Abstract
Two classes of phages yield profoundly different levels of recovery in mice experimentally infected with an Escherichia coli O18:K1:H7 strain. Phages requiring the K1 capsule for infection (K1-dep) rescue virtually all infected mice, whereas phages not requiring the capsule (K1-ind) rescue modest numbers (∼30%). To rescue infected mice, K1-ind phages require at least a 106-fold-higher inoculum than K1-dep phages. Yet their in vivo growth dynamics are only modestly inferior to those of K1-dep phages, and competition between the two phage types in the same mouse reveals only a slight growth advantage for the K1-dep phage. The in vivo growth rate seems unlikely to be the primary determinant of phage therapy success. An alternative explanation is that the success of K1-dep phages is due substantially to their proteomic composition. They encode an enzyme that degrades the K1 capsule, which has been shown in other work to be sufficient to cure infection in the complete absence of phages.
INTRODUCTION
The use of phages to treat bacterial infections, phage therapy, has a long but checkered history. Following initial popularity in the 1930s and 1940s, it was displaced in Western medicine by now-conventional antibiotics (2, 3, 9, 10, 12, 13, 18, 25, 26, 29, 32–36). Yet phage therapy, in our modern era of widespread bacterial resistance to antibiotics, is enjoying renewed interest as a possible alternative treatment. One of the chief scientific hurdles to its widespread acceptance and application is a lack of common principles that underlie phage therapy success: what attributes of a phage render it therapeutically useful? Empirically chosen cocktails of phages, often ill defined, have been used in various therapy procedures. Phase I human safety trials have been conducted using genome-sequenced but otherwise usually largely uncharacterized phages for Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus infections (4, 19, 27, 37). Trials have used between one and eight phages (4, 19, 27, 37). Removing empiricism from phage therapy would go a long way toward making it a standard treatment for disease.
Possibly the most obvious realm for predicting phage therapy success is population biology—the dynamics of phage growth in bacterial populations in the infected host. It seems obvious that phage therapy success should be directly connected to how well the phage grows: a rapidly growing phage should kill far more bacteria than one that grows slowly, all else being equal (7, 14, 15, 22, 23). Use of such a principle could lead to the rapid identification and isolation of therapeutically efficacious phages, reducing substantial in vivo experimentation and the morbidity and perhaps mortality associated with testing a large set of phages to find a few good ones. As one such example, the growth rates of phages tested on bacteria incubated in serum correlated with success of those phages in treating mouse infections (6, 8).
The purpose of the current study was to evaluate the in vivo phage growth rate as a quantitative predictor of treatment success. Our model system was phage treatment of an artificial, otherwise lethal E. coli infection of mice. It has been known since the pioneering work of Smith and Huggins (31) that treatment success in this system correlates strongly with whether the phage requires the bacterial K1 capsule for infection: phages requiring the capsule rescue nearly 100% of mice when administered at high doses, whereas phages not requiring the capsule rescue only ∼30% of the mice (31). It now appears that the critical molecular distinction between the two classes of phages is an endosialidase domain on the tail spike of capsule-requiring phages; capsule-independent phages encode a domain that degrades O antigen (8). What remains unclear is whether the endosialidase yields therapeutic success by enhancing phage growth in vivo or by another mechanism.
The work reported here evaluates correlates of treatment success from a quantitative dynamics perspective. Using the Smith-Huggins model of mice infected with E. coli O18:K1:H7, we considered three questions. What is the minimum treatment dose (MTD) for success of each phage type? Does the in vivo growth rate of each phage reflect its MTD? If a mixture of “good” and “bad” phages is coinoculated, does the good outgrow the bad phage in proportion to the MTDs? Affirmative answers to the last two questions would simplify isolation of the most beneficial phages.
MATERIALS AND METHODS
Strains.
The bacteria CAB1 (E. coli O18:K1:H7) and CAB281 (a transductional chimera of CAB1 and E. coli K-12 that lacks the K1 capsule) were used (6). Phages were designated K1-ind or K1-dep, according to whether they require the K1 capsule for infection; K1-ind phages can infect CAB281, whereas K1-dep phages cannot. Three K1-ind phages were used here: K1-ind1, K1-ind2, and K1-ind3 (8). The four K1-dep phages used were K1H, K1G (8), K1E (11), and K1-5 (28). All K1-ind phages plus K1H and K1G are members of the Siphoviridae (GenBank accession no. GU196277 to GU196281); K1E and K1-5 are members of the Podviridae. Genome sizes of all phages lie between 41.5 and 45.5 kbp. All seven phages are lytic.
