Abstract
We describe a virucidal small molecule, PD 404,182, that is effective against hepatitis C virus (HCV) and human immunodeficiency virus (HIV). The median 50% inhibitory concentrations (IC50s) for the antiviral effect of PD 404,182 against HCV and HIV in cell culture are 11 and 1 μM, respectively. The antiviral activity of PD 404,182 is due to the physical disruption of virions that is accompanied to various degrees (depending on the virus and exposure temperature/time) by the release of viral nucleic acids into the surrounding medium. PD 404,182 does not directly lyse liposomal membranes even after extended exposure, and it shows no attenuation in antiviral activity when preincubated with liposomes of various lipid compositions, suggesting that the compound inactivates viruses through interaction with a nonlipid structural component of the virus. The virucidal activity of PD 404,182 appears to be virus specific, as little to no viral inactivation was detected with the enveloped Dengue and Sindbis viruses. PD 404,182 effectively inactivates a broad range of primary isolates of HIV-1 as well as HIV-2 and simian immunodeficiency virus (SIV), and it does not exhibit significant cytotoxicity with multiple human cell lines in vitro (50% cytotoxic concentration, >300 μM). The compound is fully active in cervical fluids, although it exhibits decreased potency in the presence of human serum, retains its full antiviral potency for 8 h when in contact with cells, and is effective against both cell-free and cell-associated HIV. These qualities make PD 404,182 an attractive candidate anti-HIV microbicide for the prevention of HIV transmission through sexual intercourse.
INTRODUCTION
Human pathogenic viruses that acquire resistance to antiviral agents by rapid evolution in vivo pose a serious health problem with no simple cure. Antivirals targeting features of these viruses that can be altered through changes in the viral genetic code often exhibit limited efficacy. Hepatitis C virus (HCV) and human immunodeficiency virus (HIV) are two such viruses which cause disorders of the liver and immune system, respectively, and collectively afflict 2 to ∼3% of the world's population (40 and http://www.unaids.org/globalreport/Global_report.htm). For HCV, the current interferon/ribavirin combination therapy exhibits limited efficacy, and the two recently approved small-molecule drugs, both serine protease inhibitors—telaprevir and boceprevir (12, 23)—foster the development of resistant viral strains within days when administered alone (37, 43). For HIV, there are currently more than 20 approved antiretroviral drugs, forming the basis of highly active antiretroviral therapy (HAART). Despite the availability of this large repertoire of anti-HIV drugs, drug-resistant mutant strains of HIV still emerge over time. Approximately 4 to 5 million HIV patients are coinfected with HCV (10), and these individuals tend to exhibit a higher rate of viral persistence, increased viral load, and higher susceptibility to death compared to individuals infected with only one of these viruses (31). Thus, there is an urgent need to develop antivirals that treat and prevent infection by HCV and HIV through new modes of action.
Antiviral molecules targeting critical virus structural elements tend to be effective against several viruses and do not usually foster the emergence of drug-resistant viral isolates. One group of molecules inhibit virus-cell fusion by inducing positive membrane curvature, thus increasing the activation energy barrier for fusion with cell membranes (16, 27, 43). These molecules, which include rigid amphipathic fusion inhibitors (RAFIs) (42) and lysophosphatidylcholine (16), tend to have large hydrophilic heads and hydrophobic tails. LJ001, a recently discovered broad-spectrum small-molecule antiviral, inhibits the fusogenic activity of enveloped viruses by intercalating into the lipid membrane while leaving virion particles grossly intact (46). Alkylated porphyrins exhibit strong antiviral activity against several enveloped viruses through an unknown mechanism, perhaps by interfering with specific structures on the virus surface (17). Amphipathic peptides derived from HCV NS5A protein were shown to physically disrupt virions and were active against a variety of enveloped viruses (3, 7). Another approach to interfering with membrane elements required for virus infection is to target exposed anionic phospholipids widely expressed on infected host cells and viral envelopes, as was done with bavituximab, a chimeric antibody which rescues mice from Pichinde virus and mouse cytomegalovirus infection (41).
Previously, we identified a small-molecule inhibitor of HCV entry, PD 404,182 (PD), from a screen of 1,280 small-molecule compounds (LOPAC1280) known to be pharmacologically active in a variety of cellular processes (8). Here, we report that PD, an inhibitor of bacterial 2-keto-3-deoxyctulosonic acid (KDO) 8-P synthase (1a), is a virucidal compound that compromises the structural integrity of both HCV and HIV, likely by interacting with a nonlipid structural element of these viruses. In vitro studies revealed that PD physically disrupts variously pseudotyped lentiviruses and exposes the viral genomic RNA in a time- and temperature-dependent manner. Viral lysis is much less pronounced with cell culture-produced HCV (HCVcc), despite a clear inactivation of HCVcc infectivity on the preincubation of PD with viral supernatants. PD strongly inactivates multiple isolates of primary HIV-1 that utilize different coreceptors, HIV-2, and simian immunodeficiency virus (SIV), with a 50% inhibitory concentration (IC50) of ∼1 μM and a selectivity index (50% cytotoxic concentration [CC50]/IC50) of >300. A high antiviral potency and low cytotoxicity, combined with a unique mode of action, make PD a welcome addition to our current arsenal of antivirals to combat infection by HCV and HIV.
MATERIALS AND METHODS
Reagents.
PD 404,182 and Triton X-100 were purchased from Sigma-Aldrich (St. Louis, MO). C5A was synthesized at the Scripps Research Institute. PD and C5A were dissolved in 100% dimethylsulfoxide (DMSO) to final concentrations of 30 mM and 10 mg/ml, respectively, and stored at −20°C. Unless otherwise specified, growth medium for all cell culture work was Dulbecco's modified essential medium (DMEM) containing 4,500 mg/liter glucose, 4.0 mM l-glutamine, and 110 mg/liter sodium pyruvate (Thermo Scientific HyClone), and it was supplemented with 10% fetal bovine serum (FBS) (Atlanta Biologicals) and 1× nonessential amino acids (Thermo Scientific HyClone). Conditioned complete growth medium (DMEM plus 10% FBS) was harvested on day 3 (with cells at 100% confluence) postseeding from Huh-7.5 cells initially seeded at ∼20% confluence.
Production of HCVcc and pseudotyped lentiviruses.
The production and titer determination of Jc1 HCVcc (32) was performed as previously described (8). Unless otherwise specified, all lentiviral pseudoparticles were generated from 293T cells by the cotransfection of plasmids carrying HIV gag-pol, a provirus (pTRIP-Gluc, pV1-Gluc, or pV1-B), and an appropriate envelope protein. For the production of envelope glycoproteins of murine leukemia virus (MLVpp), Sindbis virus (SINVpp), and HIV (HIVpp), plasmids encoding the viral envelope proteins pHIT456 (5), pIntron-SINV-env (29), and HIV BaL.01 (25), respectively, were used.
pV1 is a minimal HIV-1 provirus lacking most HIV genes except for all necessary cis acting sequences, such as Tat, Rev, and Vpu open reading frames (ORFs) (9, 13, 47). In pV1-B and pV1-Gluc, the Nef gene was replaced by an irrelevant peptide and the Gluc gene, respectively. The titers of vesicular stomatitis virus envelope glycoprotein (VSV-Gpp) and HIVpp harboring pV1-B or pV1-Gluc was measured on a TZM-bl indicator cell line using the lacZ reporter in a limiting dilution assay (36).
