| ARc | 12 | 0.424 (0.161) | 0.0117 | ||
| Telithromycin | 293 (1.4–941) | NAe |
AR, autoregressive term, which represents the past values of the monthly incidence; MA, moving average term, which represents the past variance of the monthly observations.
Delay in months before the effect is observed.
Multiplicative.
Data for the two drugs were merged because of discontinuation of gatifloxacin.
Not analyzed because of discontinuation or limited use.
In bivariate analysis, transfer function models showed significant relationships between almost all explanatory time series (respiratory viruses and antibiotic prescriptions) and CDAD incidence (Table 2), with the exception of ciprofloxacin, ofloxacin, and norfloxacin, for which no significant parameter was detected.
Table 2.
Bivariate Box-Jenkins transfer function models used to estimate time series having impact on CDAD incidencea
| Time series | CDAD incidence |
||
|---|---|---|---|
| Orderb | Parameter value (SE) | P value | |
| Influenza virus | 0 | 0.06408 (0.02694) | 0.0174 |
| RSV | 1 | 0.05176 (0.01932) | 0.0074 |
| Fluoroquinolones | 1 | 0.000622 (0.000194) | 0.0014 |
| Macrolides | 1 | 0.000310 (0.000067) | <0.0001 |
| Levofloxacin | 2 | 0.0048905 (0.0009544) | <0.0001 |
| Gatifloxacin-moxifloxacinc | 1 | 0.0012259 (0.0003124) | <0.0001 |
| Erythromycin | 2 | 0.00930 (0.0045) | 0.0402 |
| Clarithromycin | 1 | 0.0003645 (0.00008389) | <0.0001 |
| Azithromycin | 1 | 0.00216 (0.00041) | <0.0001 |
Reported parameters for influenza virus, RSV, and antibiotics describe the transfer functions.
Delay in months before the effect is observed.
Data for the two drugs were merged because of discontinuation of gatifloxacin.
Multivariate time series analysis showed that temporal variations in CDAD incidence followed temporal variations in FQ use with a lag of 2 months (P = 0.01) (Table 3). This means that, on average, an increase (or decrease) of FQ use by 1,000 DDDs/100,000 inhabitants resulted in an increase (or decrease) of the CDAD incidence by 0.38/1,000 discharges 2 months later. In addition, temporal variations in CDAD incidence followed temporal variations in influenza virus and RSV time series with a lag of 1 month (P = 0.043 and P = 0.004, respectively) (Table 3). A trend for the relationship between CDAD incidence and ML use with a lag of 1 month was detected (P = 0.05). Globally, this model explained 95.64% of the CDAD incidence variance over the study period.
Table 3.
Multivariable Box-Jenkins transfer function model used to estimate times series having impact on CDAD incidencea
| Time series | CDAD incidence |
|||||
|---|---|---|---|---|---|---|
| January 2005-December 2008 |
August 2005-December 2008 |
|||||
| Orderb | Parameter value (SE) | P value | Order | Parameter value (SE) | P value | |
| CDAD incidence | AR1c | 0.97774 (0.02868) | <0.0001 | AR1 | 0.90329 (0.06822) | <0.0001 |
| Influenza virus | 1 | 0.03975 (0.01964) | 0.043 | 1 | 0.03587 (0.01808) | 0.0472 |
| RSV | 1 | 0.05093 (0.0434) | 0.0040 | 1 | 0.03885 (0.01328) | 0.0034 |
| Fluoroquinolones | 2 | 0.00038 (0.0136) | 0.0136 | 2 | 0.00029 (0.00014) | 0.0433 |
| Macrolides | 1 | 0.00012 (0.00006) | 0.0542 | 1 | 0.00011 (0.00006) | 0.0484 |
Reported parameters for influenza virus, RSV, and antibiotics describe the transfer functions.
Delay in months before the effect is observed.
AR1, autoregressive term of first order representing the past values of the CDAD incidence.
In sensitivity analyses restricted to the period without an epidemic peak (August 2005 to December 2008), similar results were obtained for Spearman correlation coefficients and time series analyses (data not shown). Results of multivariate sensitivity analysis are presented in Table 3.
