Abstract
Tuberculosis (TB) is a major infectious disease problem: 1.7 million people annually die due to TB. Emergence of drug-resistant Mycobacterium tuberculosis and the lack of new antibiotics have exacerbated the situation. There is an urgent need to develop or repurpose drugs against TB. We evaluated inhaled gentamicin as direct respiratory system-targeted therapy in a murine model of TB. Aerosolized-gentamicin-treated mice showed significantly reduced lung M. tuberculosis loads and fewer granulomas relative to untreated controls. These results suggest that direct delivery of antibiotics to the respiratory system may provide therapeutic benefit to conventional treatment regimes for treatment of pulmonary TB.
INTRODUCTION
Tuberculosis (TB) is a chronic disease resulting from infection with Mycobacterium tuberculosis. TB is a global health pandemic, leading to the death of over 1.7 million people annually (5). This situation is further exacerbated by the synergy between TB and AIDS (7), the failure of anti-TB vaccination, and the emergence of M. tuberculosis strains with single, multiple, or extensive resistance to frontline antitubercular drugs (ATDs) (1).
Oral therapies using the current regimen of ATDs are effective but are associated with a number of significant drawbacks. Only a small fraction of ATDs reach the infected pulmonary regions, particularly those with extensive cavitation (16). Hence, high doses of ATDs are required to treat TB. Therefore, pulmonary system-targeted ATDs may be an effective method to avoid the high daily dosing regimen. Targeted pulmonary drug delivery can help deliver the ATDs directly into the infected areas and target the actual site of bacillary replication and persistence, as well as reduce systemic toxicity and improve patient compliance.
Pulmonary drug delivery for TB treatment has been extensively explored (3, 15). Aerosol adjunctive therapy with different aminoglycosides can improve outcomes (15). Aerosol delivery of gamma interferon (IFN-γ), a key cytokine in the immunological response against M. tuberculosis, as an adjunct to systemic therapy has also been beneficial in particularly refractory cases (3). Aerosol therapy with the aminoglycoside streptomycin in patients with ulcerative endobronchial TB prevents severe bronchial stenosis (13). Aerosol drug delivery is also beneficial for treating Pneumocystis carinii pneumonia (9) and cystic fibrosis (12).
Gentamicin is a member of the aminoglycoside family of compounds. Several in vitro studies suggest that aminoglycosides are effective against mycobacteria at levels (concentrations) greater than those attainable by systemic administration (6). Studies in animal models confirm that gentamicin administered by aerosol is not systemically absorbed and is considered safe (14). The objective of our current study was to assess if inhaled gentamicin is effective for therapy against active pulmonary TB in a murine model of TB.
(This work was presented in part at the American Thoracic Society International Conference, New Orleans, LA, May 2010.)
MATERIALS AND METHODS
Animals.
Commercially purchased female C57BL/6 mice (Charles River Laboratories) were used for this study. The mice weighed between 18 and 20 g at 5 to 6 weeks of age and were randomized into three groups. Each group contained 30 animals. One group was administered gentamicin by small-particle aerosol daily at the nominal presented inhaled dose of 5 mg/kg of body weight. Drug dose estimates were derived from average aerosol concentrations determined from integrated air samples collected daily during aerosol treatment and calculated according to methods previously described (2). The second group received isoniazid (INH) daily at 15 mg/kg via oral gavage; a third group received no intervention and served as controls. All treatments in all groups were initiated 2 weeks postinfection; the duration of all treatments regardless of route was 20 study days (5 days/week for 4 weeks). This study was approved by the Institutional Animal Care and Use Committee at Tulane University, and all animals were handled in accordance with the American Association for Accreditation of Laboratory Animal Care.
M. tuberculosis cultures and infection of mice.
M. tuberculosis H37Rv was grown to mid-log phase in Middlebrook 7H9-albumin-dextrose-catalase-Tween 80-glycerol broth. A commercial nose-only aerosol exposure system (CH Technologies, Westwood, NJ) was used to perform the experimental infections. The entire exposure system was housed within a class III biological safety cabinet within a biosafety level 3 containment. Aerosols were generated into the exposure system using a three-jet Collison nebulizer (BGI Inc., Waltham, MA) operated at 20 lb/in2 gauge with an output flow of 7.5 liters/min. Aerosols were dried by mixing dry secondary dilution air at 2.5 liters/min. Time-of-flight aerodynamic particle size distribution was measured by using the Aerodynamic Particle Sizer (model 3321; TSI Inc., St. Paul, MN); results of physical characterization of the particles indicated a highly respirable particle size distribution (mass mean aerodynamic diameter [MMAD] = 1 μm; geometric standard deviation [σg] = 1.4). An all-glass impinger (AGI-4; Ace Glass Inc., Vineland, NJ) operated at the flow rate of 6 liters/min was used to isokinetically sample the aerosolized M. tuberculosis for 10 min. Contents of the AGI were cultured as a basis for determining the aerosol concentration in the chamber; results were used in conjunction with the cumulative respiratory estimates of the mice to derive an inhaled infectious dose. Animals were randomized and experimentally infected, 23 at a time; a total of seven discrete runs of the aerosol infection system were performed to complete all infections. The resulting inhaled infectious dose across all exposures was 384 ± 76 M. tuberculosis CFU/animal.
