Abstract
Amino acid substitutions at position 89 or 91 in GyrA of fluoroquinolone-resistant Mycobacterium leprae clinical isolates have been reported. In contrast, those at position 94 in M. tuberculosis, equivalent to position 95 in M. leprae, have been identified most frequently. To verify the possible contribution of amino acid substitutions at position 95 in M. leprae to fluoroquinolone resistance, we conducted an in vitro assay using wild-type and mutant recombinant DNA gyrases. Fluoroquinolone-mediated supercoiling activity inhibition assay and DNA cleavage assay revealed the potent contribution of an amino acid substitution of Asp to Gly or Asn at position 95 to fluoroquinolone resistance. These results suggested the possible future emergence of quinolone-resistant M. leprae isolates with these amino acid substitutions and the usefulness of detecting these mutations for the rapid identification of fluoroquinolone resistance in leprosy.
INTRODUCTION
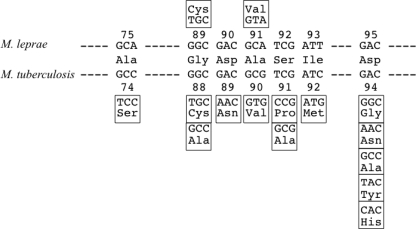
Leprosy is a chronic human infectious disease caused by Mycobacterium leprae which may cause severe disabilities due to damage to the peripheral nerves (33). The World Health Organization (WHO) reported the global number of registered new cases in 2010 to be 228,474, while during 2009 it was 244,796 (37). Although the number of new cases detected globally fell by 16,322 (6.7%) during this period, new leprosy cases are still detected every year, mainly in Asia, Latin America, and Africa (21, 37). In the 1980s, the WHO introduced multidrug therapy (MDT), composed of dapsone (DDS), rifampin (RIF), and clofazimine (36). Recently, fluoroquinolones (FQs), especially ofloxacin (OFX), have been recommended for the treatment of leprosy with a single lesion. The emergence of multidrug-resistant (MDR) leprosy, resistant to both DDS and RIF owing to therapeutic failure or low compliance, has been reported (17, 29), and FQs are thought to be important. For appropriate treatment, early assessment of drug susceptibility is essential; however, M. leprae cannot be cultivated on artificial media and a drug susceptibility test depending on in vitro growth is not available. Consequently, antibiotic susceptibility tests have relied on the mouse footpad leprosy model, requiring 8 to 12 months because of the slow growth of M. leprae (18). Recently, genetic analysis of drug-resistant M. leprae substantiated the correlation of DDS, RIF, and OFX resistance with mutations in folP1, encoding dihydropteroate synthetase (5, 15, 19, 23–25, 35); rpoB (4, 6, 12, 19, 23–25, 33), encoding the beta subunit of RNA polymerase; and gyrA, encoding the A subunit of DNA gyrase (4, 19, 24, 26, 40), respectively. Among these, data for folP1 in M. tuberculosis are not available as DDS is not used for the treatment of tuberculosis. Mutations in rpoB observed in M. leprae showed good agreement with those obtained from RIF-resistant M. tuberculosis. In contrast, the distribution of mutations in gyrA of FQ-resistant M. tuberculosis was distinct from that in gyrA of OFX-resistant M. leprae (Fig. 1). Namely, amino acid substitutions at position 94 in GyrA were found in approximately half of FQ-resistant M. tuberculosis isolates, whereas no amino acid substitutions at position 95, equivalent to position 94 in M. tuberculosis, have been reported in M. leprae, and 11 cases with amino acid substitutions at position 91, equivalent to position 94 in M. tuberculosis, were reported from a total of six countries (4, 19, 24, 26, 40). Thus, elucidation of the contribution of amino acid substitutions at position 95 of GyrA in M. leprae to FQ resistance is important for the gene-based detection of fluoroquinolone resistance.
Fig 1.
Nucleotide substitutions encoding the quinolone resistance-determining region in gyrA of WT and FQ-resistant M. leprae and M. tuberculosis. Nucleotide sequences encoding the quinolone resistance-determining region of WT M. leprae and M. tuberculosis GyrA were aligned with the amino acid sequence at the corresponding positions indicated by the numbers. Altered amino acids and the corresponding nucleotide substitutions of M. leprae and M. tuberculosis are placed above and below WT sequences, respectively.
FQs inhibit type II DNA topoisomerases, DNA gyrase, and topoisomerase IV, which play crucial roles in DNA replication during cell division (8). As M. leprae has only DNA gyrase, this is the sole target of FQs. DNA gyrase, consisting of two GyrA and two GyrB subunits, catalyzes the negative supercoiling of the circular bacterial chromosome by cleaving double strands and passing the enwrapped DNA, followed by resealing the double strands (8, 13). To reveal the significance of amino acid substitution at position 95 to FQ resistance, we conducted the FQ-mediated supercoiling activity inhibition assay and DNA cleavage assay using recombinant DNA gyrases having an amino acid substitution in GyrA at position 95, Asp to Gly (GyrA-Asp95Gly) or Asp to Asn (GyrA-Asp95Asn). These mutations are frequently found in FQ-resistant M. tuberculosis strains (1, 7, 9, 10, 32, 34, 39) but not in FQ-resistant M. leprae strains.
