Abstract
Staphylococcus epidermidis is a frequent cause of device-associated infections. In this study, we compared the efficacy of daptomycin versus vancomycin against biofilm-producing methicillin-resistant S. epidermidis (MRSE) strains in a murine model of foreign-body and systemic infection. Two bacteremic biofilm-producing MRSE strains were used (SE284 and SE385). The MIC of daptomycin was 1 mg/liter for both strains, and the MICs of vancomycin were 4 and 2 mg/liter for SE284 and for SE385, respectively. The in vitro bactericidal activities of daptomycin and vancomycin were evaluated by using time-kill curves. The model of foreign-body and systemic infection of neutropenic female C57BL/6 mice was used to ascertain in vivo efficacy. Animals were randomly allocated into three groups (n = 15): without treatment (controls) or treated with daptomycin at 50 mg/kg/day or vancomycin at 440 mg/kg/day. In vitro, daptomycin showed concentration-dependent bactericidal activity, while vancomycin presented time-dependent activity. In the experimental in vivo model, daptomycin and vancomycin decreased liver and catheter bacterial concentrations (P < 0.05) and increased the survival and the number of sterile blood cultures (P < 0.05) using both strains. Daptomycin produced a reduction in the bacterial liver concentration higher than 2.5 log10 CFU/g compared to vancomycin using both strains, with this difference being significant (P < 0.05) for infection with SE385. For the catheter bacterial concentrations, daptomycin reduced the concentration of SE284 3.0 log10 CFU/ml more than did vancomycin (P < 0.05). Daptomycin is more effective than vancomycin for the treatment of experimental foreign-body and systemic infections by biofilm-producing methicillin-resistant S. epidermidis.
INTRODUCTION
Staphylococcus epidermidis is a common nosocomial and health care-associated pathogen in several infections, causing important morbidity, mortality, and/or health care costs. Thus, it is the most important cause of infections of orthopedic prostheses, accounting for approximately 40% of all cases, and between 30% and 50% of catheter-related bacteremias are caused by coagulase-negative Staphylococcus (CNS) strains (19, 26). Other severe and/or frequent nosocomial and health care-associated infections are also caused by CNS, being the etiology in the 22.7% of endocarditis infections (9, 13) and in 37 to 78% of cerebrospinal fluid shunt-associated infections (4, 11).
Moreover, the high frequency of methicillin-resistant S. epidermidis (MRSE) is an important therapeutic problem. Thus, data from the National Nosocomial Infections Surveillance System (NNIS) in the United States from 1992 to 2004 revealed that 89.1% of the coagulase-negative staphylococci isolated in intensive care units (ICUs) presented resistance to methicillin (16). Vancomycin is the recommended treatment for infections caused by MRSE (14), but the emergence of strains with reduced susceptibility to vancomycin (1) makes the evaluation of other therapeutic alternatives necessary. Among them, daptomycin, a cyclic lipopeptide with bactericidal activity against Gram-positive bacteria (http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021572s022s023s024s027s030s032lbl.pdf), represents a clinical therapeutic option (14).
On the other hand, the ability of S. epidermidis to produce biofilms provides significant resistance to antibiotics and impairs the host innate immune response (23). In these situations, the use of an antimicrobial able to cross the biofilm enhances the efficacy of the treatment. In this context, the ability of daptomycin to penetrate rapidly into the biofilm produced by S. epidermidis is well known (21), in contrast to the reduced ability of vancomycin to do so (20). Moreover, the higher level of activity of daptomycin than that of vancomycin was demonstrated previously with an in vitro dynamic system using a polyurethane intravascular catheter as a substrate for biofilm growth (7).
Although the efficacy of daptomycin against MRSE has been analyzed in several experimental studies, such as a model of experimental endocarditis in rabbits (8) and a model of tissue cage infection in guinea pigs (17), the role of daptomycin against biofilm-producing MRSE in catheter-related infections remains unknown.
Thus, the aim of the present study was to compare the efficacy of daptomycin to that of vancomycin in an experimental model of foreign-body infection in neutropenic mice, causing bacterial systemic infection, by biofilm-producing MRSE strains with reduced susceptibility to vancomycin.