HCVcc infection assay.
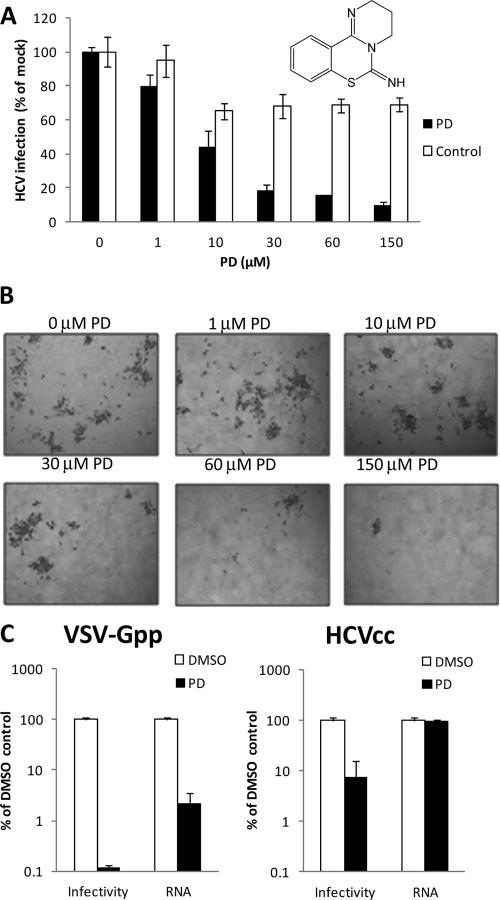
As shown in Fig. 1A, Jc1 Gluc HCVcc (∼105 50% tissue culture infectious doses [TCID50]/ml) (28) was concentrated 4-fold using an ultracentrifugation column with a 100-kDa cutoff membrane and washed twice with phenol red-free DMEM to remove any PD-inactivating molecules present in the virus supernatant. Concentrated virus was incubated with PD or DMSO at 37°C for 30 min, diluted 1,000-fold with fresh complete growth medium, and used to infect naïve Huh-7.5 cells in 24-well (105 cells/well) or 96-well (2.8 × 104 cells/well) plates 4 to 6 h after seeding. The control samples contain virus and PD of the same final titers/concentrations, but with the virus and PD separately diluted 1,000-fold prior to mixing. Viral infectivity was quantified by measuring the supernatant activity of the Gluc reporter or immunostaining infected cells for NS5A with 9E10 (anti-NS5A) antibody (26) 72 h postinfection.
Fig 1.
PD is virucidal against HCVcc and pseudotyped lentivirus. (A and B) Effect on HCVcc infectivity. Jc1 Gluc HCVcc was incubated with PD or 0.5% DMSO at 37°C for 30 min, diluted 1,000-fold, and used to infect Huh-7.5 cells. The control samples contain virus and PD of the same final titers/concentrations but with the virus and PD separately diluted 1,000-fold prior to mixing. The infectivity was quantified by measuring the supernatant activity of the Gluc reporter 72 h postinfection (A) and immunostaining for NS5A (B). HCV-infected cells are dark brown after immunostaining. The inset in panel A shows the chemical structure of PD. (C) Effect on extracellular VSV-Gpp and HCVcc. VSV-Gpp (harboring pV1-B; ∼106 TCID50/ml; undiluted) or Jc1 HCVcc (∼104 TCID50/ml; 10-fold diluted) was incubated with PD (150 μM in 0.5% DMSO) or 0.5% DMSO in the presence of 7 ng/ml RNase A at 37°C for 30 min. The viral RNA levels of the virus-PD and virus-DMSO mixtures were quantified by qRT-PCR, while the infectivity of the same mixtures was determined by the spinoculation of Huh-7.5 cells and the quantification of intracellular viral RNA by qRT-PCR 48 h later. All data are the means ± standard deviations (SD) from two independent experiments carried out in duplicate.
Spinoculation.
PD-treated virus samples (HCVcc or VSV-Gpp) were cooled on ice for 5 to 10 min and added to chilled target cells seeded in 96-well plates. Spinoculation was carried out at 300 × g for 2 h at 4°C. After centrifugation, cells were washed 4 times with cold complete growth medium to remove any residual compound/unbound virus and returned to 37°C and 5% CO2.
Viral RNA quantification.
For the direct quantification of HCVcc/VSV-Gpp RNA, the total RNA from PD-treated HCVcc/VSV-Gpp and cells infected with these viruses was isolated using the EZNA viral RNA kit (Omega Bio-Tek) and total RNA kit (Omega Bio-Tek), respectively. The amount of HCV RNA was quantified via TaqMan quantitative reverse transcription-PCR (qRT-PCR) (qScript one-step fast kit; Quanta Biosciences, Gaithersburg, MD) using previously described primers (44). The amount of lentiviral RNA was quantified using SYBR green qRT-PCR (one-step SYBR green kit; Quanta Biosciences) with primers pV1-qPCR-F (5′-ACGGCCTCTAGAATGAGC-3′) and pV1-qPCR-R (5′-ACAGCTGCTCGAGGTT-3′).
Due to the large amount of residual provirus-encoding DNA present in the pseudoparticle preparations obtained from transfected 293T cells, we were not able to directly quantify the viral RNA. Instead, repackaged pseudoparticles were used in all experiments involving the direct quantification of viral RNA by qRT-PCR. Briefly, VSV-Gpp constructed from pV1-B (9, 13, 47) were used to transduce Huh-7.5 cells. Three days later, these Huh-7.5 cells were transfected with plasmids carrying HIV gag-pol and vesicular stomatitis virus (VSV)-G envelope protein to produce freshly repackaged pseudoparticles. Pseudoparticles serially repackaged in this manner at least 3 times were used in experiments requiring the direct quantification of viral RNA.
Gluc reporter assay.
Supernatant Gluc activities were quantified 48 or 72 h postinfection with the relevant virus using a BioLux Gaussia luciferase assay kit (New England BioLabs) and normalized to viable cell levels as determined via the CellTiter-Glo luminescent cell viability assay (Promega).
Liposome dye release assay.
Liposomes composed of 36 mg POPC (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine), 39 mg DPPC (1,2-dipalmitoyl-sn-glycero-3-phosphocholine), 4 mg POPS (1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-l-serine), and 21 mg cholesterol per 100 mg, without or with 100 mM sulforhodamine B (SulfoB; Avanti Polar Lipids, Inc.), were prepared as described previously (7) and sized via repeated extrusion through a 100-nm polycarbonate membrane filter (Avanti Polar Lipids, Inc.). Dye release assays were performed in a Gemini EM spectrofluorometer (Molecular Devices, San Francisco, CA). One μl PD (30 mM), 0.24 μl C5A (10 mg/ml), or 1 μl DMSO was added to 100 μl liposomes (100 μM; 0.06 mg/ml) in phosphate-buffered saline (PBS) in 384-well plates, and membrane disruption was gauged from the increase in SulfoB fluorescence at excitation and emission wavelength settings of 544 and 590 nm, respectively, 5 min posttreatment. The fluorescence intensity corresponding to 100% SulfoB release was obtained by liposome disruption with 0.1% Triton X-100.