MATERIALS AND METHODS
Materials.
The Thai-53 strain of M. leprae (22), maintained at the Leprosy Research Center, National Institute of Infectious Diseases (Tokyo, Japan), was used to prepare M. leprae DNA. Escherichia coli strains TOP-10 (Life Technologies Corp., Carlsbad, CA), Rosetta-gami 2, and BL21(DE3)(pLysS) (Merck KGaA, Darmstadt, Germany) were used for cloning and protein expression. GyrA and GyrB expression plasmids were constructed on the basis of pET-20b (+) (Merck KGaA). OFX and gatifloxacin (GAT) were purchased from LKT Laboratories, Inc. (St. Paul, MN); moxifloxacin (MXF) was from Toronto Research Chemicals Inc. (Toronto, Ontario, Canada). Sitafloxacin (SIT) was a gift from Daiichisankyo Pharmaceutical, Co., Ltd. (Tokyo, Japan). Ampicillin was purchased from Meiji Seika Pharma, Ltd. (Tokyo, Japan). Oligonucleotide primers were synthesized by Life Technologies Corp. Restriction enzymes were obtained from New England BioLabs, Inc. (Ipswich, MA). The supercoiling assay kit and supercoiled and relaxed pBR322 DNA were purchased from John Innes Enterprises Ltd. (Norwich, United Kingdom).
Construction of recombinant wild-type (WT) and mutant DNA gyrase expression plasmids.
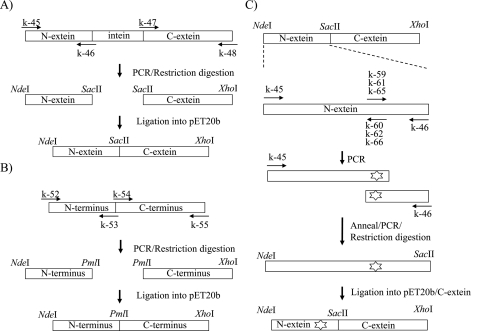
DNA gyrase expression vectors were constructed basically as previously described (16), and Fig. 2 presents an overview of the procedure. The sequences of the primers used in the study are shown in Table 1. All PCRs were carried out in a thermal cycler (Applied Biosystems) under the following conditions: predenaturation at 98°C for 2 min; 35 cycles of denaturation at 98°C for 10 s, annealing at 50 to 60°C for 15 s, and extension at 68°C for 1 to 2.5 min; and then a final extension at 68°C for 2 min. The nucleotide sequences of the DNA gyrase genes in the plasmids were confirmed using a BigDye Terminator (version 3.1) cycle sequencing kit (Life Technologies Corp.) and an ABI Prism 3130xl genetic analyzer (Life Technologies Corp.) according to the manufacturer's protocol.
Fig 2.
Construction of WT and mutant DNA gyrase expression plasmid. (A) DNA fragments encoding N-extein (amino acids 1 to 130) and C-extein of GyrA (amino acids 125 to 830) were amplified by PCR with primer pairs k-45/k-46 and k-47/k-48 (Table 1), respectively. Similarly, those encoding the N-terminal (amino acids 1 to 428) and C-terminal (amino acids 424 to 679) regions of GyrB were amplified with primer pairs k-52/k-53 and k-54/k-55 (Table 1), respectively. PCR products encoding N-extein and C-extein of GyrA were digested by NdeI-SacII and SacII-XhoI, respectively, and introduced simultaneously into NdeI-XhoI-digested plasmid pET-20b (+). (B) DNA fragments encoding the N-terminal and C-terminal regions of GyrB were digested by NdeI-PmaCI and PmaCI-XhoI, respectively, and introduced into pET20b as described above. (C) Primer pairs consisting of primer k-45 and primer k-60, k-62, or k-66 (Table 1) were used for amplifying the DNA fragment encoding the N-terminal portion (amino acids 1 to 94) of N-extein carrying Ala91Val, Asp95Gly, and Asp95Asn, respectively. Primer pairs consisting of primer k-46 and primer k-59, k-61, or k-65 (Table 1) were used for amplifying the DNA fragment encoding the C-terminal portion (amino acids 88 to 130) of N-extein carrying Ala91Val, Asp95Gly, and Asp95Asn, respectively. To complete the N-extein-encoding cassette, DNA fragments encoding the N-terminal and C-terminal regions of N-extein of GyrA were annealed and reamplified by PCR using the primer pair k-45/k-46. The mutated gyrA-N cassettes were digested with NdeI and SacII restriction endonucleases and ligated into the expression plasmid containing WT gyrA C-extein DNA fragment digested by the same enzymes.