(This work was presented at the 21st European Congress of Clinical Microbiology and Infectious Diseases/27th International Congress of Chemotherapy, Milan, Italy, 7 to 10 May 2011 [5a].)
MATERIALS AND METHODS
Bacterial strains.
Two MRSE strains (SE284 and SE385) were selected from 20 consecutive bacteremic isolates based on susceptibility to daptomycin and vancomycin and their ability to produce biofilms (3, 2, 6).
Antibiotics.
For the in vitro studies, standard laboratory daptomycin powder was supplied by Novartis Pharma AG (Basel, Switzerland), and vancomycin was supplied by Sigma-Aldrich (Madrid, Spain). For the in vivo studies, commercial vials of daptomycin (Novartis Pharma AG, Basel, Switzerland) and vancomycin (Laboratorios Normon S.A., Tres Cantos, Spain) were used.
Animals.
Female C57BL/6 mice weighing 18 to 20 g were obtained from the University of Seville, having a sanitary status of murine pathogen free. Animals were housed in regulation cages and given access to food and water ad libitum. Mice were rendered neutropenic by the injection of cyclophosphamide (Baxter Oncology GmbH, Halle, Germany) intraperitoneally (i.p.) 4 days (150 mg/kg of body weight) and 1 day (100 mg/kg) before the experiments (5) and were housed and manipulated in sterile conditions. The study was approved by the Ethics and Clinical Research Committee of the University Hospitals Virgen del Rocío, Seville, Spain.
In vitro studies. (i) Susceptibility testing.
The MIC was determined in duplicate for each bacterial strain using a standard broth microdilution method according to CLSI reference methods (3). Staphylococcus aureus ATCC 29213 was used as a control strain.
(ii) Time-kill studies.
The bactericidal activities of daptomycin and vancomycin were evaluated by using concentrations of 1× MIC and the maximal serum concentration in mice (Cmax). The initial inocula were 106 CFU of each strain/ml. Bacterial growths were quantified at 0, 2, 4, 8, and 24 h after incubation at 37°C by plating 10-fold dilutions onto plates of Columbia agar with 5% sheep blood. The limit of detection was 10 CFU/ml, corresponding to 1 log10 CFU/ml. An antimicrobial was considered bactericidal when a 3-log10 decrease in the CFU/ml was reached compared with the CFU/ml of the initial inoculum (15). In vitro daptomycin studies were performed with Mueller-Hinton broth (MHB), cation adjusted to a final Ca2+ concentration of 50 μg/ml according to standard methodologies (3).
(iii) Biofilm characterization.
The ability of the MRSE strains to produce a biofilm in vitro was determined by using phenotypic methods. Phenotypic characterization was performed by use of tryptic soy agar plates with sucrose (50 g/liter) and Congo red (0.8 g/liter) (6). Biofilm-producing strains grew on this medium as black colonies, whereas biofilm-negative strains grew as red colonies. Also, quantitative biofilm measurements were performed by a microtiter plate assay, modified from a method described previously by Christensen et al. (2). Briefly, bacteria were grown overnight in tryptic soy broth (TSB) and then diluted 1:100 in TSB with sucrose (50 g/liter), and finally, 96-well culture plates were filled. After 24 h of incubation at 37°C without shaking, the medium and nonadhered bacteria were discarded. Plates were then washed three times with phosphate-buffered saline (PBS), and the remaining bacteria were fixed by air drying. After staining with a 0.4% solution of crystal violet, the optical density at 580 nm (OD580) of the adherent biofilm was determined with a spectrometer plate reader after biofilm solubilization with 95% ethanol. OD580 values of >0.120 were considered biofilm positive (2). S. epidermidis ATCC 35983 and S. epidermidis ATCC 35984 were used as controls for biofilm-positive strains, and S. epidermidis ATCC 27626 was used as a negative control.
In vivo studies. (i) Pharmacokinetic/pharmacodynamic studies.