In prior work, in vitro growth rates of these K1-dep phages measured on CAB1 in serum ranged from ∼15 to 20 doublings/h; growth rates of the K1-ind phages ranged from ∼7 to 10 doublings/h (8). Growth rates on CAB1 in broth were moderately higher for both types of phages. The burst size, latent period, and adsorption rate were obtained for one of the K1-ind phages (when grown on CAB1 in serum) but were not pursued systematically when it was realized that most phage attachments apparently do not lead to infections (8).
In vitro assays.
Some in vivo assays required determining the frequencies of K1-dep phages and K1-ind phages in a mixed population. These assays involved plating the mixed population to obtain isolated plaques, followed by diagnosing the type of each plaque. Initial platings were on LB agar (10 g NaCl, 10 g Bacto tryptone, 5 g Bacto yeast extract, and 15 g Bacto agar per liter) using CAB1 suspended in overlays of 3 ml soft agar (7 g/liter Bacto agar). CAB1 plates both types of phages. To determine the K1-dep or K1-ind status of individual plaques on CAB1, isolated plaques were then stabbed with a sterile toothpick onto CAB281, which supports growth only of K1-ind phages.
In vivo assays.
Mouse work conformed to NIH guidelines and University of Texas IACUC protocol approval (2010-00012). Mice were 4- to 6-week-old females, NIH Swiss outbred (Hsd:NIHS mice; Harlan Laboratories Inc.). All mouse assays involved the inoculation of approximately (2 to 3) × 108 CAB1 bacteria into the posterior thigh. Inoculation volumes were 40 to 50 μl per thigh.
For some assays, the phages were mixed with cells before inoculation. With this design, the initial dynamics of infection are simplified and accelerated, avoiding unknown stochastic elements in phage loss and delay as phage migrate to the contralateral side and encounter bacteria. One implementation of this design was to estimate minimum treatment doses (MTDs), the lowest inoculum size needed to rescue 50% of mice with a particular phage; a broad range of phage doses was used in some of these tests. The other utilization of mixing cells and phages was to estimate growth rates of individual phages over short treatment times (3 or 6 h) from an inoculum of 100 phages (PFU).
Other assays administered mixtures of phages into individual mice; for these assays, phages and cells were inoculated into opposite thighs. The combined phage dose in these assays was approximately 106 PFU per mouse (range of 3 × 105 to 5 × 106).
Infected mice were monitored at least every 12 h; mice in rescue studies either died between observation times or were euthanized when they demonstrated signs of being moribund. For assays of phage titers at fixed times, mice were euthanized at scheduled times, the appropriate tissues were homogenized in buffer, and supernatants were plated on the appropriate host.
Modeling the phage growth rate.
In vivo phage growth was treated as an exponential (geometric) process:
| (1) |
where xt is the phage density at time t (in hours) and Gx is the hourly growth rate of the phage in vivo, the net growth accounting for phage loss as well as phage burst. This model is readily extended to the relative growths of two phages (densities x and y):
| (2) |
or
| (3) |
This two-phage model was used for comparisons of two phage types, either in the same host or between separate hosts. Although x and y are formally densities (e.g., phage/ml), ratios of phage types can be determined empirically from the proportions of each type of phage without knowing the phage density per se. Thus, if p is the proportion of phage type X and 1 − p is the proportion of type Y in a suspension [p = x/(x + y) and 1 − p = y/(x + y)], then x/y = p/(1 − p).
This model is clearly simplistic. A more accurate model of phage dynamics would consider cell and phage codynamics (14, 15, 22, 23). Yet this simple model suits our purpose, because it is intuitively tractable and provides insight at a level that might be applied broadly. If the true relationship between dynamics and treatment success is heavily detail specific, then it will be difficult to identify and apply generalities to phage therapy broadly. We do not assert that this simple model is accurate; rather, we ask if it is sufficiently adequate to be useful. Even so, one caveat is worth noting. The phage growth rate necessarily depends on bacterial density. Cell density is constant between mice at the outset in the experimental system, so density can be neglected during early phases of treatment as long as the bacteria outnumber the phage. However, this growth rate model will not apply once the infection comes under control.
RESULTS
Basics.