Table 1.
Nucleotide sequences of primers used in PCR
| Primer name | Primer sequence (nucleotide positions)a |
|---|---|
| k-45 | 5′-GGCATATGACTGATATCACGCTGCCACCAG-3′ (1–25) |
| k-46 | 5′-ATAACGCATCGCCGCGGGTGGGTCATTACC-3′ (361–390) |
| k-47 | 5′-CACCCGCGGCGATGCGTTATACCGAGGCTCGGCTTACTCC-3′ (371–410) |
| k-48 | 5′-GGCTCGAGTTAATGATGATGATGATGATGACCGACACCGCCGTCGG-3′ (2471–2490) |
| k-52 | 5′-GGCATATGGCTGCCCAGAGGAAG-3′ (1–18) |
| k-53 | 5′-CTAACTCACGTGCTTTACGTGCAGCTATTC-3′ (1259–1288) |
| k-54 | 5′-CGTAAAGCACGTGAGTTAGTGCGTCGAAAAAGTGCC-3′ (1270–1305) |
| k-55 | 5′-GGCTCGAGCTAATGATGATGATGATGATGGACATCCAGGAAACGAACATCC-3′ (2013–2037) |
| k-59 | 5′-GCACGGCGACGTGTCGATTTATG-3′ (261–283) |
| k-60 | 5′-CATAAATCGACACGTCGCCGTGC-3′ (261–283) |
| k-61 | 5′-CATCGATTTATGGCACGTTAGTGC-3′ (272–295) |
| k-62 | 5′-GCACTAACGTGCCATAAATCGATG-3′ (272–295) |
| k-65 | 5′-CATCGATTTATAACACGTTAGTGC-3′ (272–295) |
| k-66 | 5′-GCACTAACGTGTTATAAATCGATG-3′ (272–295) |
Six-histidine tag codons are underlined, and mutated codons are shown in bold type.
Expression and purification of recombinant DNA gyrase.
DNA gyrase subunits were purified as previously described (2, 3, 16, 20, 21, 31). Expression plasmids carrying the gyrA (WT and mutants) and WT gyrB genes of M. leprae were transformed into E. coli Rosetta-gami 2 and BL21(DE3)(pLysS), respectively. Expression of GyrA and GyrB was induced with the addition of 1 mM isopropyl-β-d-thiogalactopyranoside (Wako Pure Chemical Industries Ltd., Tokyo, Japan), followed by further incubation at 14°C for 16 h. The recombinant DNA gyrase subunit in the supernatant of the sonication lysate (by Sonifier 250; Branson, Danbury, CT) was purified by nickel-nitrilotriacetic acid (Ni-NTA) agarose resin (Life Technologies Corp.) column chromatography. The protein fractions were examined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
DNA supercoiling activities and inhibition by FQs.
ATP-dependent and quinolone-inhibited DNA supercoiling assays were carried out as previously described (2, 3, 16, 20, 21, 31) with the following modifications. DNA supercoiling activity was examined with a reaction mixture (total volume, 30 μl) consisting of DNA gyrase reaction buffer, relaxed pBR322 DNA (300 ng), and purified GyrA and GyrB (50 ng each) subunits. Reactions were performed at 30°C for 1.5 h and stopped by adding an equal volume of chloroform-isoamyl alcohol (24:1 mixture) and 3 μl of 10× DNA loading dye. The total reaction mixtures were subjected to electrophoresis in a 1% agarose gel in 1× Tris-borate-EDTA (TBE) buffer, followed by ethidium bromide (0.7 μg/ml) staining. Supercoiling activity was evaluated by tracing the brightness of the bands with the software ImageJ (http://rsbweb.nih.gov/ij). Gyrase bearing an Ala91Val amino acid substitution in GyrA was used as a positive control for all assays (20). The inhibitory effect of FQs on DNA gyrases was assessed by determining the drug concentrations required to inhibit the supercoiling activity of the enzyme by 50% (IC50s). All enzyme assays were performed at least three times to confirm reproducibility.
Quinolone-mediated DNA cleavage assay.
DNA cleavage assays were carried out as previously described (16, 20, 21, 31). The reaction mixture (total volume, 30 μl) contained DNA gyrase reaction buffer, recombinant DNA gyrase subunits (50 ng), supercoiled pBR322 DNA (300 ng), and 2-fold serially increasing concentrations of FQs. After incubation for 2 h at 30°C, 3 μl of 2% SDS and 3 μl proteinase K (1 mg/ml) were added to the reaction mixture. After subsequent incubation for 30 min at 30°C, reactions were stopped by the addition of 3 μl of 0.5 mM EDTA, 30 μl chloroform-isoamyl alcohol (24:1 mixture), and 3 μl of 10× DNA loading dye. The total reaction mixtures were subjected to electrophoresis in 0.8% agarose gels in 1× TBE buffer, followed by ethidium bromide staining. The extent of DNA cleavage was quantified with ImageJ, and the quinolone concentrations required to induce 25% of the maximum DNA cleavage (CC25s) were determined.