Serum antibiotic concentrations in neutropenic noninfected mice were determined after a single intraperitoneal administration of either 50 mg/kg daptomycin or 110 mg/kg vancomycin. For groups of three mice for every time point, blood was extracted from the periorbital plexus in anesthetized mice after 5, 10, 15, 30, 60, 90, 120, 240, 360, 600, 720, 840, 1,080, and 1,440 min. Concentrations of daptomycin were determined by using a high-performance liquid chromatography (HPLC) method (22). Concentrations of vancomycin were determined by using an agar diffusion bioassay with Bacillus subtilis ATCC 6633 as a control strain (12). The Cmax (mg/liter), the area under the concentration-time curve (AUC) (mg · h/liter), and the terminal half-life (t1/2) (h) were calculated by a computer-assisted method (PK Functions for Microsoft Excel; J. L. Usansky, A. Desai, and D. Tang-Liu, Department of Pharmacokinetics and Drug Metabolism, Allergan, Irvine, CA [http://www.boomer.org/pkin/soft.html]).
A daptomycin dose of 50 mg/kg was chosen to obtain an AUC from 0 to 24 h (AUC0–24) similar to that in humans after a dose of 6 mg/kg (25). In the case of vancomycin, a dose of 110 mg/kg was chosen to target an AUC0–24 similar to that in humans after doses of 1 g every 12 hours (q12h) (454 mg · h/liter) (10).
(ii) Animal model.
A modified murine model of foreign-body infection described previously (18) was used to evaluate the efficacies of daptomycin and vancomycin. Briefly, mice were rendered neutropenic, and 1 cm of a sterile polyfluorinated ethylene-propylene catheter was then aseptically implanted into the abdominal cavity. Experimental infection was produced by the intraperitoneal injection of 0.5 ml of a bacterial suspension (8.04 ± 0.45 log10 CFU/ml) into the lateral abdominal wall opposite the operation wound, 15 min after the insertion of the catheter implant. The treatments started 4 h after the infection. At this time point, the bacterial burdens were 7.84 ± 0.22 and 8 ± 0.29 log10 CFU/g in liver and 5.01 ± 0.26 and 4.10 ± 0.21 log10 CFU/ml in catheter for SE284 and SE385, respectively. Also, 100% of blood cultures were positive for both strains. Animals were randomly included in three different therapeutic groups of 15 mice each, control (without treatment), daptomycin at 50 mg/kg/day, or vancomycin at 440 mg/kg/day, for both strains. Animals were treated and monitored during 72 h.
Before the experiment, in order to discard the toxicity of the treatments, two groups of 5 uninfected neutropenic mice received daptomycin or vancomycin during 72 h.
Immediately after death or sacrifice at the end of the 72-h period, samples were obtained and processed as follows. Aseptic thoracotomy was performed, and blood samples were obtained for qualitative blood cultures through cardiac puncture. The peritoneal cavity was aseptically opened; catheter and liver were then aseptically removed. The catheter was slowly flushed with 1 ml of sterile saline solution to remove blood and nonadhered bacteria. Later, the catheter was transferred into 1 ml of sterile saline solution in a tube, which was sonicated for 10 min at 40 to 60 kHz (Ultrasons 513 water bath sonicator; P-Selecta, Barcelona, Spain). The liver was homogenized (Stomacher 80; Tekmar Co., Cincinnati, OH) in 2 ml of sterile saline solution. Ten-fold dilutions of homogenized liver and the resulting catheter-sonicated solution were performed, and the mixtures were plated onto Columbia agar with 5% sheep blood for quantitative cultures.
Statistical analysis.
Means of bacterial concentrations in liver (log10 CFU/g of tissue) and catheter (log10 CFU/ml) were compared by analysis of variance (ANOVA) and, when required, by Dunnett and Tukey post hoc tests. The frequencies of sterile blood cultures and survival were analyzed by a chi-square test. A P value of <0.05 was considered significant. The statistical package SPSS, version 15.0, was used (SPSS Inc., Chicago, IL).
RESULTS
In vitro studies. (i) MIC.