Mice were inoculated in a thigh with ∼2 × 108 to 3 × 108 cells of CAB1, generally following the method of Smith and Huggins (31). Untreated, the infection is nearly always lethal within 48 h and sometimes within 24 h. The phages used here are all lytic and are distinguished by whether they require the bacterial K1 capsule; phages requiring the capsule are referred to as K1 dependent (K1-dep), while the others are K1 independent (K1-ind). Prior to this study, only 2 in this set of 7 phages were tested for efficacy in mouse infections (8), although the inference from the work of Smith and Huggins (31) is that all four K1-dep phages would be highly effective and that all three K1-ind phages would be poor.
Minimum treatment doses vary by 6 orders of magnitude.
High levels of mouse recovery were obtained with very low doses of all K1-dep phages (e.g., often less than 100 phages per mouse) (Fig. 1). In striking contrast, mice were not rescued with doses of up to 107 and 108 K1-ind phages. For the most extreme K1-ind doses, the number of phages inoculated was only a few fold less than the number of bacteria inoculated, yet the mouse generally died.
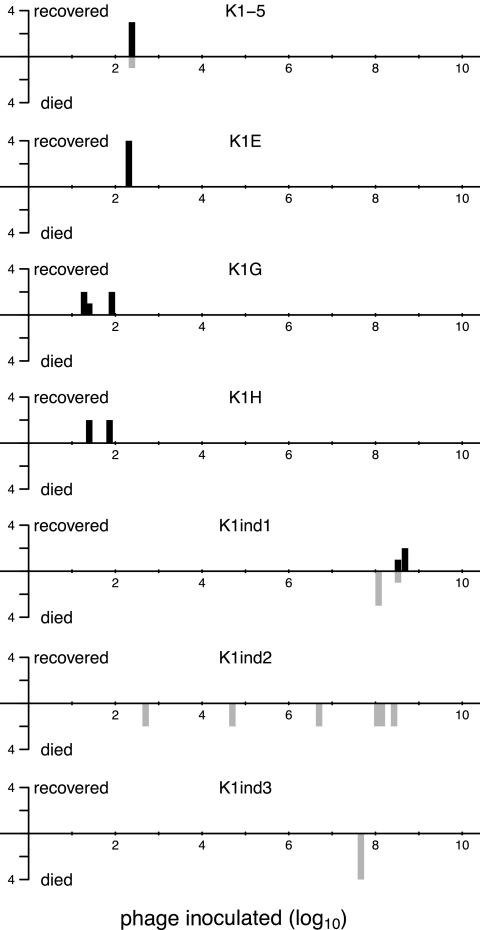
Fig 1.
Mouse recovery per dose of phages inoculated. Phages and ∼2 × 108 to 3 × 108 CAB1 bacteria were mixed and, within 2 min, injected into the left thigh. Recovery was monitored for 5 days. For each panel, the horizontal axis scale is the log10 phage dose, and the vertical axis gives the number of mice rescued from the infection (black bars above the axis) or not rescued (gray bars below the axis). Phages are named at the top of each panel. The top 4 panels are K1-dep; the bottom 3 are K1-ind phages. Mice represented within a single bar, as defined by its position along the horizontal axis, were inoculated at the same time.
An approximate assignment of the minimum treatment dose (MTD) is 100 for the K1-dep phages and 108 for the K1-ind phages, with a 106-fold difference. This difference is conservative for at least some combinations: all four mice treated with K1H recovered with an input dose of 25 phages or less, and four mice died when treated with at least 1.4 × 108 K1-ind2 phages; the MTD difference in this case is minimally 5.6 × 106-fold, since the actual MTDs lie outside the doses tested. (The difference in survival of 4/4 versus 0/4 is statistically significant at P values of <0.03 using a 2-tailed Fisher exact test.) Our MTDs for K1-dep phages are somewhat lower than that reported by Smith and Huggins (31) for a single K1-dep phage, but that study inoculated phages and cells into separate legs, so a lower MTD is to be expected with our design. Our MTDs for K1-ind phages are also broadly consistent with the work of Smith and Huggins, in which 3 × 108 phages inoculated into the contralateral leg typically rescued fewer than half the mice and sometimes rescued none.
If phage therapy success is determined solely by in vivo phage growth, it must be that K1-ind phage growth is almost as slow as cell growth if mice do not recover when inoculated with only a few fold fewer phages than cells. Furthermore, the MTD comparison indicates that K1-dep phages must grow much faster than K1-ind phages.
K1-ind phages grow rapidly in vivo, much faster than cells.