DISCUSSION
To our knowledge, this is the first study to assess the impact of two respiratory viruses (influenza virus and RSV) on CDAD while controlling for antibiotic prescription. Circulation of influenza virus was previously shown to be associated with changes in CDAD rates (35). Our study suggests that in addition to influenza virus, RSV is also associated with seasonal variations in CDAD incidence. RSV is a common wintertime respiratory virus that affects persons of all ages and is the major cause of serious lower respiratory tract infections in young children. Detection of RSV in young children contributes importantly to the epidemic curve describing RSV circulation. However, epidemic cycles of respiratory viruses consistently occur 1 to 2 weeks later in adults than in children (24), and as such, a time series of aggregated 1-month RSV circulation is representative of overall RSV seasonal variations. Also, influenza virus and RSV surveillance data precede the peak in hospitalizations associated with influenza virus and RSV infections by 1 to 2 weeks (17, 36). Thus, monthly aggregated data capture variations in both community and hospitalized influenza virus and RSV infections.
Unexpectedly, influenza virus and RSV time series had a significant impact on CDAD independently of antibiotic prescription. The force of impact of influenza virus and RSV time series on CDAD rates decreased in multivariate models controlling for the impact of antibiotic prescription compared to models with only respiratory viruses. This means that the effect of viruses may be partially explained by antibiotics. However, there is an independent role for viruses. This may be related to close sequestering of individuals, coupled with likely changes in diet and outdoor activity, hospital overcrowding, shortages of medical staff, deficiencies in applying infection control measures during winter months, or other unknown factors.
Other factors which could potentially influence CDAD rates include seasonality in C. difficile contamination of the food supply, such as ground beef (37), and increased C. difficile carriage by calves and swine in winter (33, 38). A relationship between CDAD and norovirus outbreaks (which tend to occur during winter months) has also been reported (6, 41). Additional studies are needed to assess the degree to which these candidate determinants contribute to CDAD seasonality.
It is of note that significant correlations with CDAD were observed for both examined classes of drugs and specific drugs, as well as for influenza virus and RSV. In the bivariate transfer function models, some of the examined time series (ciprofloxacin, ofloxacin, and norfloxacin) did not show a significant impact on CDAD time series. This suggests that some of the significant correlations between time series detected may be due only to similar patterns or a correlation of consecutive observations in the examined time series and not necessarily to a real association. Correlation of consecutive observations in time series is especially true when these are cases of infection. In contrast to the positive association between ciprofloxacin in outpatients and CDAD detected in Germany by using Spearman's correlation coefficient (8), we detected a negative significant correlation between ciprofloxacin prescription and CDAD when applying the same statistical method. However, this association was no longer significant when using the transfer function model. The absence of an association between ciprofloxacin and CDAD in our study could be due to the poor activity of ciprofloxacin against respiratory pathogens, which makes it a less than ideal candidate to treat respiratory infections. The divergence in results obtained in our study and the study from Germany may also be explained by methodology differences, differences in antibiotic prescription practices, or differences in the genotypes and antibiotic susceptibility profiles of circulating C. difficile strains in different geographical regions. For example, the NAP1 strain is the predominating strain in Québec, while other strains predominate in European countries (7, 21).
The temporal relationships between FQ and ML prescriptions in the community and CDAD incidence in hospitals detected in our study are congruent with those observed in other ecologic studies using similar statistical methods relating antibiotic use within hospitals and CDAD incidence in hospitals (2, 40). In our study, an increase in FQ and ML prescriptions in the community was associated with an increase in the CDAD incidence with delays of 2 months and 1 month, respectively. This is in line with the findings of other studies that detected an association between health care-acquired or community-acquired CDAD and the antibiotics used prior to hospitalization (14, 27). Our results add to the evidence that when assessing factors associated with an increased risk of CDAD in hospitalized patients, exposure to antibiotics before admission should be considered in addition to the antibiotics received during hospitalization. In addition to FQ and ML classes, we explored the effects of specific drugs on CDAD. Our results suggest that differences in the effects of different specific drugs may be observed and that an aggregated class effect may be observed but the effect does not allow generalization to all its representatives. This observation is of particular importance for setting priorities in settings with different antibiotic prescribing practices.