Temperature sensitivity of M. leprae DNA gyrase.
The reactions with mixtures (total volume, 30 μl) consisting of DNA gyrase reaction buffer, relaxed pBR322 DNA (300 ng), and recombinant DNA gyrase subunits (50 ng) were run at 25, 30, 33, 37, and 42°C for 1.5 h. Supercoiling activities of recombinant DNA gyrases were evaluated at each reaction temperature as described above.
RESULTS
Construction and purification of recombinant His-tagged GyrA and GyrB proteins.
DNA fragments, including the gyrA and gyrB genes, were successfully amplified from M. leprae Thai-53 strain DNA and inserted in frame downstream of a T7 promoter in pET-20b (+). GyrA and GyrB were expressed as C-terminal hexahistidine-tagged proteins for ease of purification, as the His tag has been shown not to interfere with the catalytic functions of GyrA and GyrB (2, 3, 16, 20, 21, 31). Expressed recombinant WT and mutant DNA gyrase subunits were purified as 0.3 to 1.5 mg soluble His-tagged 80-kDa protein of GyrA and 75-kDa protein of GyrB from 500-ml cultures. The purity of the recombinant proteins was confirmed by SDS-PAGE (Fig. 3). All of the recombinant proteins were obtained with high purity (>95%).
Fig 3.
SDS-PAGE analysis of purified M. leprae DNA gyrases. The His-tagged recombinant DNA gyrases were overexpressed in E. coli and purified by Ni-NTA affinity resin chromatography. Lanes: M, protein marker (NEB); 1, WT GyrA; 2, GyrA-Ala91Val; 3, GyrA-Asp95Gly; 4, GyrA-Asp95Asn; 5, WT GyrB. Three hundred nanograms of each protein was loaded onto a 5 to 20% gradient polyacrylamide gel.
DNA supercoiling activities.
Combinations of GyrA WT, Ala91Val, Asp95Gly, or Asp95Asn and WT GyrB subunits were examined for DNA supercoiling activities using relaxed pBR322 DNA as a substrate in the presence or absence of ATP (Fig. 4). DNA supercoiling activities were observed in the presence of ATP and recombinant DNA gyrase subunits (Fig. 4A to D, lane 3), while neither subunit alone exhibited DNA supercoiling activity (Fig. 4A to D, lanes 4 and 5). In addition, no supercoiling activity was observed when ATP was omitted from the reaction mixture (Fig. 4A to D, lane 6).
Fig 4.
DNA supercoiling assay. Supercoiling activities of WT DNA gyrase (A) and DNA gyrases bearing GyrA-Ala91Val (B), GyrA-Asp95Gly (C), and GyrA-Asp95Asn (D) were analyzed. Relaxed pBR322 (0.3 μg) was incubated with GyrA (50 ng) or GyrB (50 ng), or both. Lanes: 1, relaxed pBR322 alone; 2, relaxed pBR322 and ATP; 3, relaxed pBR322, ATP, GyrA, and GyrB; 4, relaxed pBR322, ATP, and GyrA; 5, relaxed pBR322, ATP, and GyrB; 6, relaxed pBR322, GyrA, and GyrB.
Inhibition of DNA gyrase activities by FQs.
The IC50s of FQs were determined using the quinolone-inhibited DNA supercoiling assay (Fig. 5). Representative data showing the inhibitory effects of OFX against DNA gyrase are shown in Fig. 5, and data for other FQs are presented in Fig. S1 in the supplemental material. IC50s of each FQ against WT and mutant DNA gyrases are summarized in Table 2. Each FQ showed dose-dependent inhibition, with IC50s ranging from 0.4 to 262.3 μg/ml. DNA gyrases bearing GyrA-Asp95Gly and -Asp95Asn showed significantly higher IC50s to quinolones (Table 2; Fig. 5; see Fig. S1 in the supplemental material) than WT gyrase (Table 2). These DNA gyrases also showed higher resistance than DNA gyrase bearing GyrA-Ala91Val, which was simultaneously analyzed as a positive control for resistance to FQs. Inhibitory effects of FQs were ranked SIT > GAT > MXF > OFX in all DNA gyrases.
Fig 5.
OFX-inhibited DNA supercoiling assay. Relaxed pBR322 (0.3 μg) was incubated with GyrA (50 ng) and GyrB (50 ng) in the presence of the indicated concentration of OFX. Quinolone-inhibited supercoiling activity assay was performed with combinations consisting of WT GyrB-WT GyrA (A), GyrA-Ala91Val (B), GyrA-Asp95Gly (C), and GyrA-Asp95Asn (D). R and SC, relaxed and supercoiled pBR322 DNA, respectively.