Smith and Huggins (31) reported the quantitative dynamics of phage growth in mice but only for a K1-dep phage. They suggested that K1-ind phages grew poorly in culture, but since their relatively poor performance in rescuing mice was not a major focus of the study, K1-ind in vivo dynamics were reported only qualitatively and for just one K1-ind phage. They indicated that this phage failed to reach high densities early in the infection; even later in the infection, it never reached densities much higher than those of the bacterium. One question, therefore, is whether growth of K1-ind phages is slow enough to explain why the MTD is possibly as high as the number of cells inoculated.
Total phage counts were obtained from the inoculated leg for two K1-ind phages at 6 h after inoculation and for one of those phages 3 h after inoculation (Fig. 2). The 6-h means of total phage counts in the infected leg exceeded 9 × 109 for both phages; the 3-h mean was 30-fold less. Approximately 100 phages were injected into the thigh, so amplifications were almost 8 logs after 6 h and 6.5 logs at 3 h. Based on the 6-h values, the phages were increasing at 20-fold per hour; based on the 3-h value, the increase was 150-fold per hour. There was thus a slowdown in growth of the phages between 3 and 6 h, possibly due either to exhaustion of the cell population or to a change in the state of the cells (6).
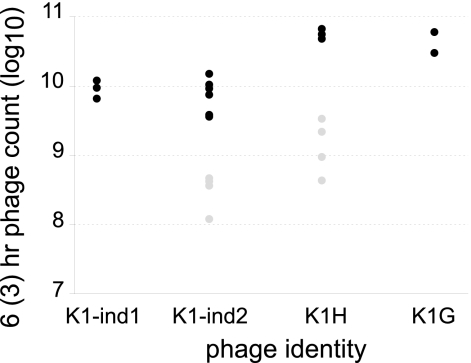
Fig 2.
In vivo growth of phages in separate infections. Left thighs of mice were inoculated with (∼2 to 3) × 108 cells of CAB1 mixed with ∼100 phage (PFU). Six hours after infection (black circles) or 3 h after infection (gray circles), a total phage count was made from the left leg. K1H and K1G are K1-dep phages. Three replicates were performed for the 6-h times except for K1-ind2 (six) and K1G (two), and four replicates were performed for the 3-h times.
By either the 6-h or 3-h growth rate measure, K1-ind phages grew much faster than cells. Smith and Huggins (31) reported that cell density in the inoculated leg increased 2 logs over 8 h, whereas our K1-ind phages increased 6 logs in 3 h and increased another 2 logs in the following 3 h. If survival was based solely on phages outnumbering cells, the K1-ind phages should have overwhelmed cells rapidly at many of the doses tested. Yet mice died, except for K1-ind1 at some doses exceeding 1 × 108. This analysis therefore suggests that K1-ind phages grow too fast to explain their poor treatment success.
Only modest superiority of K1-dep phages across single-phage treatments.
If therapy success depends solely on phage concentrations, then K1-dep phages must grow more rapidly than K1-ind phages because of the large difference in MTD values. Specifically, K1-dep density must increase fast enough to exceed K1-ind density soon after inoculation, even when the K1-ind inoculum has a millionfold excess. Figure 2 includes both types of phages and thus enables a comparison of their in vivo growth rates. We may use equation 1 to calculate in vivo growth rates and then use equation 2 to examine how long it would take a K1-dep phage inoculated at 100 phages per mouse to attain the same titer as a K1-ind phage inoculated at 108 phages per mouse. Applying this method to K1H versus K1-ind2 using the 6-h growth rates, we found that 44 h would be required for the K1-dep phage density to merely catch up to the K1-ind phage density. The growth rates from 3 h suggest the K1-dep phage could overtake the K1-ind phage in 18 to 26 h (assuming that the K1-dep phage at 3 h has either a 10-fold-higher density or a 5-fold-higher density than the K1-ind phage). Yet despite this superior growth rate, the data suggest that the superiority of K1-dep phages is manifest in the first 3 h but not from 3 to 6 h, since the relative difference in densities has not increased after 3 h. Thus, K1-dep phages do have a modestly superior growth rate in vivo under some conditions, but this advantage is not easily interpreted as supporting a model in which therapy success is strictly a function of the in vivo titer or growth rate, regardless of phage type.
Only modest superiority of K1-dep phages during in vivo competitions.
A third test of whether phage success depends on the growth rate is provided by coinoculated mixtures of “good” and “bad” phages. Changes in frequencies during treatment indicate relative differences in growth rates. Growth rates measured separately (as described in the previous section) should match relative growth rates measured in competitions if both assays capture the same basic principles of phage therapy. If growth rates measured for individual phages are fundamentally flawed in some way. as might be suggested from their failure to match MTD differences, the competitions provide an independent means of exposing any such error and would then show that phages with low MTDs vastly outpace phages with high MTDs during treatment of the same mouse.