HIV-1, HIV-2, and SIV infectivity assays.
TZM-bl cells (5) (100,000 cells/ml) were exposed to HIV or SIV (1 ng of p24/p27) for 4 h together with increasing concentrations of PD or DMSO control and washed, and then infection was measured 48 h later by β-galactosidase activity. Primary HIV-1, HIV-2, and SIV were obtained through the National Institutes of Health (NIH) AIDS Research and Reference Reagent Program and amplified in activated human peripheral blood mononuclear cells (PBMC; activated by phytohemagglutinin/interleukin-2 treatment). To determine the anti-HIV effect of PD in genital fluids, the same TZM cells were exposed to HIV strain JR-CSF(1 ng of p24) for 4 h together with increasing concentrations of PD diluted in cervical fluids (pool of four donors) (2).
HIV-1 sedimentation assay.
Purified HIV-1 (20 ng of p24 of NL4.3) was microcentrifuged for 90 min at 4°C to remove free capsid, resuspended in PBS, exposed to PD or DMSO medium control, and loaded over a 20 to 70% sucrose gradient. After ultracentrifugation at 20,000 rpm for 24 h in an SW-41 T rotor, fractions (1 ml) were collected and tested for their content of viral proteins. HIV-1 capsid was detected by p24 enzyme-linked immunosorbent assay (ELISA). Reverse transcriptase (RT) activity was measured using a polyribonucleotide template (exo-RT assay) (1). The density of each sucrose gradient fraction was determined by measuring the refractive index.
HIV-1 cell-to-cell transfer assay.
Blood-derived immature dendritic cells (DC) were plated at 50,000 cells per well in 96-well V-bottom plates (BD Biosciences). Cells were incubated with wild-type NL4.3-eGFP (X4), NL4.3-BaL-eGFP (R5), or the single-round NL4.3ΔEnv-eGFP pseudotyped virus with NL4.3 gp160 (X4) (25 ng of p24) for 2 h at 37°C. Medium supplemented with either PD or DMSO then was added and incubated with DC for 2 h. Cells were washed three times with warm medium, and CCR5 Jurkat T cells (100,000 cells) were added. Cells were cultured in a flat-bottom 96-well plate, harvested after 3 days, and fixed in 4% paraformaldehyde-PBS, and green fluorescent protein (GFP) expression was measured by a fluorescence-activated cell sorter (FACS). The percentage of infected Jurkat T cells was selectively quantified by gating T cells using an anti-CD3 antibody. Virus isolates used in this study were pNL4.3-BaL (R5), in which wild-type NL4.3 envelope was switched for the R5 BaL envelope; pNL4.3ΔEnv, which lacks gp160; pNL4.3-eGFP (X4); and pNL4.3-BaL-eGFP (R5), which encode the GFP gene instead of the Nef gene (14).
RESULTS
PD 404,182 is virucidal against HCV and pseudotyped lentiviruses.
Previously, we showed that PD alleviates a HCVcc-induced cytopathic effect and inhibits the cellular entry of HIV lentivirus pseudotyped with envelope glycoproteins from the H77 isolate of HCV and vesicular stomatitis virus (VSV). In this study, we show that PD inhibits HCV infection by inactivating extracellular virions. As shown in Fig. 1A and B, PD dose dependently inactivates cell-free HCVcc with an IC50 of 11 μM. To explore the possibility that the antiviral activity of PD is independent of the viral envelope protein, we incubated PD with HIV lentiviruses pseudotyped with three additional envelope proteins derived from murine leukemia virus (MLV) (5), Sindbis virus (SINV) (29), and HIV (25). PD exhibits similar antiviral activity against all of these pseudotyped lentiviruses (see Fig. S1 in the supplemental material), indicating that the antiviral activity derives from interference with a viral structural component other than the envelope proteins.
We next set out to evaluate whether PD treatment causes the lysis of the virus membrane/capsid. Supernatant containing lentivirus pseudotyped with vesicular stomatitis virus envelope glycoprotein (VSV-Gpp) or HCVcc was treated with PD or with the corresponding concentration of the solvent DMSO at 37°C for 30 min in the presence of RNase A prior to the quantification of viral RNA and infectivity. As shown in Fig. 1C, treatment with 150 μM PD induces the RNase-mediated degradation of VSV-Gpp RNA by ∼30-fold (3.7% remaining) and inhibits supernatant infectivity by ∼1,000-fold (0.1% remaining) relative to that of DMSO-treated virus. The significant fold difference between virus inactivation and virion lysis, which becomes more pronounced with shorter virus-PD preincubation times (see Fig. 4B), suggests that virion lysis is not required for PD-mediated virus inactivation. A similar effect was observed with the virucidal peptide C5A, which showed ∼100-fold more virion inactivation than lysis (7). Little to no virion lysis was observed in 150 μM PD-treated HCVcc, despite the >10-fold inhibition of virus infectivity. A low but reproducible level of HCVcc virion lysis (27%) was observed (see Fig. S2 in the supplemental material) only when a higher concentration of PD (300 μM) was used in combination with a prolonged incubation at 37°C (90 min). Since PD appears to significantly inactivate but only poorly lyse HCVcc, we asked ourselves whether PD-treated HCVcc particles that resist lysis by PD retain their ability to attach to the surface of cells. The measurement of cell surface-associated virus via qRT-PCR revealed that PD-treated HCVcc binds to cells comparably to control DMSO-treated virus, suggesting that treatment with the compound inhibits a postattachment step for virions that remain intact (see Fig. S2). The ability of PD to physically inactivate both VSV-Gpp and HCVcc, two very different viruses, combined with the observation that the compound does not seem to significantly distinguish between lentiviruses pseudotyped with different envelope proteins (see Fig. S1), suggests that its antiviral activity is mediated through a common non-envelope protein structural component.
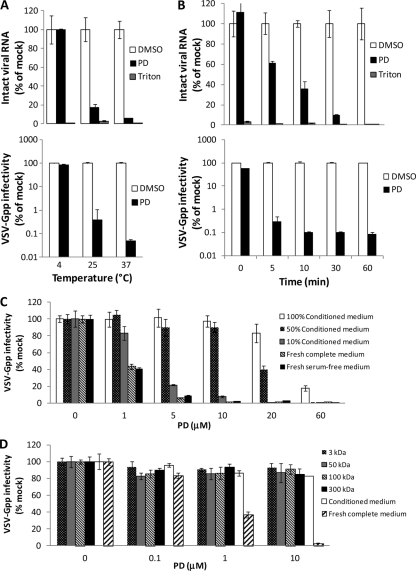
Fig 4.