The strengths of our study include the use of a robust multivariable analysis method allowing assessment of independent temporal relationships between circulating respiratory viruses, antibiotic prescriptions, and CDAD incidence. In addition, the Box-Jenkins transfer function method assumes that each additional percentage of virus positivity and each additional DDD of antibiotic prescription contribute additively to CDAD incidence (17). As such, detection of significant associations in our study is indicative of a dose-response relationship between respiratory virus activity, antibiotic prescriptions, and CDAD. Also, we used CDAD incidence from a surveillance program including the great majority of provincial acute care hospitals and using a standardized definition.
Our study has several limitations. First, an ecologic design with aggregated group-level data may not represent individual-level associations. However, the association between FQs and MLs as a class and their representatives with CDAD is in line with what is observed in individual-level case-control and cohort studies (28, 34). Furthermore, temporal relationships with circulating viruses at a regional level may be indicative of other phenomena at the local (hospital) or individual level and merit further exploration. Second, we did not consider the antibiotics prescribed in hospitals. One can expect that seasonal variations in prescribing FQs and MLs in the community and in hospitals are similar; however, this may be different for specific drugs. Although the strength of association that we found may not necessarily quantify exactly the relation between antibiotics and hospitalized CDAD cases, we think that the significance and delays in the temporal relationships with FQs and MLs detected are probably true. Third, we cannot exclude the role of other antibiotic classes and other respiratory viruses which we did not consider in our study. For example, we did not assess the impact of beta-lactam antibiotics on CDAD incidence, as local clinical practice guidelines do not advise their use in lower respiratory tract infections in adults (11). Finally, the number of observations in time (48 monthly observations) limited the power of the transfer function models to detect significant associations.
In conclusion, our study attempted to clarify the temporal association of influenza virus, RSV, FQs, and MLs and the incidence of CDAD. While the initial hypothesis was that the role of winter viruses is no longer significant after controlling for the antibiotics prescribed against respiratory infections, we detected an independent impact of influenza virus and RSV on CDAD time series. This suggests that measures aimed at preventing influenza virus and RSV infection may help decrease the risk of CDAD. However, this association was observed at an aggregated level (ecologic study) and may be indicative of other antibiotic classes or other phenomena occurring during wintertime. More research including longer periods of time and more antibiotic classes is needed to confirm these findings.
ACKNOWLEDGMENT
We appreciate the help of Louis Dumont, pharmacologist at the Institut Universitaire de Cardiologie et de Pneumologie de l'Université Laval, Québec, Canada, for useful comments during the preparation of the manuscript.
Footnotes
Published ahead of print 21 November 2011
REFERENCES
- 1. Ackermann G, Degner A, Cohen SH, Silva J, Rodloff AC. 2003. Prevalence of association of macrolide-lincosamide-streptogramin B (MLSb) resistance with resistance to moxifloxacin in Clostridium difficile. J. Antimicrob. Chemother. 51: 599–603 [DOI] [PubMed] [Google Scholar]
- 2. Aldeyab MA, et al. 2009. Quasiexperimental study of the effects of antibiotic use, gastric acid-suppressive agents, and infection control practices on the incidence of Clostridium difficile-associated diarrhea in hospitalized patients. Antimicrob. Agents Chemother. 53: 2082–2088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aldeyab MA, et al. 2008. Modelling the impact of antibiotic use and infection control practices on the incidence of hospital-acquired methicillin-resistant Staphylococcus aureus: a time-series analysis. J. Antimicrob. Chemother. 62: 593–600 [DOI] [PubMed] [Google Scholar]