From the perspective of common treatment practices, our design for competitions was more realistic than was the design of separate assays: the treatment period was 48 h, phages were inoculated into the contralateral thigh from cells, and a larger phage dose was used. Relative to K1-ind phages, the K1-dep phage increases in concentration (measured as the ratio on the left side of equation 3) were 5.3 ± 1.4, 3.5 ± 2.4, and 1.3 ± 0.12 (for K1H versus K1-ind1, K1G versus K1-ind1, and K1G versus K1-ind2, respectively). None of these means differs significantly from 1.0 (a value of 1.0 would apply if there was no difference in growth rates between the two phages).
The competition protocols are not quantitatively comparable to the separate growth protocols chiefly because it is unknown how long the in vivo bacterial populations will support rapid phage growth. Thus, we do not know whether phage growth during a 48-h competition reaches stationarity in 3 h, 6 h, 10 h, or some other interval. It is nonetheless interesting to compare the K1-dep increases observed in the competitions with that calculated for 6 h using growth rates from the separate assays. The 6-h values calculated from separate infections—6.6, 3.8, and 4.8, respectively—were remarkably similar to the competition values. Therefore, all three growth rate assays were consistent in showing that K1-ind phages grew far better than they should have based on their high MTDs.
DISCUSSION
Phage therapy is an old idea being investigated anew in the West, motivated by the accelerating incidence of drug-resistant bacteria. The concept of phage therapy seems infallible: viruses that evolved to kill bacteria in nature should be able to kill those bacteria in our bodies and should even be able to keep pace with bacterial evolution of resistance. The science behind phage therapy is still in its early stages, however, and general principles that determine treatment success and failure are hard to identify. Perhaps the most oft-assumed principle is that the rate at which phages kill bacteria is critical to success; the principle underlies all theoretical endeavors into phage therapy (7, 14, 15, 22, 23) and several experimental ones (9, 13, 17, 20, 30, 31).
Although it may be broadly true that, for any particular phage being used in treatment, higher numbers of phages will enhance treatment success over that with lesser numbers, it remains to be seen whether the same principle operates across different phages—that treatment efficacy of one phage compared to another's correlates with the density each phage attains in an infection. If such a principle could be established, it would greatly facilitate and expedite the discovery of efficacious phages and reduce experimentation. Evaluation of this (or any other) principle requires a set of phages differing in efficacies for the same infection.
Smith and Huggins (31) discovered that two classes of phages were differentially effective in treating mice receiving an artificial infection of E. coli O18:K1:H7. Treatment with phages requiring the K1 capsule for infection led to recovery in 90% to 100% of mice, whereas treatment with phages not requiring the capsule was effective in close to 30%; nearly all mice receiving no treatment died. The dynamical basis of this difference between the two types of phages was not reported in detail. Both phages formed plaques on the bacterium, but K1-ind phages were reported to have been poor at lysing cultures in vitro; the description of in vivo studies using one K1-ind phage suggested that it had a poor growth rate in the mouse, but numbers were not provided (see p. 314 of reference 31). Thus, there was a clear suggestion from that study that the poor treatment efficacy of K1-ind phages was due to their poor growth but there was no basis for a quantitative comparison of the two types of phages.
Bull et al. in 2002 (6) and more extensively in 2010 (8) showed that K1-ind phages were not universally inferior at growth in vitro if the medium was broth, but they were inferior when the medium was serum, a surrogate in vitro medium for a systemic infection in vivo. This observation raised the possibility that in vivo growth was the primary determinant of treatment success. The latter study also revealed that the molecular distinction between K1-dep and K1-ind phages likely resided in the enzymatic domain of the tail spike protein: 4 of 4 K1-dep phages had a sialidase domain, while 3 of 3 K1-ind phages did not (they instead had an enzyme that degrades an O antigen). Remarkably, except for tailspike, the three K1-ind and two K1-dep phage genomes were almost identical, and the gene order was totally conserved. Furthermore, adding free sialidase to a serum culture of a K1-ind phage raised its growth rate to that of a K1-ind phage. There was thus a feasible causal connection between the K1-dep phenotype and its superior growth rate in the pseudo-in vivo environment.