Table 2.
IC50s and CC25s of FQs against WT and mutant DNA gyrasesa
| Drug | IC50 |
CC25 |
||||||
|---|---|---|---|---|---|---|---|---|
| WT | Ala91Val | Asp95Gly | Asp95Asn | WT | Ala91Val | Asp95Gly | Asp95Asn | |
| OFX | 6.8 ± 0.8 | 39.4 ± 15.5 (5.8) | 161.2 ± 44.2 (23.7) | 262.3 ± 105.8 (38.6) | 7.3 ± 0.5 | 75.5 ± 16.8 (10.1) | 240.5 ± 30.7 (32.1) | 269.5 ± 76.5 (35.9) |
| GAT | 1.0 ± 0.1 | 3.1 ± 0.7 (3.1) | 7.5 ± 1.6 (7.5) | 13.8 ± 1.6 (13.8) | 1.1 ± 0.2 | 4.3 ± 0.2 (3.9) | 15.6 ± 3.6 (14.2) | 13.5 ± 3.1 (12.3) |
| MXF | 1.5 ± 0.3 | 5.2 ± 1.0 (3.5) | 21.5 ± 4.7 (14.3) | 34.7 ± 3.1 (23.1) | 1.0 ± 0.1 | 4.5 ± 1.0 (4.5) | 25.5 ± 3.7 (25.5) | 20.8 ± 5.0 (20.8) |
| SIT | 0.4 ± 0.0 | 1.0 ± 0.2 (2.5) | 2.2 ± 0.5 (5.5) | 3.9 ± 0.6 (9.8) | 0.3 ± 0.0 | 0.9 ± 0.0 (3.0) | 2.2 ± 0.6 (7.3) | 2.3 ± 0.4 (7.7) |
IC50s and CC25s are in μg/ml, and data in parentheses represent the fold increase compared to WT.
FQ-mediated DNA-cleavable complex formation.
The CC25s of FQs were determined. Figure 6 shows the result of a DNA cleavage assay using OFX, and Fig. S2 in the supplemental material presents the results using GAT, MXF, and SIT. Table 2 summarizes the CC25s of each DNA gyrase. DNA gyrases bearing GyrA-Asp95Gly and -Asp95Asn showed significantly higher CC25s to quinolones than WT gyrase (Table 2). These DNA gyrases also showed higher CC25s than gyrase bearing GyrA-Ala91Val (Table 2). Effects on cleavable complex formation were ranked SIT > GAT > MXF > OFX in all DNA gyrases.
Fig 6.
OFX-mediated DNA cleavage assay. Supercoiled pBR322 (0.3 μg) was incubated with GyrA (50 ng) and GyrB (50 ng) in the presence of the indicated concentration of OFX. DNA cleavage assay was performed with combinations consisting of WT GyrB-WT GyrA (A), GyrA-Ala91Val (B), GyrA-Asp95Gly (C), and GyrA-Asp95Asn (D). R, L, and SC, relaxed, linear, and supercoiled pBR322 DNA, respectively.
Temperature sensitivity of M. leprae DNA gyrase.
Figure 7 shows the effects of temperature on DNA gyrase activities. The highest DNA supercoiling activities were observed at 33°C in all DNA gyrases. WT and GyrA-A91V DNA gyrases showed reduced DNA supercoiling activities at 37°C, whereas Gyr-Asp95Gly and Asp95Asn DNA gyrases maintained activities comparable to those at 33°C. No supercoiling activities were observed in any of the DNA gyrases at 42°C.
Fig 7.
Temperature-dependent DNA supercoiling activity of DNA gyrases. Relaxed pBR322 (0.3 μg) was incubated with WT GyrB-WT GyrA (A), GyrA-Ala91Val (B), GyrA-Asp95Gly (C), and GyrA-Asp95Asn (D) at the temperatures (in °C) indicated above the lanes. The proportion of supercoiled DNA compared to that of WT DNA gyrase at 33°C is plotted for each incubation temperature.
DISCUSSION
Mutations in the gyrA gene of quinolone-resistant M. leprae clinical isolates have predominantly been reported at codon 91, and a smaller number have been reported at codon 89 (4, 19, 24, 26, 40). Amino acid substitutions at other positions have not been reported, in strong contrast to the substitutions reported in M. tuberculosis, with predominant mutations in codon 94 (1, 7, 9, 10, 32, 34, 39), equivalent to codon 95 in M. leprae (Fig. 1). This study aimed to obtain basic data for the rapid detection of FQ-resistant leprosy by elucidating the correlation between mutations at codon 95 and quinolone resistance.