PD exhibits virucidal activity that is temperature- and virus dilution-dependent and virus lysis activity that is time dependent. Undiluted VSV-Gpp (harboring pV1-B or pV1-Gluc; ∼5 ×105 TCID50/ml) was treated with 300 μM PD, 0.1% Triton X-100, or 1% DMSO in the presence of 7 ng/ml RNase A for 60 min at different temperatures (A) or at 37°C for different times (B). Viral RNA was isolated thereafter and quantified using qRT-PCR. Viral infectivity was determined by measuring the supernatant Gluc reporter activity of Huh-7.5 cells 48 h postinoculation with 1,000-fold-diluted virus-PD mixtures. For virus dilution studies, VSV-Gpp stock (harboring pV1-Gluc; ∼1.7 × 107 TCID50/ml) was diluted 500-fold in medium comprising different proportions of conditioned and fresh complete media (all containing 10% FBS) or fresh serum-free medium (C), or the flowthrough of conditioned medium size-fractionated through membranes with pores of the indicated size prior to pretreatment with PD at 37°C for 30 min (D). PD-treated viral samples were used to spinoculate naïve Huh-7.5 cells. Infectivity was quantified 2 days later by measuring the supernatant activity of the Gluc reporter. All data are the means ± SD from two independent experiments carried out in duplicate.
To evaluate the antiviral specificity of PD, we tested the effect of the compound on two other enveloped viruses, SINV, an alphavirus (38), and Dengue virus (DenV), a flavivirus closely related to HCV (18). As shown in Fig. S3 in the supplemental material, PD exhibits no significant inhibitory effect on the infectivity of SINV and DenV at 300 μM. Interestingly, despite the observed absence of antiviral activity against SINV, PD was found to exhibit strong antiviral activity against lentivirus pseudotyped with SINV envelope protein (see Fig. S1), underscoring the nonspecific nature of the antiviral effect on pseudotyped lentiviruses. The neutrality of PD toward SINV and DenV suggests that PD exerts its antiviral effect by specifically interfering with a structural feature common to HCVcc and pseudotyped lentiviruses but not present on SINV and DenV.
PD inactivates a broad range of primary HIV isolates and related retroviruses.
Since PD strongly inactivates all of the pseudotyped lentiviruses we tested regardless of the envelope protein (see Fig. S1 in the supplemental material), we asked ourselves whether PD also inactivates primary HIV and related retroviruses. Using CD4+ HeLa cells (TZM-bl cells [34]) that produce β-galactosidase in response to HIV infection, we determined the antiviral activity of PD on 14 isolates of HIV-1 which represent various subtypes and which use different coreceptors, either CCR5 (R5 viruses) or CXCR4 (X4 viruses), to infect cells, as well as isolates of other retroviruses, including HIV-2 and simian immunodeficiency virus (SIV). Viruses were added to TZM-bl cells together with PD for 4 h, cells were washed, and infection was scored 48 h later. As shown in Table 1, PD effectively inhibits all of the tested isolates of HIV and SIV at submicromolar to low-micromolar concentrations, on par with the potency of the virucidal amphipathic peptide C5A (3, 7). Similar anti-HIV potency was observed when PD was diluted in cervical fluids (Table 1).
Table 1.
PD 404,182 inhibits a broad spectrum of HIV and related viruses
| Isolate | Clade | Coreceptor usage | IC of PD (μM) ina: |
|||
|---|---|---|---|---|---|---|
| DMEMb |
Cervical Fluidc |
|||||
| IC50 | IC90 | IC50 | IC90 | |||
| Primary HIV-1 | ||||||
| 92RW021 | A | R5 | 0.43 ± 0.03 | 3.8 ± 0.3 | 0.67 ± 0.04 | 4.4 ± 0.2 |
| 92UG029 | A | X4 | 1.18 ± 0.02 | 4.4 ± 0.2 | 1.47 ± 0.1 | 5.3 ± 0.3 |
| 92TH026 | B | R5 | 0.35 ± 0.01 | 2.8 ± 0.2 | 0.61 ± 0.03 | 3.3 ± 0.1 |
| 92HT599 | B | X4 | 1.8 ± 0.1 | 5.9 ± 0.3 | 2.2 ± 0.04 | 6.4 ± 0.5 |
| 93IN101 | C | R5 | 1.5 ± 0.2 | 5.1 ± 0.3 | 1.8 ± 0.1 | 5.5 ± 0.2 |
| 98IN017 | C | X4 | 0.4 ± 0.1 | 1.9 ± 0.2 | 0.7 ± 0.05 | 2.4 ± 0.2 |
| 92UG005 | D | R5 | 1.26 ± 0.2 | 5.3 ± 0.4 | 1.39 ± 0.11 | 5.7 ± 0.3 |
| 92UG024 | D | X4 | 0.33 ± 0.02 | 1.4 ± 0.2 | 0.55 ± 0.04 | 1.8 ± 0.2 |
| 92TH006 | E | R5 | 1.8 ± 0.2 | 6.6 ± 0.4 | 2.3 ± 0.1 | 7.6 ± 0.6 |
| 93TH053 | E | X4 | 1.4 ± 0.2 | 5.3 ± 0.3 | 1.8 ± 0.2 | 6.4 ± 0.4 |
| 93BR029 | F | R5 | 0.7 ± 0.1 | 3.5 ± 0.2 | 1.2 ± 0.1 | 4.6 ± 0.3 |
| 93BR020 | F | X4 | 1.4 ± 0.2 | 6.9 ± 0.4 | 1.9 ± 0.2 | 7.5 ± 0.4 |
| RU132 | G | R5 | 0.6 ± 0.2 | 3.1 ± 02 | 0.9 ± 0.2 | 3.9 ± 0.5 |
| Jv1083 | G | R5 | 1.1 ± 0.2 | 3.9 ± 0.3 | 1.5 ± 0.2 | 4.7 ± 0.1 |
| Other retroviruses | ||||||
| SIVmac251 32H | 1.1 ± 0.2 | 6.2 ± 0.4 | ||||
| SIVsyk1.2 | 1.9 ± 0.3 | 5.4 ± 0.2 | ||||
| HIV-2CDC310342 | 1.8 ± 0.2 | 4.7 ± 0.2 | ||||
| HIV-27312A | 2.2 ± 0.3 | 6.8 ± 0.3 | ||||
Errors represent the SD from 2 independent experiments carried out in duplicate.
IC50 is measured with PD diluted in DMEM with 10% fetal bovine serum.
IC50 is measured with PD diluted in cervical fluids (pool of 4 donors).
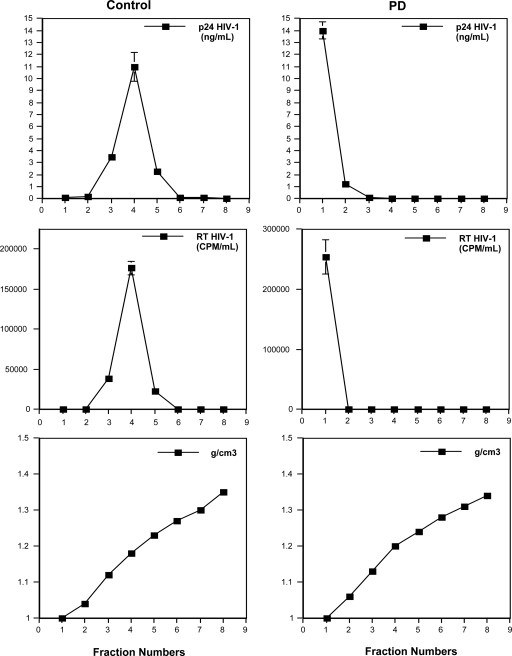
To probe the effect of PD on the structural integrity of HIV particles, we carried out a virus sedimentation assay. Purified HIV-1 (X4 NL4.3) (20 ng of p24 in PBS) was incubated in the presence or absence of PD (10 μM) for 30 min at 37°C and loaded onto a 20 to 70% sucrose gradient. Each fraction was analyzed for the amount of HIV capsid and reverse transcriptase (RT) (Fig. 2). Untreated virus (capsid and RT proteins) sediments at a density of 1.16 g/cm3. In contrast, viral capsid and RT relocate to the top of the gradient in PD-treated virus preparations, indicating that PD exerts its virucidal effect on HIV and retroviral particles by compromising virion integrity. This observation is consistent with the lysis of pseudotyped lentivirus shown in Fig. 1C.