The present study attempted to evaluate the growth rate model quantitatively in vivo. Mouse recovery curves were evaluated for seven different phages, four K1-dep wild isolates and three K1-ind wild isolates. The wild K1-dep phages were able to rescue mice consistently with inocula of as few as 100 (some as low as 20) particles; K1-ind phages failed with up to 108 phages in the inoculum. These results led naturally to the minimum treatment dose (MTD) as a measure of phage success. Exact MTDs were not determined, but it was established that the MTDs of some K1-ind phages were at least 106-fold higher than those for some wild K1-dep phages.
We considered whether the high MTDs of K1-ind phages were due to correspondingly lower in vivo growth rates, both on an absolute scale and also relative to those of K1-dep phages. The general findings were that in vivo growth rates of K1-ind phages were higher than would be expected from their MTDs. K1-ind growth was far above that of the cells, so an initial high dose of those phages should have had no problem in outgrowing cells and thus in rescuing the mouse. When they were the only phage used in treatment, their titers after 6 h were only ∼5-fold lower than those of the K1-dep phages (5- to 10-fold lower after 3 h). When a K1-ind phage was competed with a K1-dep phage in the same mouse, the two grew almost equally well. Growth rates in mixed competitions need not mirror growth rates observed separately because of strong interactions (e.g., see reference 1), but here the two methods gave indistinguishable results. Collectively, the results offer little support for a model in which the poor therapeutic success of K1-ind phages is due primarily to poor growth in vivo.
Our conclusion might thus appear to be somewhat at odds with the preliminary assessment of Smith and Huggins (31), but a quantitative comparison with that study is not possible. We did observe slower growth in vivo of K1-ind phages than of K1-dep phages (as did they), and in any case, their in vivo test was limited to a single K1-ind phage that may have had growth characteristics different from those of ours. The fact that a K1-ind phage grows at least moderately well in vivo, as observed here, suggests that phage therapy success involves more than just phage growth to a high density. If the profound difference in treatment success observed between K1-dep and K1-ind phages is due solely to the modest difference in growth dynamics observed here, it will indeed be difficult to apply a priori criteria to choose the best phages for treatment.
An alternative to the MTD model can be inferred from the work of Mushtaq et al. (21), who showed that the sialidase enzyme alone can cure the infection (in rats)—phages were not needed at all. The likely mechanism is that sialidase stripped the bacteria of their capsule, enabling the immune system to clear the infection. This result raises the possibility that phage killing per se is not the basis of K1-dep success but rather that phages are merely acting to deliver a “drug” that augments immune-mediated clearance. A set of mass action differential equations helps illustrate this point:
| (4) |
where C is cell density, P is phage density, E is enzyme density, and I is an immune effect on cells (subscripted to indicate the possibility of different effects on free cells and enzyme-bound cells). The terms k, γ, α, δE, and δP are rate constants (phage-cell adsorption rate, enzyme-cell adsorption rate, cell growth rate, enzyme clearance rate, and phage clearance rate, respectively). The terms b and β are the respective numbers of phage progeny and enzyme molecules released when the infected host lyses. The subscript L indicates values L time units in the past; L is the time from infection to lysis of a cell (as per references 5 and 16).
The attempt to specify even this minimal level of detail surpasses our current knowledge. However, inspection of the middle equation in equation display 4 illustrates one main point: cell loss can result from two phage effects, from phage killing and from enzyme-mediated immune system clearance. Cell lysis not only releases mature phage into the growth medium but also the contents of the cell cytoplasm, which normally include partially assembled or unassembled structural proteins and other phage-encoded proteins. It is plausible that growth of K1-dep phages, which would release any unassembled sialidase, is contributing to infection clearance in both ways. From the work of Mushtaq et al. (21), we know the sialidase immune effect could be substantial, possibly explaining why K1-dep phages provide such good protection despite having marginally better growth rates than K1-ind phages. Presently, we do not know how much free enzyme is released at phage lysis, and hence we have no way of assessing whether free enzyme contributes substantially to recovery. Such studies are needed to resolve whether K1-dep superiority is due to two mechanisms instead of one.
It is easily seen from equation 4 that the phage growth rate should be important to therapy whether or not direct phage killing of cells is the primary mechanism of infection control. A higher phage growth rate means higher enzyme levels as well as higher phage levels. A corollary is that there should be a treatment equivalence between K1-dep phages and sufficiently faster-growing K1-ind phages, because enzyme substitutes for additional phages. The comparison of treatment success between a K1-dep phage and a K1-ind phage with the same in vivo growth rate would reveal the contribution of sialidase to treatment success. Unfortunately, such a phage pair is not available because natural isolates of K1-ind phages grow more slowly than K1-dep phages (at least in the surrogate in vivo environment of serum and for the few in vivo comparisons attempted). Thus, any testing of this equivalence principle awaits engineering of or the fortuitous discovery of low-fitness K1-dep phages.