Drug treatments and samples.
Fourteen days postinfection (p.i.), groups were treated with either INH at 15 mg/kg by oral gavage or gentamicin sulfate administered by small-particle aerosol (GSA) (MMAD = 1.2; σg = 1.4) at a presented dose of 5 mg/kg. Treatments were administered 5 days a week over 1 month (20 treatment days). A subset of animals (n = 3) in each of the groups were euthanized weekly for lung bacillary count and histopathologic analysis. Tissues were aseptically removed and individually placed in 1 ml 0.9% NaCl. The left lung was removed for determination of M. tuberculosis burden as previously described (8). Colonies were counted after incubation for 21 days at 37°C and reported as CFU per gram of tissue. For pathological analysis, one of the lung lobes was dissected, placed in 4% paraformaldehyde, processed, and paraffin embedded. Sections were stained with hematoxylin and eosin (H&E) and viewed by light microscopy.
Quantitative real-time RT-PCR.
Quantitative RT-PCR was performed using a Sybr green method as previously described (10). Briefly, fluorescence signals were detected with an Applied Biosystems 7900HT sequence detector. Data were captured and analyzed with sequence detector software (Life Technologies).
Statistical analysis.
Quantitative results among groups were statistically compared using parametric measures (Student's t test); P values >0.05 were considered significant.
RESULTS AND DISCUSSION
We designed experiments to evaluate the effect of repeated treatment with aerosolized gentamicin (GMA) on the progression of pulmonary TB in mice. Three groups of mice were infected with a low dose of M. tuberculosis H37Rv via the aerosol route. Treatment began 15 days postinfection. The effects of GMA were evaluated in both the treatment (30 days) and relapse (21 days) phases. As a positive control we treated a group of mice orally with INH, a frontline anti-TB drug and one against which the performance of all other such compounds is measured. As a negative control, mice were left untreated.
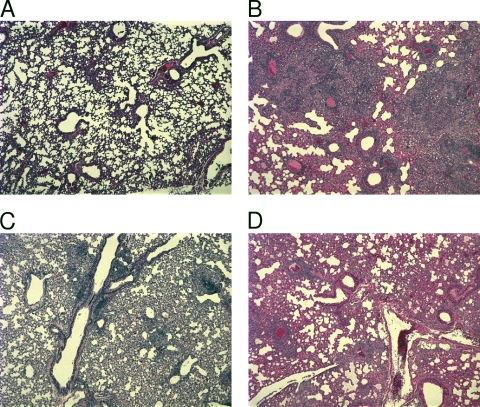
We initially examined the lungs of these mice at the time of sacrifice for pulmonary immunopathology. Toward this end, histopathology was determined in the lungs of uninfected animals as well as in those after various treatments for various times postinfection. A subset of these results (representative H&E stains) are shown in Fig. 1. The lungs of these animals were devoid of any tuberculous immunopathology at a time prior to infection (Fig. 1A). The untreated animals exhibited a progressive worsening of lung pathology over the course of the infection and exhibited severe immunopathology at day 30 postinfection (Fig. 1B). The animals treated with INH exhibited relatively few granulomatous lesions and areas of inflammation at day 30 postinfection (Fig. 1C). The animals treated with GMA exhibited a slightly higher degree of immunopathological lesions relative to INH-treated animals at the same time (Fig. 1D). However, the degree of lesions was significantly lower than that observed for untreated animals (Fig. 1B). The results from histopathology images were numerically analyzed as lesion counts for different time points postinfection and are graphically represented in Fig. 2A. Once again, INH-treated animals exhibited significantly lower lung pathologies. As little as 2 weeks after treatment began (day 30 postinfection), INH-treated animals typically had three times less lung area involved in lesions than untreated animals. At this time point, greater-than-twofold less lung involvement was also observed for GMA-treated animals relative to untreated animals (Fig. 2A). By day 37 postinfection, the lesion area percentages for the GMA-treated animals were comparable to those for the INH-treated group.
Fig 1.