Fig 2.
PD destabilizes HIV-1 particles. NL4.3 virus (20 ng of p24) was incubated in the presence or absence of 10 μM PD for 30 min at 37°C and loaded over a sucrose density gradient. The quantification of HIV-1 capsid and RT proteins was conducted by p24 ELISA and exoRT assay, respectively. All data are the means ± SD from two independent experiments carried out in duplicate.
PD does not lyse or interact with liposomal membranes.
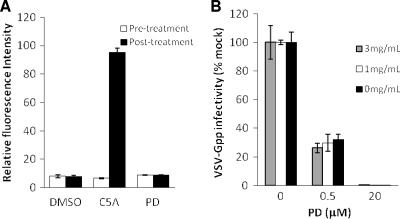
Because the antiviral potency of PD is virus envelope protein independent, we investigated the possibility that PD exerts its anti-viral activity via the disruption of the viral lipid membrane. Cholesterol-phospholipid liposomes entrapping the fluorescent dye sulforhodamine B (SulfoB) were incubated with PD, C5A, or DMSO. The disruption of the liposomes is accompanied by the dequenching of the fluorescent dye, and it was quantified by measuring the resultant fluorescence release. PD does not interfere with SulfoB fluorescence (data not shown). C5A, a peptide derived from HCV NS5A protein that has been shown to lyse liposomes, was used as a positive control (7). As shown in Fig. 3A, PD is unable to permeabilize liposomes after incubation for 5 min. No significant increase in fluorescence intensity was observed even after prolonged (up to 3 h) incubation with PD (see Fig. S4A in the supplemental material).
Fig 3.
PD does not lyse or directly interact with liposomal membranes. (A) The ability of PD (300 μM), the virucidal peptide C5A (10 μM), and solvent DMSO (1%) to permeabilize liposomes entrapping SulfoB was determined by a liposome dye release assay. A relative fluorescence intensity of 100 corresponds to SulfoB release resulting from liposome disruption with 0.1% Triton X-100. (B) VSV-Gpp (harboring pV1-Gluc; ∼1.7 × 107 TCID50/ml) was diluted 500-fold in fresh complete growth medium and preincubated with PD for 30 min at 37°C in the presence or absence of various concentrations of liposomes. The virus-PD-liposome mixtures then were used to spinoculate Huh-7.5 cells, and infectivity was quantified by measuring the supernatant activity of the Gluc reporter 48 h posttransduction. All data are the means ± SD from two independent experiments carried out in triplicate.
We next set out to determine whether PD associates with liposomal membranes without causing lysis. Since PD is not inherently fluorescent, we were unable to directly measure the interaction of PD with liposomes. Instead, we sought to determine whether the inhibitory effect of PD during infection can be reversed by the addition of liposomes. VSV-Gpp (3.4 × 104 TCID50/ml) mixed with PD and increasing concentrations of liposomes was used to infect Huh-7.5 cells. The presence of liposomes was not able to reverse PD's antiviral effect (Fig. 3B), suggesting that PD does not significantly interact with liposomes. In fact, liposomes of different lipid compositions were tested, but none were found to reverse the antiviral effect of PD (see Fig. S4B and C in the supplemental material).
The virucidal activity of PD is temperature, time, and virus dilution dependent.
To further elucidate the antiviral effect of PD, VSV-Gpp was incubated with PD (300 μM) or DMSO (1%) at various temperatures and for various times, and the amount of remaining viral RNA and the infectivity of the virus/compound mixtures were determined thereafter. The virion lysis activity of PD was found to be temperature dependent, as PD disrupts the virus following a 30-min incubation at 37°C but is less disruptive at 25°C and exhibits no measurable virion lysis at 4°C (Fig. 4A), indicating that a minimum level of membrane fluidity is required for PD to lyse the virus membrane/capsid. A similar trend was observed for viral infectivity. PD rapidly inactivates pseudotyped lentivirus at 37°C as determined by the loss of viral RNA and infectivity (Fig. 4B). More than 99.5% of the VSV-Gpp was inactivated within 5 min when in contact with 300 μM PD. However, only ∼40% of the virions were compromised to the point of genomic RNA release for the same 5-min virus-PD preincubation, indicating that virion lysis is not required for virus inactivation.
The sensitivity of virus to PD also is virus dilution dependent (see Fig. S5 in the supplemental material). The IC50s of PD for cell culture-produced VSV-Gpp virus stocks diluted 5- and 500-fold in fresh complete growth medium (DMEM plus 10% FBS) are 4.6 and 0.5 μM, respectively. Similarly, the IC50s for HIVpp (lentivirus pseudotyped with envelope protein from Bal.01 HIV) are 24.6 and 0.3 μM for undiluted and 100-fold-diluted virus. Further studies demonstrated that PD is inactivated by a molecule(s) present in conditioned cell culture medium, as virus diluted in conditioned medium is significantly less sensitive to inactivation by PD than the same virus diluted in fresh complete medium (Fig. 4C). To gauge the approximate size of the molecule(s) responsible for neutralizing the antiviral effect of PD, we fractionated conditioned medium from Huh-7.5 cells by passage through ultrafiltration membranes with different pore sizes and found that the filtrate from a 3-kDa membrane is able to inactivate PD to the same extent as the unfiltered conditioned medium (Fig. 4D). This result suggests that the molecule(s) responsible for neutralizing PD is relatively small (≤3 kDa). Analysis using liquid chromatography-mass spectrometry (LC-MS) demonstrated that PD is degraded (data not shown), possibly by reacting with a small molecule secreted by cells, rendering it less active against pseudotyped lentivirus. Although the presence of 10% fetal bovine serum appears to have no inhibitory effect on PD's antiviral activity (Fig. 4C), 10% human serum yielded a significant (50- to 80-fold) increase in IC50 (see Fig. S6 in the supplemental material), suggesting the presence of PD-inhibitory factors in human serum in addition to cell culture medium conditioned by human cancer cells. It is worth noting that similar IC50 and IC90 values were obtained with PD diluted in DMEM or cervical fluids (Table 1), indicating that cervical fluids are free of molecule(s) that detectably inhibit the antiviral activity of PD.
Antiviral effect of PD before, during, and after HIV-1 exposure and on cell-to-cell transmission of HIV-1.