A disappointing result was that K1-dep phages proved no more than mildly superior to K1-ind phages in mixed competitions. It might be hoped that competitions provide a way of identifying the best therapeutic phages (those with the lowest MTDs), because such a method would reduce the need for testing phages separately. Furthermore, phage cocktails are often used in actual therapy (24), and the components of these cocktails are changed periodically. Recovery of phages posttreatment would otherwise have been an easy way to decide which phages to maintain in the cocktail.
In the present study, phage therapy generalities based on population biology have not materialized. Despite the recognition of two classes of phages differing in treatment success, there is no suggestion that the in vivo growth rate is a strong determinant of treatment success. Rather, the two classes of phage differ in a way that appears to be vital to treatment success and may mask any effect of the growth rate: the presence of an enzyme. It may be that the growth rate will prove to be the major determinant of success in other model systems, but for now, an empirical approach to phage therapy remains warranted.
ACKNOWLEDGMENTS
We thank R. Springman for technical assistance and Craig Miller for R code used to generate Fig. 1. Two reviewers helped clarify the manuscript.
In vitro work was supported by NIH GM 57756 (to J.J.B.) and AI 07680 (to I.J.M.). Mouse work was supported by the University of Texas Miescher Regents Professorship (J.J.B.).
Footnotes
Published ahead of print 21 November 2011
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1. Adams MH. 1959. Bacteriophages. Interscience Publishers, New York, NY [Google Scholar]
- 2. Biswas B, et al. 2002. Bacteriophage therapy rescues mice bacteremic from a clinical isolate of vancomycin-resistant Enterococcus faecium. Infect. Immun. 70:204–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brussow H. 2005. Phage therapy: the Escherichia coli experience. Microbiology 151:2133–2140 [DOI] [PubMed] [Google Scholar]
- 4. Bruttin A, Brussow H. 2005. Human volunteers receiving Escherichia coli phage T4 orally: a safety test of phage therapy. Antimicrob. Agents Chemother. 49:2874–2878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bull JJ. 2006. Optimality models of phage life history and parallels in disease evolution. J. Theor. Biol. 241:928–938 [DOI] [PubMed] [Google Scholar]
- 6. Bull JJ, Levin BR, DeRouin T, Walker N, Bloch CA. 2002. Dynamics of success and failure in phage and antibiotic therapy in experimental infections. BMC Microbiol. 2:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bull JJ, Regoes RR. 2006. Pharmacodynamics of non-replicating viruses, bacteriocins and lysins. Proc. Biol. Sci. 273:2703–2712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bull JJ, Vimr ER, Molineux IJ. 2010. A tale of tails: sialidase is key to success in a model of phage therapy against K1-capsulated Escherichia coli. Virology 398:79–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carlton RM, Noordman WH, Biswas B, de Meester ED, Loessner MJ. 2005. Bacteriophage P100 for control of Listeria monocytogenes in foods: genome sequence, bioinformatic analyses, oral toxicity study, and application. Regul. Toxicol. Pharmacol. 43:301–312 [DOI] [PubMed] [Google Scholar]
- 10. Coates AR, Hu Y. 2007. Novel approaches to developing new antibiotics for bacterial infections. Br. J. Pharmacol. 152:1147–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gross RJ, Cheasty T, Rowe B. 1977. Isolation of bacteriophages specific for the K1 polysaccharide antigen of Escherichia coli. J. Clin. Microbiol. 6:548–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hanlon GW. 2007. Bacteriophages: an appraisal of their role in the treatment of bacterial infections. Int. J. Antimicrob. Agents 30:118–128 [DOI] [PubMed] [Google Scholar]
- 13. Leverentz B, et al. 2003. Biocontrol of Listeria monocytogenes on fresh-cut produce by treatment with lytic bacteriophages and a bacteriocin. Appl. Environ. Microbiol. 69:4519–4526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Levin BR, Bull JJ. 1996. Phage therapy revisited: the population biology of a bacterial infection and its treatment with bacteriophage and antibiotics. Am. Nat. 147:881–898 [Google Scholar]