- 4. Archibald LK, Banerjee SN, Jarvis WR. 2004. Secular trends in hospital-acquired Clostridium difficile disease in the United States, 1987-2001. J. Infect. Dis. 189: 1585–1589 [DOI] [PubMed] [Google Scholar]
- 5. Barbut F, et al. 2007. Clinical features of Clostridium difficile-associated infections and molecular characterization of strains: results of a retrospective study, 2000-2004. Infect. Control Hosp. Epidemiol. 28: 131–139 [DOI] [PubMed] [Google Scholar]
- 6. Barrett SP, Holmes AH, Newsholme WA, Richards M. 2007. Increased detection of Clostridium difficile during a norovirus outbreak. J. Hosp. Infect. 66: 394–395 [DOI] [PubMed] [Google Scholar]
- 7. Bauer MP, et al. 2011. Clostridium difficile infection in Europe: a hospital-based survey. Lancet 377: 63–73 [DOI] [PubMed] [Google Scholar]
- 8. Borgmann S, et al. 2010. Association of ciprofloxacin prescriptions to outpatients to Clostridium difficile infections. Euro Surveill. 15 (5): pii=19479 [PubMed] [Google Scholar]
- 9. Bourgault AM, Lamothe F, Loo VG, Poirier L. 2006. In vitro susceptibility of Clostridium difficile clinical isolates from a multi-institutional outbreak in southern Quebec, Canada. Antimicrob. Agents Chemother. 50: 3473–3475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Choi K, Thacker SB. 1981. An evaluation of influenza mortality surveillance 1962-1979. I. Time series forecasts of expected pneumonia and influenza deaths. Am. J. Epidemiol. 113: 215–226 [DOI] [PubMed] [Google Scholar]
- 11. Conseil du Médicament du Québec 2011, posting date Clinical guides in antibiotic treatment. Conseil du Médicament du Québec, Québec, Québec, Canada: http://www.cdm.gouv.qc.ca/site/English_publications.phtml [Google Scholar]
- 12. Delaney J-A, Dial S, Barkun A, Suissa S. 2007. Antimicrobial drugs and community-acquired Clostridium difficile-associated disease, UK. Emerg. Infect. Dis. 13: 761–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. De Serres G, et al. 2009. Importance of viral and bacterial infections in chronic obstructive pulmonary disease exacerbations. J. Clin. Virol. 46: 129–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dial S, Kezouh A, Dascal A, Barkun A, Suissa S. 2008. Patterns of antibiotic use and risk of hospital admission because of Clostridium difficile infection. Can. Med. Assoc. J. 179: 767–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. 2005. Respiratory syncytial virus infection in elderly and high-risk adults. N. Engl. J. Med. 352: 1749–1759 [DOI] [PubMed] [Google Scholar]
- 16. Fawley WN, et al. 2007. Efficacy of hospital cleaning agents and germicides against epidemic Clostridium difficile strains. Infect. Control Hosp. Epidemiol. 28: 920–925 [DOI] [PubMed] [Google Scholar]
- 17. Gilca R, De Serres G, Skowronski D, Boivin G, Buckeridge DL. 2009. The need for validation of statistical methods for estimating respiratory virus-attributable hospitalization. Am. J. Epidemiol. 170: 925–936 [DOI] [PubMed] [Google Scholar]
- 18. Gilca R, Hubert B, Fortin E, Gaulin C, Dionne M. 2010. Epidemiological patterns and hospital characteristics associated with increased incidence of Clostridium difficile infection in Quebec, Canada, 1998-2006. Infect. Control Hosp. Epidemiol. 31: 939–947 [DOI] [PubMed] [Google Scholar]
- 19. Helfenstein U. 1996. Box-Jenkins modelling in medical research. Stat. Methods Med. Res. 5: 3–22 [DOI] [PubMed] [Google Scholar]
- 20. Helfenstein U. 1991. The use of transfer function models, intervention analysis and related time series methods in epidemiology. Int. J. Epidemiol. 20: 808–815 [DOI] [PubMed] [Google Scholar]