Both strains were susceptible to daptomycin and vancomycin. The MICs of daptomycin were 1 mg/liter for both strains, and those of vancomycin were 4 and 2 mg/liter for SE284 and for SE385, respectively.
(ii) Time-kill studies.
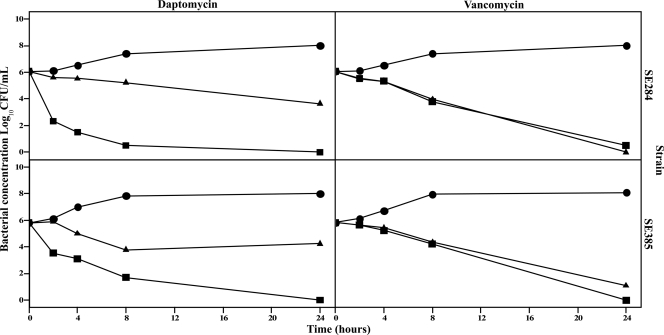
The results of the bactericidal assays are shown in Fig. 1. Daptomycin was bactericidal at Cmax at 4, 8, and 24 h for SE284 and at 8 and 24 h for SE385. Vancomycin was bactericidal at 24 h for both strains with the two concentrations used.
Fig 1.
Time-kill curves of daptomycin and vancomycin at 1× MIC and the maximal serum concentration in mice (Cmax). Circles, controls; triangles, 1× MIC; squares, Cmax.
(iii) Biofilm characterization.
Both strains were biofilm positive after the Congo red agar plate assay and showed an OD580 of >120 with the crystal violet assay.
In vivo studies. (i) Pharmacokinetic/pharmacodynamic studies.
Pharmacokinetic and pharmacodynamic parameters are shown in Table 1. In the case of daptomycin, the dose of 50 mg/kg showed an AUC0–∞ of 595.39 mg · h/liter, so a single dose was used to mimic the human dose of 6 mg/kg (AUC0–∞ of 598 mg · h/liter). The vancomycin dose of 110 mg/kg showed an AUC0–∞ of 111.36 mg · h/liter; thus four daily doses were used to achieve a similar value of AUC0–24 h after two doses of 1 g/12 h in humans (454 mg · h/liter).
Table 1.
Pharmacokinetics and pharmacodynamics of daptomycin and vancomycin
| Antimicrobial | No. of xxxd | Dose (mg/kg) | Cmax (mg/liter) | AUC0–∞ (mg · h/liter) | t1/2 (h) | AUC0-24/MIC ratio |
|---|---|---|---|---|---|---|
| Daptomycin | 42 | 50 | 162.21 | 595.39 | 1.91 | 595.39a |
| Vancomycin | 42 | 110 | 97.79 | 111.36 | 0.57 | 111.36b |
| 222.72c |
Parameter calculated for both strains using MICs of daptomycin of 1 mg/liter and a daptomycin dose of 50 mg/kg/day.
Parameter calculated for strain SE284 using MICs of vancomycin of 4 mg/liter and a vancomycin dose of 440 mg/kg/day.
Parameter calculated for strain SE385 using MICs of vancomycin of 2 mg/liter and a vancomycin dose of 440 mg/kg/day.
Three mice every time point.
(ii) Animal model.
The efficacies of the different treatments are shown in Table 2. For both strains, daptomycin reduced the liver bacterial concentration more than 7 log10 CFU/g and reduced the catheter bacterial concentration approximately 5.5 log10 CFU/ml with respect to their controls (P < 0.05). In the case of vancomycin, the treatment reduced the liver bacterial concentration approximately 5 log10 CFU/g and reduced the catheter bacterial concentration 2.87 and 4.78 log10 CFU/ml with respect to their control groups (P < 0.05) for strains SE284 and SE385, respectively. Numbers of both sterile blood cultures and survivors were increased in infections with any strain treated with daptomycin or vancomycin compared to those with their control groups (P < 0.05).
Table 2.