To explain the discrepancy described above, we first hypothesized that amino acid substitution at position 95 in GyrA of M. leprae has less of an influence on FQ resistance. Hence, we carried out a quinolone-mediated supercoiling activity inhibition assay and DNA cleavage assay at 30°C, the optimal temperature of M. leprae growth, using recombinant DNA gyrases and calculated IC50s and CC25s of four FQs, OFX, MXF, GAT, and SIT. The DNA gyrase bearing GyrA-Ala91Val, used as a control, exhibited resistance, having approximately 2- to 10-fold higher IC50s and CC25s of FQs than WT DNA gyrase, as has been reported previously (20, 21). Interestingly, DNA gyrases bearing GyrA-Asp95Gly or -Asp95Asn showed resistance, having approximately 5- to 40-fold higher IC50s and CC25s of FQs than WT DNA gyrase (Table 2). Namely, amino acid substitution from Asp to Gly or Asn at position 95 added higher resistance to DNA gyrase than that from Ala to Val at position 91. This was similar to the observation in M. tuberculosis (2, 3). These results suggested that a possible property of Asp95Gly and Asp95Asn amino acid substitutions in GyrA is to give higher FQ resistance to DNA gyrase in M. leprae.
We then hypothesized that amino acid substitutions at position 95 place a disadvantage on the enzymatic property of DNA gyrases, especially lower or abolished activity at higher temperatures, and thus, we conducted a DNA supercoiling assay at various temperatures: 25, 30, 33, 37, and 42°C. DNA supercoiling activities of WT and GyrA-Ala91Val DNA gyrase showed a similar temperature dependence, with the highest activity being at 25 to 33°C, reduced activity occurring at 37°C, and activity being completely abolished at 42°C. In contrast, DNA gyrases bearing GyrA-Asp95Gly or -Asp95Asn maintained their activities even at 37°C. Our hypothesis was rejected by these data.
The influence of the clear usage of FQs for the treatment of leprosy and tuberculosis might solve this question. For leprosy patients with a single lesion, a single application of 400 to 600 mg of OFX is used. For the treatment of MDR leprosy, two or three doses of 400 to 600 mg in combination with first-line drugs DDS and RIF (11) are applied. In contrast, for tuberculosis, OFX is taken twice daily at 400 mg each time with first-line drugs such as isoniazid and rifampin for several months (11, 36). The maximum serum concentration (Cmax) of OFX has been reported to show a dose-dependent increase. The Cmaxs achieved with administration of 100 mg, 300 mg, and 600 mg of OFX in humans were 1.00, 2.81, and 6.81 μg/ml, respectively (14). The blood concentration of OFX is low in leprosy patients and is maintained at a high level in tuberculosis patients because of the treatment regimen. Thus, M. leprae carrying DNA gyrase with lower resistance, such as GyrA-Ala91Val, might be predominantly selected for various reasons in leprosy patients, whereas GyrA-Asp94Gly or -Asp94Asn is predominantly found in M. tuberculosis-infected patients (1, 7, 9, 10, 32, 34, 39); however, the possible emergence in the future of highly FQ-resistant M. leprae having an amino acid substitution at position 95 cannot be rejected, especially when MDR leprosy is treated by repeated administration of FQs.
We investigated the inhibitory effects of OFX, GAT, MXF, and SIT against WT and mutant DNA gyrases. IC50s of OFX for WT and GyrA-Ala91Val, -Asp95Gly, and -Asp95Asn DNA gyrases were 6.8, 39.4, 161.2, and 262.3 μg/ml, respectively (Table 2). The order of FQ inhibitory activity was SIT > GAT > MXF > OFX. OFX does not have the ability to inhibit M. leprae with DNA gyrase carrying GyrA-Asp95Gly or -Asp95Asn. The IC50 of SIT was the lowest of the four quinolones, with IC50s of 0.4, 1.0, 2.2, and 3.9 μg/ml for WT, A91V, D95G, and D95N gyrases, respectively. As the Cmaxs of OFX, GAT, MXF, and SIT at the 100-mg dosage were determined in clinical trials to be 1.00, 0.87 to 5.41, 4, and 0.3 to 1.9 μg/ml, respectively (14, 27, 28, 30), SIT might strongly inhibit M. leprae carrying GyrA-Ala91Val DNA gyrase and be a promising candidate for the treatment of the majority of cases of FQ-resistant leprosy.
In conclusion, we revealed the contribution of the GyrA-Asp95Gly and -Asp95Asn amino acid substitutions to FQ resistance in M. leprae by an in vitro assay. This suggested the possible emergence in the future of FQ-resistant M. leprae carrying GyrA with these amino acid substitutions, although further analysis is needed to clarify a direct relationship to in vivo resistance. Hence, we would like to propose analysis for these amino acid substitutions to detect FQ-resistant leprosy.