Representative H&E-stained histopathology images from the lungs of various groups of mice at day 30 postinfection. (A) Uninfected murine lungs. (B) Lungs of untreated mice. (C) Lungs of mice treated with INH via oral gavage for 14 days. (D) Lungs of mice treated with gentamicin via the aerosol route for 14 days.
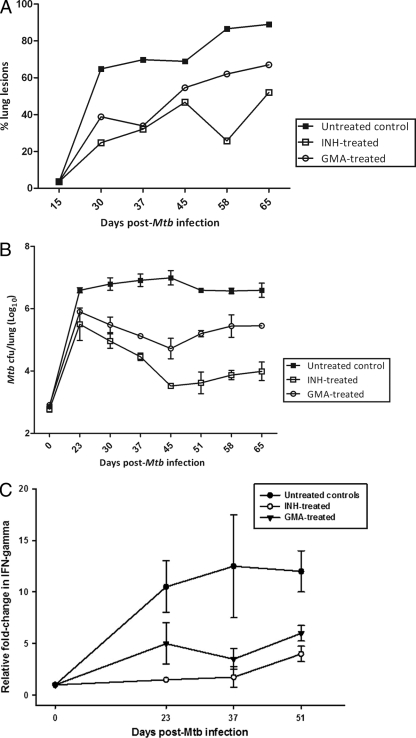
Fig 2.
(A) Percentage of lung lesions observed in treated and control animals. Lesion counts for lungs of untreated controls and GMA-treated or INH-treated mice are shown. Mtb, M. tuberculosis. (B) Bacillary load of lungs in animals treated with aerosolized gentamicin or isoniazid and in untreated controls. Data points are the mean group subsets (n = 3). (C) IFN-γ levels of expression in the lungs of mice of different groups at different time points.
We next examined the bacillary loads in the lungs of different groups of animals. Toward this end, a subset of animals was sacrificed 2 weeks postinfection and M. tuberculosis loads in whole lungs were determined. At this point, various treatments began, and three mice per group were sacrificed weekly for M. tuberculosis load analysis. Figure 2B shows the results of bacillary loads of lungs in animals treated with GMA along with those for isoniazid-treated mice and untreated controls. Mice that received GMA exhibited a significant reduction in M. tuberculosis CFU levels relative to untreated animals, although the levels of CFU reduction were less significant than that achieved by INH treatment (Fig. 2B). The maximal reduction in CFU was observed at day 44 postinfection (day 28 from the beginning of treatment). At this point, about a 1.5 log reduction in M. tuberculosis CFU could be attributed to GMA treatment. This is in contrast to approximately 3 logs of reduction in CFUs caused by INH treatment. In the relapse phase of the infection the CFU numbers showed increases in both the treatment groups. However, at the end of the experiment (day 65 postinfection or day 49 from the beginning of the treatment), the effect of GMA treatment was still significant. Animals in this group exhibited approximately 1 log lower CFU relative to untreated mice. At this time, about a 2 log CFU reduction was observed for the INH-treated mice (Fig. 2B).
We then studied the expression of a key immune marker of TB infection, IFN-γ, in the lung samples of these three groups of animals by quantitative PCR. While it is believed that IFN-γ is crucial for the control of TB infection, its expression levels can also indicate the extent of disease, particularly in those experimental models where high levels of bacilli are present. Compared to expression in a baseline of uninfected animals, the expression of IFN-γ increased progressively upon M. tuberculosis infection in the untreated animals. However, the levels IFN-γ decreased in the treatment phase of both the INH- and GMA-treated groups (Fig. 2C). At day 37 postinfection, IFN-γ expression was significantly (>4-fold) lower for GMA- as well as INH-treated animals, relative to untreated animals. Once again, these differences in the expression of IFN-γ between the treated and untreated groups narrowed during the relapse phase but remained significantly different.
Taken together, our results suggest that GMA treatment significantly reduced the burden of active TB in a mouse model. This suggests that aerosol adjunct therapy with compounds like gentamicin may be effective for targeted delivery to the lungs. This is very encouraging, since we have adapted a commercially available intravenous formulation of gentamicin sulfate with minimal modifications for pulmonary delivery. Although these results are promising, additional studies are warranted to evaluate the efficacy of inhaled gentamicin as a potential therapeutic for active pulmonary TB. These studies could also address the issue of distribution of aerosolized gentamicin in the infected tuberculoma cavities. Further, these studies could be performed in more appropriate models of TB, such as nonhuman primates, to address if adjunct aerosol therapy along with frontline drug treatment would significantly outperform conventional treatment. We have developed models of acute as well as chronic TB and TB/AIDS coinfection using rhesus macaques, which can be used for this purpose (4, 11).