Efficacies of daptomycin and vancomycin in a murine model of foreign-body infection caused by strains SE284 and SE385
| Strain | Treatment | No. of xxx | Bacterial concn (mean ± SD) |
Sterile blood cultures (%) | Survival (%) | |
|---|---|---|---|---|---|---|
| Liver (log10 CFU/g) | Catheter (log10 CFU/ml) | |||||
| SE284 | Control | 15 | 9.39 ± 0.52 | 6.56 ± 0.65 | 0 | 0 |
| Daptomycin | 14 | 1.69 ± 2.28a | 0.69 ± 1.22a,b | 86.7a | 66.7a | |
| Vancomycin | 14 | 4.20 ± 3.12a | 3.69 ± 2.70a | 57.1a | 42.9a | |
| SE385 | Control | 14 | 9.93 ± 0.88 | 5.98 ± 0.90 | 0 | 0 |
| Daptomycin | 15 | 1.66 ± 2.81a,b | 0.42 ± 1.26a | 100a | 93.3a | |
| Vancomycin | 14 | 4.56 ± 2.61a | 1.20 ± 1.92a | 76.9a | 76.9a | |
P < 0.05 compared with the internal control group.
P < 0.05 compared with the vancomycin group.
When the efficacies of both antimicrobials were compared, there was a trend toward better results with daptomycin than with vancomycin in terms of the reduction of the bacterial concentration in liver or catheter, sterile blood cultures, and survival for both strains. These differences were significant for the reduction of the bacterial catheter concentration of strain SE284 (P < 0.05) and for the decrease of the bacterial liver concentration of strain SE385 (P < 0.05).
No mortality was observed for the uninfected neutropenic mice included in the toxicity studies receiving daptomycin or vancomycin during 72 h.
DISCUSSION
The present study evaluates the in vivo therapeutic efficacies of daptomycin and vancomycin against two biofilm-producing MRSE strains by using a murine model of foreign-body infection described previously (18). Using this model, both antimicrobials reduced the bacterial concentrations in catheter and liver and increased the numbers of sterile blood cultures and survival rates with any strain. On the other hand, in the comparison of both treatments, there was a trend toward better results with daptomycin than with vancomycin for any parameter, which was significant for the bacterial concentrations in the catheter and the liver in the experiments with strains SE284 and SE385, respectively.
These in vivo results are in accordance with the early in vitro bactericidal activity of daptomycin at Cmax, beginning at 4 and 8 h with strains SE284 and SE385, respectively, while vancomycin did not show bactericidal activity until 24 h with any strain. Moreover, these results may be related to the ability of daptomycin to penetrate S. epidermidis biofilms (21), compared with the reduced ability of vancomycin to penetrate biofilms (20).
Foreign-body infections caused by MRSE strains are difficult to treat, mainly because of the presence of biofilm and bacterial tolerance to antibiotics (22). To date, vancomycin is the treatment of reference against MRSE infections, but several studies have pointed out its potential toxicity, limited bactericidal killing, and low capacity to cross biofilms as well as the inability to reach optimal pharmacodynamic parameters due to the increasing MIC of vancomycin against MRSE strains (1, 14, 19). On the basis of previous experimental studies, daptomycin currently plays a key role in the treatment of skin and soft-tissue infections caused by S. aureus (18a). In this context, daptomycin would appear to be a safer and more effective alternative anti-MRSE treatment. However, its efficacy against these difficult-to-treat infections is scarce and limited to experimental models (8).
Similar results were found by Garcia-de-la-María et al. in an experimental endocarditis model in rabbits using an MRSE strain (8). Those researchers evaluated the efficacies of 6 and 10 mg/kg of daptomycin and 30 and 60 mg/kg of vancomycin, simulating the human doses, and they observed a higher percentage of sterile vegetations with daptomycin than with vancomycin (8). Other authors evaluated the efficacy of daptomycin and vancomycin in an experimental rat model of central venous catheter biofilms with antibiotic-lock therapy combined with systemic therapy against MRSE (24) and concluded that daptomycin has an activity similar to that of vancomycin for the eradication of infection in central venous catheters and in catheter-related metastatic infection or bacteremia (24).