Supplementary Material
ACKNOWLEDGMENTS
We thank Haruka Suzuki, Yukari Fukushima, and Aiko Ohnuma for their technical support.
This work was supported by grants from the U.S.-Japan Cooperative Medical Science Program, the Global Center of Excellence Program, the Establishment of International Collaboration Centers for Zoonosis Control, and the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan, and in part by grants from the Japan Initiative for Global Research Network on Infectious Diseases MEXT to Y.S. and by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science to Y.S. and C.N.
Footnotes
Published ahead of print 21 November 2011
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1. An DD, et al. 2009. Beijing genotype of Mycobacterium tuberculosis is significantly associated with high-level fluoroquinolone resistance in Vietnam. Antimicrob. Agents Chemother. 53: 4835–4839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aubry A, et al. 2006. Novel gyrase mutations in quinolone-resistant and -hypersusceptible clinical isolates of Mycobacterium tuberculosis: functional analysis of mutant enzymes. Antimicrob. Agents Chemother. 50: 104–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aubry A, Pan XS, Fisher LM, Jarlier V, Cambau E. 2004. Mycobacterium tuberculosis DNA gyrase: interaction with quinolones and correlation with antimycobacterial drug activity. Antimicrob. Agents Chemother. 48: 1281–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cambau E, Perani E, Guillemin I, Jamet P, Ji B. 1997. Multidrug-resistance to dapsone, rifampicin, and ofloxacin in Mycobacterium leprae. Lancet 349: 103–104 [DOI] [PubMed] [Google Scholar]
- 5. Cambau E, Carthagena L, Chauffour A, Ji B, Jarlierbl V. 2006. Dihydropteroate synthase mutations in the folP1 gene predict dapsone resistance in replaced cases of leprosy. Clin. Infect. Dis. 42: 238–241 [DOI] [PubMed] [Google Scholar]
- 6. Cambau E, et al. 2002. Molecular detection of rifampin and ofloxacin resistance for patients who experience relapse of multibacillary leprosy. Clin. Infect. Dis. 34: 39–45 [DOI] [PubMed] [Google Scholar]
- 7. Campbell PJ, et al. 2011. Molecular detection of mutations associated with first- and second-line drug resistance compared with conventional drug susceptibility testing of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 55: 2032–2041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Champoux JJ. 2001. DNA topoisomerases: structure, function, and mechanism. Annu. Rev. Biochem. 70: 369–413 [DOI] [PubMed] [Google Scholar]
- 9. Cui Z, Wang J, Lu J, Huang X, Hu Z. 2011. Association of mutation patterns in gyrA/B genes and ofloxacin resistance levels in Mycobacterium tuberculosis isolates from east China in 2009. BMC Infect. Dis. 11: 78–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Feuerriegel S, et al. 2009. Sequence analyses of just four genes to detect extensively drug-resistant Mycobacterium tuberculosis strains in multidrug-resistant tuberculosis patients undergoing treatment. Antimicrob. Agents Chemother. 53: 3353–3356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goto M, et al. 2006. Guideline for the treatment of Hansen's disease in Japan (second edition), Japanese leprosy. Nihon Hansenbyo Gakkai Zasshi 75: 191–226 (In Japanese.) [DOI] [PubMed] [Google Scholar]
- 12. Honore N, Cole ST. 1993. Molecular basis of rifampin resistance in Mycobacterium leprae. Antimicrob. Agents Chemother. 37: 414–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hooper DC. 2000. Mechanisms of action and resistance of older and newer fluoroquinolones. Clin. Infect. Dis. 31: S24–S28 [DOI] [PubMed] [Google Scholar]
- 14. Ichihara N. 1984. Phase I study on DL-8280 (OFX). Chemotherapy 32: 118–149 [Google Scholar]
- 15. Kai M, et al. 1999. Diaminodiphenylsulfone resistance of Mycobacterium leprae due to mutations in the dihydropteroate synthase gene. FEMS Microbiol. Lett. 177: 231–235 [DOI] [PubMed] [Google Scholar]
- 16. Kim H, et al. 2011. Impact of the E540V amino acid substitution in GyrB of Mycobacterium tuberculosis on quinolone resistance. Antimicrob. Agents Chemother. 55: 3661–3667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kyaw UK, Aye DKS. 2006. A case of multi-drug resistant leprogy-relapse or re-infection, Myanmar J. Curr. Med. Pract. 10: 41–43 [Google Scholar]
- 18. Levy L, Ji B. 2006. The mouse foot-pad technique for cultivation of Mycobacterium leprae. Lepr. Rev. 77: 5–24 [PubMed] [Google Scholar]