- 15. Levin BR, Bull JJ. 2004. Population and evolutionary dynamics of phage therapy. Nat. Rev. Microbiol. 2:166–173 [DOI] [PubMed] [Google Scholar]
- 16. Levin BR, Stewart FM, Chao L. 1977. Resource-limited growth, competition, and predation—a model and experimental studies with bacteria and bacteriophage. Am. Nat. 111:3–24 [Google Scholar]
- 17. Matsuda T, et al. 2005. Lysis-deficient bacteriophage therapy decreases endotoxin and inflammatory mediator release and improves survival in a murine peritonitis model. Surgery 137:639–646 [DOI] [PubMed] [Google Scholar]
- 18. Mattey M, Spencer J. 2008. Bacteriophage therapy—cooked goose or phoenix rising? Curr. Opin. Biotechnol. 19:608–612 [DOI] [PubMed] [Google Scholar]
- 19. Merabishvili M, et al. 2009. Quality-controlled small-scale production of a well-defined bacteriophage cocktail for use in human clinical trials. PLoS One 4:e4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Merril CR, et al. 1996. Long-circulating bacteriophage as antibacterial agents. Proc. Natl. Acad. Sci. U. S. A. 93:3188–3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mushtaq N, Redpath MB, Luzio JP, Taylor PW. 2004. Prevention and cure of systemic Escherichia coli K1 infection by modification of the bacterial phenotype. Antimicrob. Agents Chemother. 48:1503–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Payne RJ, Jansen VA. 2003. Pharmacokinetic principles of bacteriophage therapy. Clin. Pharmacokinet. 42:315–325 [DOI] [PubMed] [Google Scholar]
- 23. Payne RJ, Jansen VA. 2001. Understanding bacteriophage therapy as a density-dependent kinetic process. J. Theor. Biol. 208:37–48 [DOI] [PubMed] [Google Scholar]
- 24. Pirnay JP, et al. 2011. The phage therapy paradigm: pret-a-porter or sur-mesure? Pharm. Res. 28:934–937 [DOI] [PubMed] [Google Scholar]
- 25. Projan S. 2004. Phage-inspired antibiotics? Nat. Biotechnol. 22:167–168 [DOI] [PubMed] [Google Scholar]
- 26. Radetsky P. 1996. The good virus. Discover 17:52 [Google Scholar]
- 27. Rhoads DD, et al. 2009. Bacteriophage therapy of venous leg ulcers in humans: results of a phase I safety trial. J. Wound Care 18:237–238, 240–243 [DOI] [PubMed] [Google Scholar]
- 28. Scholl D, Rogers S, Adhya S, Merril CR. 2001. Bacteriophage K1-5 encodes two different tail fiber proteins, allowing it to infect and replicate on both K1 and K5 strains of Escherichia coli. J. Virol. 75:2509–2515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schoolnik GK, Summers WC, Watson JD. 2004. Phage offer a real alternative. Nat. Biotechnol. 22:505–507 [DOI] [PubMed] [Google Scholar]
- 30. Smith HW, Huggins MB. 1983. Effectiveness of phages in treating experimental Escherichia coli diarrhoea in calves, piglets and lambs. J. Gen. Microbiol. 129:2659–2675 [DOI] [PubMed] [Google Scholar]
- 31. Smith HW, Huggins MB. 1982. Successful treatment of experimental Escherichia coli infections in mice using phage: its general superiority over antibiotics. J. Gen. Microbiol. 128:307–318 [DOI] [PubMed] [Google Scholar]
- 32. Soothill J, Hawkins C, Anggard E, Harper D. 2004. Therapeutic use of bacteriophages. Lancet Infect. Dis. 4:544–545 [DOI] [PubMed] [Google Scholar]
- 33. Sulakvelidze A. 2005. Phage therapy: an attractive option for dealing with antibiotic-resistant bacterial infections. Drug Discov. Today 10:807–809 [DOI] [PubMed] [Google Scholar]
- 34. Sulakvelidze A, Alavidze Z, Morris JG., Jr 2001. Bacteriophage therapy. Antimicrob. Agents Chemother. 45:649–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sulakvelidze A, Morris JG., Jr 2001. Bacteriophages as therapeutic agents. Ann. Med. 33:507–509 [DOI] [PubMed] [Google Scholar]
- 36. Summers WC. 2001. Bacteriophage therapy. Annu. Rev. Microbiol. 55:437–451 [DOI] [PubMed] [Google Scholar]
- 37. Wright A, Hawkins CH, Anggard EE, Harper DR. 2009. A controlled clinical trial of a therapeutic bacteriophage preparation in chronic otitis due to antibiotic-resistant Pseudomonas aeruginosa: a preliminary report of efficacy. Clin. Otolaryngol. 34:349–357 [DOI] [PubMed] [Google Scholar]