Using a model of tissue cage infection in guinea pigs, Olson et al. showed a failure of treatment with daptomycin and vancomycin against two isogenic S. epidermidis strains, a biofilm-producing strain and a non-biofilm-producing strain (17). However, those authors did not determine the susceptibility of the strains and used lower doses of daptomycin and vancomycin (5 and 15 mg/kg, respectively), without previous pharmacokinetic and pharmacodynamic studies (17). On the contrary, the addition of rifampin to daptomycin or vancomycin sterilized 5/6 tissues cages colonized with biofilm-producing S. epidermidis.
In summary, the present study shows that daptomycin is more effective than vancomycin for the treatment of experimental foreign-body infection causing bacterial systemic infection by biofilm-producing MRSE strains with reduced susceptibility to vancomycin. However, only randomized clinical trials may elucidate whether daptomycin is better than vancomycin for the treatment of foreign-body infections caused by methicillin-resistant S. epidermidis.
ACKNOWLEDGMENTS
This study was supported by a research grant from Novartis Farmacéutica S.A. (Barcelona, Spain) and by the Ministerio de Ciencia e Innovación, Instituto de Salud Carlos III, cofinanced by the European Development Regional Fund “A Way to Achieve Europe” ERDF, Spanish Network for the Research in Infectious Diseases (REIPI RD06/0008/0000).
Footnotes
Published ahead of print 5 December 2011
REFERENCES
- 1. Ahlstrand E, Svensson K, Persson L, Tidefelt U, Söderquist B. 2011. Glycopeptide resistance in coagulase-negative staphylococci isolated in blood cultures from patients with hematological malignancies during three decades. Eur. J. Clin. Microbiol. Infect. Dis. 30:1349–1354 [DOI] [PubMed] [Google Scholar]
- 2. Christensen GD, et al. 1985. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J. Clin. Microbiol. 22:996–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Clinical and Laboratory Standards Institute 2010Performance standards for antimicrobial susceptibility testing; 20th informational supplement.CLSI document M100-S20. ; CLSI, Wayne, PA [Google Scholar]
- 4. Conen A, et al. 2008. Characteristics and treatment outcome of cerebrospinal fluid shunt-associated infections in adults: a retrospective analysis over an 11-year period. Clin. Infect. Dis. 47:73–82 [DOI] [PubMed] [Google Scholar]
- 5. Craig WA, Andes DR. 2008. In vivo pharmacodynamics of ceftobiprole against multiple bacterial pathogens in murine thigh and lung infection models. Antimicrob. Agents Chemother. 52:3492–3496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5a. Domínguez-Herrera J, Docobo-Perez F, López-Rojas R, Pichardo C, Pachón J. 2011. Abstr. 21st Eur. Congr. Clin. Microbiol. Infect. Dis./27th Int. Congr. Chemother., Milan, Italy, 7 to 10 May 2011, abstr P1069. [Google Scholar]
- 6. Freeman DJ, Falkiner FR, Keane CT. 1989. New method for detecting slime production by coagulase negative staphylococci. J. Clin. Pathol. 42:872–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garcia I, del Carmen Conejo M, Ojeda A, Rodriguez-Bano J, Pascual A. 2010. A dynamic in vitro model for evaluating antimicrobial activity against bacterial biofilms using a new device and clinical-used catheters. J. Microbiol. Methods 83:307–311 [DOI] [PubMed] [Google Scholar]
- 8. Garcia-de-la-Maria C, et al. 2010. Daptomycin is effective for treatment of experimental endocarditis due to methicillin-resistant and glycopeptide-intermediate Staphylococcus epidermidis. Antimicrob. Agents Chemother. 54:2781–2786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garnacho-Montero J, et al. 2008. Risk factors and prognosis of catheter-related bloodstream infection in critically ill patients: a multicenter study. Intensive Care Med. 34:2185–2193 [DOI] [PubMed] [Google Scholar]