ACKNOWLEDGMENTS
Author contributions: research design, D.K., C.J.R., N.K.D., and S.M.; research, C.J.R., N.K.D., S.M., S.K.S., N.A.G., and S.K.; novel research reagent/method, J.D.T. and B.E.H.; veterinary pathology, P.J.D.; data analysis, D.K., C.J.R., N.K.D., S.M.; writing, D.K. with input from C.J.R.
This study was funded in part by National Institutes of Health contract HHSN27220070030C, by Nanotherapeutics, Inc., by U.S. Public Health Service grants RR000164 (Tulane National Primate Research Center), AI089323, HL106790, AI091457 and RR026006 (to D.K.), by grants from the Louisiana State Board of Regents and Louisiana Vaccine Center (to D.K.), by Tulane Research Enhancement Funds (to D.K. and C.J.R.), and by the Tulane Center of Infectious Diseases (S.M. and N.K.D.).
C.J.R. has received research support from Nanotherapeutics. J.D.T. is an employee of Nanotherapeutics. B.E.H. is a former employee of Nanotherapeutics. D.K., S.M., N.K.D., S.K.S., N.A.G., S.K., and P.J.D. have no disclosures to report.
Footnotes
Published ahead of print 5 December 2011
REFERENCES
- 1. Andrews JR, et al. 2010. Predictors of multidrug- and extensively drug-resistant tuberculosis in a high HIV prevalence community. PLoS One 5:e15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bray M, et al. 2002. Treatment of aerosolized cowpox virus infection in mice with aerosolized cidofovir. Antiviral Res. 54:129–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Condos R, Rom WN, Schluger NW. 1997. Treatment of multidrug-resistant pulmonary tuberculosis with interferon-gamma via aerosol. Lancet 349:1513–1515 [DOI] [PubMed] [Google Scholar]
- 4. Dutta NK, et al. 2010. Genetic requirements for the survival of tubercle bacilli in primates. J. Infect. Dis. 201:1743–1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dye C, et al. 2009. Trends in tuberculosis incidence and their determinants in 134 countries. Bull. World Health Organ. 87:683–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gangadharam PR, et al. 1995. Therapy of Mycobacterium avium complex infections in beige mice with streptomycin encapsulated in sterically stabilized liposomes. Antimicrob. Agents Chemother. 39:725–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Granich R, et al. 2010. Prevention of tuberculosis in people living with HIV. Clin. Infect. Dis. 50:S215–S222 [DOI] [PubMed] [Google Scholar]
- 8. Kaushal D, et al. 2002. Reduced immunopathology and mortality despite tissue persistence in a Mycobacterium tuberculosis mutant lacking alternative sigma factor, SigH. Proc. Natl. Acad. Sci. U. S. A. 99:8330–8335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Leoung GS, et al. 2001. Aerosolized pentamidine for prophylaxis against Pneumocystis carinii pneumonia. The San Francisco Community Prophylaxis Trial. N. Engl. J. Med. 323:769–775 [DOI] [PubMed] [Google Scholar]
- 10. Mehra S, et al. 2010. Transcriptional reprogramming in nonhuman primate (rhesus macaque) tuberculosis granulomas. PLoS One 5:e12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mehra S, et al. 2011. Reactivation of latent tuberculosis in rhesus macaques by coinfection with simian immunodeficiency virus. J. Med. Primatol. 40:233–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ramsey BW, et al. 1999. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis: cystic fibrosis inhaled tobramycin study group. N. Engl. J. Med. 340:23–30 [DOI] [PubMed] [Google Scholar]
- 13. Rikimaru T, et al. 2001. Treatment of ulcerative endobronchial tuberculosis and bronchial stenosis with aerosolized streptomycin and steroids. Int. J. Tuberc. Lung Dis. 5:769–774 [PubMed] [Google Scholar]
- 14. Riviere JE, et al. 1981. Gentamicin aerosol therapy in 18 dogs: failure to induce detectable serum concentrations of the drug. J. Am. Vet. Med. Assoc. 179:166–168 [PubMed] [Google Scholar]
- 15. Sacks LV, et al. 2001. Adjunctive salvage therapy with inhaled aminoglycosides for patients with persistent smear-positive pulmonary tuberculosis. Clin. Infect. Dis. 32:44–49 [DOI] [PubMed] [Google Scholar]
- 16. Telzak EE, et al. 1997. Factors influencing time to sputum conversion among patients with smear-positive pulmonary tuberculosis. Clin. Infect. Dis. 25:666–670 [DOI] [PubMed] [Google Scholar]