- 21. Hubert B, et al. 2007. A portrait of the geographic dissemination of the Clostridium difficile North American pulsed-field strain and the epidemiology of Clostridium difficile-associated disease in Quebec. Clin. Infect. Dis. 44: 238–244 [DOI] [PubMed] [Google Scholar]
- 22. Jagai J, Naumova E. 2009. Clostridium difficile-associated disease in the elderly, United States. Emerg. Infect. Dis. 15: 343–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Johnson S, Gerding DN. 1998. Clostridium difficile-associated diarrhea. Clin. Infect. Dis. 26: 1027–1034 [DOI] [PubMed] [Google Scholar]
- 24. Johnston NW. 2007. The similarities and differences of epidemic cycles of chronic obstructive pulmonary disease and asthma exacerbations. Proc. Am. Thorac. Soc. 4: 591–596 [DOI] [PubMed] [Google Scholar]
- 25. Kherad O, et al. 2010. Upper-respiratory viral infection, biomarkers, and COPD exacerbations. Chest 138: 896–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kuijper EJ, Coignard B, Tull P. 2006. Emergence of Clostridium difficile-associated disease in North America and Europe. Clin. Microbiol. Infect. 12 (Suppl 6): 2–18 [DOI] [PubMed] [Google Scholar]
- 27. Loo V, et al. 2011. Host and pathogen factors for Clostridium difficile infection and colonization. N. Engl. J. Med. 365: 1693–1703 [DOI] [PubMed] [Google Scholar]
- 28. Loo VG, et al. 2005. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N. Engl. J. Med. 353: 2442–2449 [DOI] [PubMed] [Google Scholar]
- 29. Lopez-Lozano JM, et al. 2000. Modelling and forecasting antimicrobial resistance and its dynamic relationship to antimicrobial use: a time series analysis. Int. J. Antimicrob. Agents 14: 21–31 [DOI] [PubMed] [Google Scholar]
- 30. McDonald LC, et al. 2005. An epidemic, toxin gene-variant strain of Clostridium difficile. N. Engl. J. Med. 353: 2433–2441 [DOI] [PubMed] [Google Scholar]
- 31. Mohan A, et al. 2010. Prevalence of viral infection detected by PCR and RT-PCR in patients with acute exacerbation of COPD: a systematic review. Respirology 15: 536–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Monnet DL, et al. 2004. Antimicrobial drug use and methicillin-resistant Staphylococcus aureus, Aberdeen, 1996-2000. Emerg. Infect. Dis. 10: 1432–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Norman KN, et al. 2009. Varied prevalence of Clostridium difficile in an integrated swine operation. Anaerobe 15: 256–260 [DOI] [PubMed] [Google Scholar]
- 34. Pepin J, et al. 2005. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin. Infect. Dis. 41: 1254–1260 [DOI] [PubMed] [Google Scholar]
- 35. Polgreen PM, Yang M, Bohnett LC, Cavanaugh JE. 2010. A time-series analysis of Clostridium difficile and its seasonal association with influenza. Infect. Control Hosp. Epidemiol. 31: 382–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Quenel P, Dab W, Hannoun C, Cohen JM. 1994. Sensitivity, specificity and predictive values of health service based indicators for the surveillance of influenza A epidemics. Int. J. Epidemiol. 23: 849–855 [DOI] [PubMed] [Google Scholar]
- 37. Rodriguez-Palacios A, et al. 2009. Possible seasonality of Clostridium difficile in retail meat, Canada. Emerg. Infect. Dis. 15: 802–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rodriguez-Palacios A, et al. 2006. Clostridium difficile PCR ribotypes in calves, Canada. Emerg. Infect. Dis. 12: 1730–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Spigaglia P, et al. 2008. Fluoroquinolone resistance in Clostridium difficile isolates from a prospective study of C. difficile infections in Europe. J. Med. Microbiol. 57: 784–789 [DOI] [PubMed] [Google Scholar]
- 40. Vernaz N, et al. 2008. Temporal effects of antibiotic use and hand rub consumption on the incidence of MRSA and Clostridium difficile. J. Antimicrob. Chemother. 62: 601–607 [DOI] [PubMed] [Google Scholar]
- 41. Wilcox M, Fawley W. 2007. Viral gastroenteritis increases the reports of Clostridium difficile infection. J. Hosp. Infect. 66: 395–396 [DOI] [PubMed] [Google Scholar]