- 10. Healy DP, Polk RE, Garson ML, Rock DT, Comstock TJ. 1987. Comparison of steady-state pharmacokinetics of two dosage regimens of vancomycin in normal volunteers. Antimicrob. Agents Chemother. 31:393–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jimenez-Mejias ME, Garcia-Cabrera E. 2008. Infection of cerebrospinal fluid shunt systems. Enferm. Infecc. Microbiol. Clin. 26:240–251. (In Spanish.) [DOI] [PubMed] [Google Scholar]
- 12. Klassen M, Edberg S. 1996. Measurement of antibiotic in human body fluids: techniques and significance, p 230–295. In Lorian V. (ed), Antibiotics in laboratory medicine, 4th ed. Williams & Wilkins, Baltimore, MD [Google Scholar]
- 13. Lomas JM, et al. 2010. Healthcare-associated infective endocarditis: an undesirable effect of healthcare universalization. Clin. Microbiol. Infect. 16:1683–1690 [DOI] [PubMed] [Google Scholar]
- 14. Mermel LA, et al. 2009. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 49:1–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. National Committee for Clinical Laboratory Standards 1999. Methods for determining bactericidal activity of antimicrobial agents.NCCLS document M26-A. ; National Committee for Clinical Laboratory Standards, Wayne, PA [Google Scholar]
- 16. National Nosocomial Infections Surveillance System 2004. National Nosocomial Infections Surveillance (NNIS) System report, data summary from January 1992 through June 2004, issued October 2004. Am. J. Infect. Control 32:470–485 [DOI] [PubMed] [Google Scholar]
- 17. Olson ME, Slater SR, Rupp ME, Fey PD. 2010. Rifampicin enhances activity of daptomycin and vancomycin against both a polysaccharide intercellular adhesin (PIA)-dependent and -independent Staphylococcus epidermidis biofilm. J. Antimicrob. Chemother. 65:2164–2171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pichardo C, et al. 2011. Local imipenem activity against Pseudomonas aeruginosa decreases in vivo in the presence of siliconized latex. Eur. J. Clin. Microbiol. Infect. Dis. 30:289–291 [DOI] [PubMed] [Google Scholar]
- 18a. Quist SR, Fierlbeck G, Seaton RA, Loeffler J, Chaves RL. 2012. Comparative randomised clinical trial against glycopeptides supports the use of daptomycin as first-line treatment of complicated skin and soft-tissue infections. Int. J. Antimicrob. Agents. 39:90–91 [DOI] [PubMed] [Google Scholar]
- 19. Rogers KL, Fey PD, Rupp ME. 2009. Coagulase-negative staphylococcal infections. Infect. Dis. Clin. North Am. 23:73–98 [DOI] [PubMed] [Google Scholar]
- 20. Singh R, Ray P, Das A, Sharma M. 2010. Penetration of antibiotics through Staphylococcus aureus and Staphylococcus epidermidis biofilms. J. Antimicrob. Chemother. 65:1955–1958 [DOI] [PubMed] [Google Scholar]
- 21. Stewart PS, Davison WM, Steenbergen JN. 2009. Daptomycin rapidly penetrates a Staphylococcus epidermidis biofilm. Antimicrob. Agents Chemother. 53:3505–3507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tobin CM, Darville JM, Lovering AM, Macgowan AP. 2008. An HPLC assay for daptomycin in serum. J. Antimicrob. Chemother. 62:1462–1463 [DOI] [PubMed] [Google Scholar]
- 23. Uckay I, et al. 2009. Foreign body infections due to Staphylococcus epidermidis. Ann. Med. 41:109–119 [DOI] [PubMed] [Google Scholar]
- 24. Van Praagh AD, et al. 2011. Daptomycin antibiotic lock therapy in a rat model of staphylococcal central venous catheter biofilm infections. Antimicrob. Agents Chemother. 55:4081–4089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Woodworth Jr, Nyhart EH, Jr, Brier GL, Wolny JD, Black HR. 1992. Single-dose pharmacokinetics and antibacterial activity of daptomycin, a new lipopeptide antibiotic, in healthy volunteers. Antimicrob. Agents Chemother. 36:318–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zimmerli W, Trampuz A, Ochsner PE. 2004. Prosthetic-joint infections. N. Engl. J. Med. 351:1645–1654 [DOI] [PubMed] [Google Scholar]