- 19. Maeda S, et al. 2001. Multidrug resistant Mycobacterium leprae from patients with leprosy. Antimicrob. Agents Chemother. 45: 3635–3639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Matrat S, Cambau E, Jarlier V, Aubry A. 2008. Are all the DNA gyrase mutations found in Mycobacterium leprae clinical strains involved in resistance to fluoroquinolones? Antimicrob. Agents Chemother. 52: 745–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Matrat S, et al. 2007. Expression and purification of an active form of the Mycobacterium leprae DNA gyrase and its inhibition by quinolones. Antimicrob. Agents Chemother. 51: 1643–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Matsuoka M. 2010. The history of Mycobacterium leprae Thai-53 strain. Lepr. Rev. 81: 137. [PubMed] [Google Scholar]
- 23. Matsuoka M, et al. 2007. The frequency of drug resistance mutations in Mycobacterium leprae isolates in untreated and replaced leprosy patients from Myanmar, Indonesia and the Philippines. Lepr. Rev. 78: 343–352 [PubMed] [Google Scholar]
- 24. Matsuoka M, Kashiwabara Y, Namisato M. 2000. A Mycobacterium leprae isolate resistant to dapsone, rifampin, ofloxacin and sparfloxacin. Int. J. Lepr. Other Mycobact. Dis. 68: 452–455 [PubMed] [Google Scholar]
- 25. Matsuoka M, Kashiwabara Y, Liangfen Z, Goto M, Kitajima S. 2003. A second case of multidrug resistant Mycobacterium leprae isolated from a Japanese patient with relapsed lepromatous leprosy. Int. J. Lepr. Other Mycobact. Dis. 71: 240–243 [DOI] [PubMed] [Google Scholar]
- 26. Matsuoka M, et al. 2010. Possible mode of emerging drug resistant leprosy cases revealed in Mexican samples' analysis. Jpn. J. Infect. Dis. 63: 412–416 [PubMed] [Google Scholar]
- 27. Nakashima M, et al. 1995. Pharmacokinetics and tolerance of DU-6859a, a new fluoroquinolone, after single and multiple oral doses in healthy volunteers. Antimicrob. Agents Chemother. 39: 170–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nakashima M, et al. 1995. Single- and multiple-dose pharmacokinetics of AM-1155, a new 6-fluoro-8-methoxy quinolone, in humans. Antimicrob. Agents Chemother. 39: 2635–2640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Norman G, Joseph G, Ebenezer G, Rao PSSS, Job CK. 2003. Secondary rifampin resistance following multi-drug therapy–a case report. Int. J. Lepr. Other Mycobact. Dis. 71: 18–21 [DOI] [PubMed] [Google Scholar]
- 30. Ohnishi N, et al. 2005. Safety, pharmacokinetics and influence on the intestinal flora of BAY 12-8039 (moxifloxacin hydrochloride) after oral administration in healthy male subjects. Jpn. Pharmacol. Ther. 33: 1029–1045 [Google Scholar]
- 31. Pan XS, Yague G, Fisher LM. 2001. Quinolone resistance mutations in Streptococcus pneumoniae GyrA and ParC proteins: mechanistic insights into quinolone action from enzymatic analysis, intracellular levels, and phenotypes of wild-type and mutant proteins. Antimicrob. Agents Chemother. 45: 3140–3147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ramaswamy S, Musser JM. 1998. Molecular genetic basis of antimicrobial agent resistance in Mycobacterium tuberculosis 1998 update. Tuber. Lung Dis. 79: 3–29 [DOI] [PubMed] [Google Scholar]
- 33. Scollard DM, et al. 2006. The continuing challenges of leprosy. Clin. Microbiol. Rev. 19: 338–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sun Z, et al. 2008. Comparison of gyrA gene mutations between laboratory-selected ofloxacin-resistant Mycobacterium tuberculosis strains and clinical isolates. Int. J. Antimicrob. Agents 31: 115–121 [DOI] [PubMed] [Google Scholar]
- 35. Williams DL, Spring L, Harris E, Roche P, Gillis TP. 2000. Dihydropteroate synthase of Mycobacterium leprae and dapsone resistance. Antimicrob. Agents Chemother. 44: 1530–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. World Health Organization 2004. Multidrug therapy against leprosy. Report WHO/CDS/CPE/CEE/2004.46 World Health Organization, Geneva, Switzerland [Google Scholar]
- 37. World Health Organization 2011. Leprosy update, 2011. Wkly. Epidemiol. Rec. 86: 389–399 [PubMed] [Google Scholar]
- 38. Reference deleted.
- 39. Yin X, Yu Z. 2010. Mutation characterization of gyrA and gyrB genes in levofloxacin-resistant Mycobacterium tuberculosis clinical isolates from Guangdong province in China. J. Infect. 61: 150–154 [DOI] [PubMed] [Google Scholar]
- 40. You E-Y, Kang TJ, Kim S-K, Lee S-B, Chae G-T. 2010. Mutations in genes related to drug resistance in Mycobacterium leprae isolates from leprosy patients in Korea. J. Infect. 50: 6–11 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.