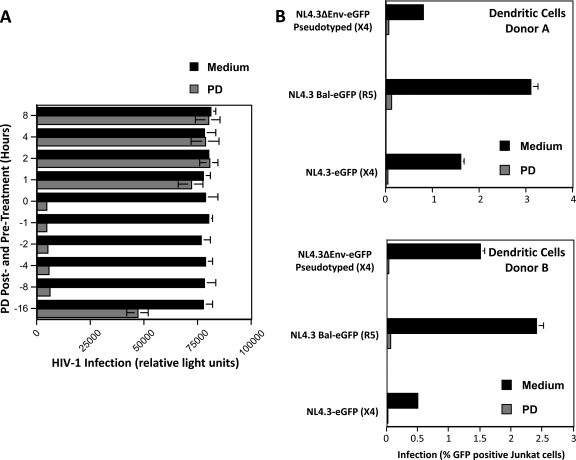
PD exhibits strong virucidal activity against pseudotyped lentivirus and primary HIV, raising the possibility of its use as a topical microbicide for preventing the sexual transmission of HIV-1. To shed light on this possibility, we investigated the antiviral effect of PD when the compound was added to cells at various time points relative to the addition of HIV-1. PD was added to TZM-bl cells at 1, 2, 4, or 16 h before the addition of HIV-1 (R5 JR-CSF) (1 ng of p24), together with the virus (time zero), and at 1, 2, 4, or 8 h after the addition of the virus, and infectivity was quantified 48 h after virus addition. As shown in Fig. 5A, PD significantly inhibits HIV-1 infection when added together with the virus (time zero) and retains its full potency up to 8 h before the addition of the virus. However, PD loses its antiviral effect when added to cells after virus inoculation (Fig. 5A, 1, 2, 4, and 8 h after treatment). This result suggests that PD is not able to disrupt intracellular virus. In addition, extended (>16 h) preincubation of PD with cells prior to virus inoculation also significantly reduces the compound's antiviral efficacy.
Fig 5.
Effect of PD on HIV-1 infection. (A) Antiviral effect of PD before, during, and after virus exposure. PD (10 μM) or just growth medium (DMSO control) was added to TZM-bl cells 1, 2, 4, 8, or 16 h before (negative values on the y axis) or after (positive values on the y axis) the addition of HIV-1 (R5 JR-CSF) (1 ng of p24) or together (time zero) with the virus. Infection was quantified 48 h later via the measurement of β-galactosidase activity. (B) DC (105 cells) were incubated for 2 h at 37°C with wild-type NL4.3-eGFP (X4) and NL4.3-BaL-eGFP (R5) viruses or with the pseudotyped NL4.3ΔEnv-eGFP/gp160 X4 Env virus (25 ng of p24). PD (10 μM) or control DMSO medium was added 2 h later. DC were washed 2 h after adding PD, Jurkat T cells (100,000 cells) were added for 3 days, and the percentage of infected Jurkat T cells (GFP+) was analyzed by flow cytometry. Error bars represent standard errors of duplicates from two independent experiments.
Since HIV-1 can be transmitted either as a cell-free or cell-associated virus, we examined the effect of PD on cell-to-cell transmission. Specifically, we examined the capacity of PD to prevent the dendritic cell (DC)-mediated transmission of HIV-1. We took advantage of a replication-defective virus (NL4.3ΔEnv-eGFP) which does not encode Env but which has been pseudotyped with HIV-1 Env. (These pseudotyped viruses infect cells because they contain Env.) However, cells that have been infected by pseudotyped viruses cannot produce infectious viruses, because de novo viruses do not encode Env. Thus, the use of pseudotyped viruses allowed the analysis of the effect of PD on the transmission of infectious particles from DC to T cells independently of DC infection. DC were incubated with wild-type NL4.3-eGFP (X4 virus) and NL4.3-BaL-eGFP (R5 virus) or pseudotyped NL4.3ΔEnv-eGFP/gp160 Env viruses (25 ng of p24). Two hours later, at which time the attachment of the virus onto DC is completed (15), PD (10 μM) was added. After 2 h, DC were washed to remove both free virus and PD. To measure DC-T cell transmission, Jurkat T cells were added for 3 days, and the percentage of infected T cells (gated with an anti-CD3 antibody) was analyzed by FACS. Only pseudotyped viruses that have been rapidly transferred from DC to T cells through the virological synapse (independently of DC infection) can infect T cells. Indeed, progeny viruses from DC infected by pseudotyped viruses can no longer infect T cells because they do not encode Env. Because DC were washed before adding T cells, the T-cell infection by the pseudotyped virus observed in Fig. 5B could arise only from pseudotyped particles that were transferred from DC to T cells. Importantly, PD added to DC prevents subsequent T-cell infection with the pseudotyped virus. This finding suggests that PD also can inactivate DC-bound virus, preventing HIV transmission from DC to T cells. These results suggest that, unlike neutralizing antibodies (14), PD blocks the cell-to-cell transfer of HIV even when transmission occurs via the virological synapse.
DISCUSSION
In this study, we report a small molecule, PD 404,182 (PD), that renders extracellular cell culture-produced HCV, pseudotyped lentiviruses, and several primary isolates of HIV and SIV noninfectious. In the case of pseudotyped lentivirus and primary HIV, the antiviral activity of PD appears to be due to the physical disruption of the virion. The antiviral action of PD is very rapid, as >99.5% of the lentivirus becomes inactivated within 5 min of contact with 300 μM PD at 37°C. However, only ∼40% of the lentiviruses were lysed in the same period, indicating that PD inactivates pseudotyped lentiviruses and HIV by physical disruption that does not necessitate the complete lysis of virions. PD exposure does not appear to significantly rupture HCV or inhibit its attachment to cells, even with 90 min of exposure at 37°C (see Fig. S2 in the supplemental material), despite the inactivation of extracellular virus, suggesting a subtle disruption of virions (e.g., by irreversibly interfering with membrane fluidity or curvature) that causes the inhibition of a postattachment step, such as endocytosis or fusion with the endosomal membrane. Encouragingly, PD exhibits very low cytotoxicity in several human cell lines (CC50, >300 μM; see Fig. S7 in the supplemental material). The selectivity index (CC50/IC50) of PD is >300 for HIV and >27 for HCV. PD was originally synthesized by Birck et al. as an inhibitor of bacterial KDO 8-P synthase (1a) and was recently found to also affect angiogenesis (21) and mammalian circadian rhythms (19).
Intriguingly, despite exhibiting strong lysis of virions derived from the HIV capsid, PD does not directly lyse liposomes and shows no attenuation in antiviral activity when preincubated with liposomes, which is suggestive of little to no direct interaction with lipid membranes. The antiviral action of PD thus appears to be different from that reported for the amphipathic virucidal peptide C5A (3, 7), which lyses both virions and liposomal membranes, and the membrane-intercalating virucidal molecule LJ001 (46), whose antiviral effect is attenuated by preincubation with liposomes. It is conceivable that PD disrupts the structural integrity of virions by selectively interacting with a feature of virions that involves an interplay between two or more structural components (e.g., lipid membrane and envelope protein/capsid). We also cannot rule out the possibility that PD interferes with other virion structural components not represented in the liposome model, for example, sites that are glycosylated or phosphorylated (4, 30, 35).
The virion lysis activity of PD is temperature dependent, suggesting that a minimal level of viral membrane fluidity is required to sufficiently compromise virion integrity to the point of viral RNA release. PD is inactivated by human serum and medium conditioned by human cell culture, possibly by interacting with one or more small molecules/peptides secreted by humans but not bovine cells.
A striking feature of PD is its highly specific inactivation of certain viruses (only HCV, HIV, and related retroviruses were found to be inactivated in this study) without strong association directly with or disruption of lipid membranes in general, as evident from our liposome studies. PD exhibited no significant antiviral effect on Dengue virus, an enveloped flavivirus closely related to HCV, or cell culture-produced Sindbis virus, an enveloped alphavirus (see Fig. S3 in the supplemental material). Like HCV, Dengue virus acquires the viral envelope by budding into the endoplasmic reticulum lumen and is able to undergo intensive structural rearrangement in an infected cell (45). On the other hand, Sindbis virus, like HIV, buds from the plasma membrane and contains an envelope rich in cholesterol and sphingolipid molecules (20). We have yet to determine the antiviral effect of PD on other enveloped or nonenveloped viruses. The narrow target spectrum of PD as determined by our studies on a limited range of viruses, combined with the absence of nonspecific lysis of or association with lipid membranes may, at least in part, account for the molecule's very low cytotoxicity.
Our studies bring to light some limitations of PD as an antiviral agent. Although PD effectively inactivates extracellular virus, it appears to be ineffective against intracellular virus, possibly due to a poor ability to enter cells and/or intracellular conversion to an inactive metabolite. Furthermore, human serum and the extended (>16 h) preincubation of PD with cells prior to virus inoculation appears to significantly reduce its antiviral efficacy. Our observation that the antiviral effect of PD is suppressed by a molecule(s) secreted by cells into the surrounding growth medium may be responsible for the latter phenomenon. Since we found that PD retains its full antiviral activity in cervical fluids, the PD-neutralizing molecule(s) present in human serum and conditioned cell culture growth medium is likely physiologically irrelevant in the case of the development of PD as a topical microbicide for the treatment/prevention of HIV infection.
It is estimated that there are approximately 4 million new incidences of HIV infection each year, mostly transmitted through heterosexual intercourse (6). The development of a vaginal (or rectal) microbicide against HIV would represent a major stride toward slowing the global spread of HIV (22). Despite the poor antiviral efficacy of PD against intracellular virus and in medium conditioned by human cell growth, this compound possesses several desirable attributes that make it an attractive candidate anti-HIV microbicide. Most notably, PD (i) exhibits antiviral activity against a broad range of primary HIV-1 isolates, HIV-2, and SIV; (ii) retains full anti-HIV potency for 8 h when in contact with cells; (iii) is effective against both cell-free and cell-associated HIV and inhibits HIV transmission from DC to T cells; (iv) retains full anti-HIV potency in cervical fluids; and (v) irreversibly inactivates HIV predominantly through virion disruption with an activity that appears to be independent of specific virus envelope proteins. Drugs that target viral proteins mediating the replication of viral nucleic acids or virus attachment to target cells often foster the emergence of escape mutants (33). The antiviral action of PD on critical components of the virus other than specific virus envelope proteins makes the development of drug-resistant mutant viruses less likely.
Several candidate anti-HIV microbicides exist (11, 24, 39), but only a handful exhibit an ability to strongly and irreversibly disrupt virions without being detrimental to cells (7, 17). PD 404,182 is an anti-HIV compound with a unique mode of action and represents a useful molecular scaffold for the generation of new anti-HIV-1 microbicides. Finally, the observation that PD 404,182 is able to inactivate both HCV and HIV and the unique antiviral action of this small molecule justify further studies of PD 404,182 and derivatives thereof to determine antiviral effects upon other enveloped and nonenveloped viruses.
Supplementary Material
ACKNOWLEDGMENTS
We thank Charles M. Rice (Rockefeller University) for providing Huh-7.5 and BHK-J cells, 9E10 antibody, and plasmids for generating HCV and SINV in cell culture; Matthew J. Evans (Mount Sinai Medical Center) and Irvin S. Chen (UCLA School of Medicine) for providing plasmid encoding the MLV and SINV envelope protein, respectively; and Da Huang for assisting with liposome preparation. Plasmid for expressing HIV envelope protein (2) was obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH; HIV-1 clone Bal.01 (catalog no. 11445) was from J. Mascola.
Financial support for this work came from new faculty start-up funds from the Artie McFerrin Department of Chemical Engineering, Texas A&M University, National Institutes of Health grant 1R21AI083965-01 (to A.M.C., K.C., R.S., and Z.C.), and U.S. Public Health Service grants AI071952 and AI076005 (to P.A.G. and M.B.).
Footnotes
Published ahead of print 14 November 2011
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1. Aiken C, Troro D. 1995. Nef stimulates human immunodeficiency virus type 1 proviral RNA synthesis. J. Virol. 69:5048–5056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1a. Birck MR, Holler TP, Woodard RW. 2000. Identification of a slow tight-binding inhibitor of 3-deoxy-D-manno-octulosonic acid 8-phosphate synthase. J. Am. Chem. Soc. 122:9334–9335 [Google Scholar]
- 2. Bobardt MD, Chatterji U, Schaffer L, de Witte L, Gallay PA. 2010. Syndecan-Fc hybrid molecule as a potent in vitro microbicidal anti-HIV-1 agent. Antimicrob. Agents Chemother. 54:2753–2766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bobardt MD, et al. 2008. Hepatitis C virus NS5A anchor peptide disrupts human immunodeficiency virus. Proc. Natl. Acad. Sci. U. S. A. 105:5525–5530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bremer CM, Bung C, Kott N, Hardt M, Glebe D. 2009. Hepatitis B virus infection is dependent on cholesterol in the viral envelope. Cell Microbiol. 11:249–260 [DOI] [PubMed] [Google Scholar]
- 5. Cannon PM, Kim N, Kingsman SM, Kingsman AJ. 1996. Murine leukemia virus-based Tat-inducible long terminal repeat replacement vectors: a new system for anti-human immunodeficiency virus gene therapy. J. Virol. 70:8234–8240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. CDC 2006. The global HIV/AIDS pandemic, 2006. MMWR Morb. Mortal. Wkly. Rep. 55:841–844 [PubMed] [Google Scholar]
- 7. Cheng G, et al. 2008. A virocidal amphipathic alpha-helical peptide that inhibits hepatitis C virus infection in vitro. Proc. Natl. Acad. Sci. U. S. A. 105:3088–3093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chockalingam K, Simeon RL, Rice CM, Chen Z. 2010. A cell protection screen reveals potent inhibitors of multiple stages of the hepatitis C virus life cycle. Proc. Natl. Acad. Sci. U. S. A. 107:3764–3769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cowan S, et al. 2002. Cellular inhibitors with Fv1-like activity restrict human and simian immunodeficiency virus tropism. Proc. Natl. Acad. Sci. U. S. A. 99:11914–11919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Deming P, McNicholl IR. 2011. Coinfection with human immunodeficiency virus and hepatitis C virus: challenges and therapeutic advances insights from the society of infectious diseases pharmacists. Pharmacotherapy 31:357–368 [DOI] [PubMed] [Google Scholar]
- 11. Dhawan D, Mayer KH. 2006. Microbicides to prevent HIV transmission: overcoming obstacles to chemical barrier protection. J. Infect. Dis. 193:36–44 [DOI] [PubMed] [Google Scholar]
- 12. Enserink M. 2011. Infectious diseases. First specific drugs raise hopes for hepatitis C. Science 332:159–160 [DOI] [PubMed] [Google Scholar]
- 13. Evans MJ, et al. 2007. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature 446:801–805 [DOI] [PubMed] [Google Scholar]
- 14. Ganesh L, et al. 2004. Infection of specific dendritic cells by CCR5-tropic human immunodeficiency virus type 1 promotes cell-mediated transmission of virus resistant to broadly neutralizing antibodies. J. Virol. 78:11980–11987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Geijtenbeek TB, et al. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100:587–597 [DOI] [PubMed] [Google Scholar]
- 16. Günther-Ausborn S, Praetor A, Stegmann T. 1995. Inhibition of influenza-induced membrane fusion by lysophosphatidylcholine. J. Biol. Chem. 270:29279–29285 [DOI] [PubMed] [Google Scholar]
- 17. Guo H, et al. 2011. Alkylated porphyrins have broad antiviral activity against hepadnaviuses, flaviviruses, filoviruses and arenaviruses. Antimicrob. Agents Chemother. 55:478–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Henchal EA, Putnak JR. 1990. The dengue viruses. Clin. Microbiol. Rev. 3:376–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Isojima Y, et al. 2009. CKIepsilon/delta-dependent phosphorylation is a temperature-insensitive, period-determining process in the mammalian circadian clock. Proc. Natl. Acad. Sci. U. S. A. 106:15744–15749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jose J, Snyder JE, Kuhn RJ. 2009. A structural and functional perspective of alphavirus replication and assembly. Future Microbiol. 4:837–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kalén M, et al. 2009. Combination of reverse and chemical genetic screens reveals angiogenesis inhibitors and targets. Chem. Biol. 16:432–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Klasse PJ, Shattock R, Moore JP. 2008. Antiretroviral drug-based microbicides to prevent HIV-1 sexual transmission. Annu. Rev. Med. 59:455–471 [DOI] [PubMed] [Google Scholar]
- 23. Kolykhalov AA, Mihalik K, Feinstone SM, Rice CM. 2000. Hepatitis C virus-encoded enzymatic activities and conserved RNA elements in the 3′ nontranslated region are essential for virus replication in vivo. J. Virol. 74:2046–2051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lederman MM, Offord RE, Hartley O. 2006. Microbicides and other topical strategies to prevent vaginal transmission of HIV. Nat. Rev. Immunol. 6:371–382 [DOI] [PubMed] [Google Scholar]
- 25. Li Y, et al. 2006. Characterization of antibody responses elicited by human immunodeficiency virus type 1 primary isolate trimeric and monomeric envelope glycoproteins in selected adjuvants. J. Virol. 80:1414–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lindenbach BD, et al. 2005. Complete replication of hepatitis C virus in cell culture. Science 309:623–626 [DOI] [PubMed] [Google Scholar]
- 27. Martin I, Ruysschaert JM. 1995. Lysophosphatidylcholine inhibits vesicles fusion induced by the NH2-terminal extremity of SIV/HIV fusogenic proteins. Biochim. Biophys. Acta 1240:95–100 [DOI] [PubMed] [Google Scholar]
- 28. Marukian S, et al. 2008. Cell culture-produced hepatitis C virus does not infect peripheral blood mononuclear cells. Hepatology 48:1843–1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Morizono K, Bristol G, Xie YM, Kung SK, Chen IS. 2001. Antibody-directed targeting of retroviral vectors via cell surface antigens. J. Virol. 75:8016–8020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moss B. 2006. Poxvirus entry and membrane fusion. Virology 344:48–54 [DOI] [PubMed] [Google Scholar]
- 31. Operskalski EA, Kovacs A. 2011. HIV/HCV co-infection: pathogenesis, clinical complications, treatment, and new therapeutic technologies. Curr. HIV/AIDS Rep. 8:12–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pietschmann T, et al. 2006. Construction and characterization of infectious intragenotypic and intergenotypic hepatitis C virus chimeras. Proc. Natl. Acad. Sci. U. S. A. 103:7408–7413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pillay D. 2007. The priorities for antiviral drug resistance surveillance and research. J. Antimicrob. Chemother. 60(Suppl. 1):i57–i58 [DOI] [PubMed] [Google Scholar]
- 34. Platt EJ, Wehrly K, Kuhmann SE, Chesebro B, Kabat D. 1998. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J. Virol. 72:2855–2864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rando RF, et al. 2006. Critical design features of phenyl carboxylate-containing polymer microbicides. Antimicrob. Agents Chemother. 50:3081–3089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Reed LJ, Muench H. 1938. A simple method of estimating fifty percent end-points. Am. J. Hyg. (London) 27:493–497 [Google Scholar]
- 37. Reesink HW, et al. 2006. Rapid decline of viral RNA in hepatitis C patients treated with VX-950: a phase Ib, placebo-controlled, randomized study. Gastroenterology 131:997–1002 [DOI] [PubMed] [Google Scholar]
- 38. Rice CM, Levis R, Strauss JH, Huang HV. 1987. Production of infectious RNA transcripts from Sindbis virus cDNA clones: mapping of lethal mutations, rescue of a temperature-sensitive marker, and in vitro mutagenesis to generate defined mutants. J. Virol. 61:3809–3819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shattock RJ, Moore JP. 2003. Inhibiting sexual transmission of HIV-1 infection. Nat. Rev. Microbiol. 1:25–34 [DOI] [PubMed] [Google Scholar]
- 40. Shepard CW, Finelli L, Alter MJ. 2005. Global epidemiology of hepatitis C virus infection. Lancet Infect. Dis. 5:558–567 [DOI] [PubMed] [Google Scholar]
- 41. Soares MM, King SW, Thorpe PE. 2008. Targeting inside-out phosphatidylserine as a therapeutic strategy for viral diseases. Nat. Med. 14:1357–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. St. Vincent MR, et al. 2010. Rigid amphipathic fusion inhibitors, small molecule antiviral compounds against enveloped viruses. Proc. Natl. Acad. Sci. U. S. A. 107:17339–17344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Susser S, et al. 2009. Characterization of resistance to the protease inhibitor boceprevir in hepatitis C virus-infected patients. Hepatology 50:1709–1718 [DOI] [PubMed] [Google Scholar]
- 44. Takeuchi T, et al. 1999. Real-time detection system for quantification of hepatitis C virus genome. Gastroenterology 116:636–642 [DOI] [PubMed] [Google Scholar]
- 45. Welsch S, et al. 2009. Composition and three-dimensional architecture of the dengue virus replication and assembly sites. Cell Host Microbe 5:365–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wolf MC, et al. 2010. A broad-spectrum antiviral targeting entry of enveloped viruses. Proc. Natl. Acad. Sci. U. S. A. 107:3157–3162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zennou V, Bieniasz PD. 2006. Comparative analysis of the antiretroviral activity of APOBEC3G and APOBEC3F from primates. Virology 349:31–40 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.