Abstract
Methicillin-resistant Staphylococcus aureus (MRSA) is a common cause of complicated bacteremia (CB) and infective endocarditis (IE). The gold standard treatment for these infections is vancomycin. A vancomycin area under the concentration-time curve from 0 to 24 h (AUC24)/MIC ratio of >400 has been suggested as a target to achieve clinical effectiveness, and yet to date no study has quantitatively investigated the AUC24/MIC ratio and its association with attributable mortality (AM). We performed a review of patients treated for MRSA CB and IE from 1 July 2006 to 30 June 2008. AM was defined as deaths where CB or IE was documented as the main cause or was mentioned as the main diagnosis. Classification and regression tree analysis (CART) was used to identify the AUC24/MIC ratio associated with AM. Mann-Whitney and Fisher exact tests were used for univariate analysis, and logistic regression was used for multivariate modeling. The MICs were determined by Etest, and the AUC24 was determined using a maximum a posteriori probability-Bayesian estimator. A total of 32 CB and 18 IE patients were enrolled. The overall crude mortality and AM were 24 and 16%, respectively. The CART-derived partition for the AUC24/MIC ratio and AM was <211. Patients with an AUC24/MIC ratio of <211 had a >4-fold increase in AM than patients who received vancomycin doses that achieved an AUC24/MIC ratio of ≥211 (38 and 8%, respectively; P = 0.02). In bivariate analysis the APACHE-II score and an AUC24/MIC ratio of <211 were significantly associated with AM. In the multivariate model, the APACHE-II score (odds ratio, 1.24; P = 0.04) and a vancomycin AUC/MIC ratio of <211 (odds ratio, 10.4; P = 0.01) were independent predictors of AM. In our analysis, independent predictors of AM were the APACHE-II score and an AUC24/MIC ratio of <211. We believe further investigations are warranted.
INTRODUCTION
Despite advances in medical and surgical interventions, endovascular infections, including complicated bacteremia and infective endocarditis, continue to be a cause of considerable morbidity and mortality (3, 9, 10, 12, 16, 21). Mortality estimates vary, but even the most conservative estimates suggest a crude mortality of ca. 30% (10). Interestingly, the predominant etiology of these infections has changed over the past 4 decades, with Streptococcus spp. formerly being the most common, whereas now Staphylococcus aureus is the most frequently isolated pathogen (4). This change has brought with it an increase in resistance such as methicillin-resistant S. aureus (MRSA) and substantial changes in antibiotic strategies.
For empirical and confirmed MRSA treatment in these types of endovascular infections, vancomycin has become the gold standard. With the increased use of vancomycin, a slow yet steady increase in fulminant resistance isolates have accumulated. However, phenotypes with heteroresistant characteristics (i.e., heteroresistant vancomycin-intermediate S. aureus [hVISA]) and accessory gene regulator (agr) dysfunction are even more startling. This is because hVISA and agr mutant testing is not routinely performed in most clinical laboratories due to workload and feasibility issues (7). Other microbiological issues, such as MIC values of >1 μg/ml, are also important and have been shown to have an impact on infection-related mortality (15, 19, 20).
Such microbiological dilemmas have created pharmacodynamic challenges for clinicians when faced with high mortality infections such as endovascular infections which are commonly caused by S. aureus (23). This has led investigators to question the pharmacodynamic target best correlated with clinical effectiveness. Moise-Broder et al. specifically address this question in a cohort of patients with S. aureus pneumonia and found a vancomycin area under the concentration-time curve from 0 to 24 h (AUC24)/MIC ratio of ≥400 to be best correlated with clinical effectiveness (22). However, that study is probably only pertinent to the investigator-specified success definitions and patients with S. aureus pneumonia. Furthermore, that study did not specifically test for hVISA or agr dysfunction, which likely has an impact on patient outcomes and attributable mortality. Our aim was to quantitatively investigate the relationship between the vancomycin AUC24/MIC ratio and attributable mortality in patients with complicated bacteremia and infective endocarditis in well-characterized MRSA isolates.
MATERIALS AND METHODS
We performed a retrospective review of patients treated for confirmed complicated bacteremia and infective endocarditis due to MRSA from 1 July 2006 to 30 June 2008. Our primary study objective was to quantitatively determine the relationship between the vancomycin AUC/MIC ratio and attributable mortality in patients with complicated bacteremia and infective endocarditis in well-characterized MRSA isolates. Patients with catheter-related bacteremia were not included except when catheter removal was not possible. Patients with complicated bacteremia and definite or possible infective endocarditis as defined by the Modified Duke Criteria were included (18). In this case, complicated bacteremia was defined as follows: patients with two positive blood cultures from distinct draw sites within 24 h and without definite or possible infective endocarditis as defined by the Modified Duke Criteria, those in which catheter removal is not possible and patients with metastatic foci of infection (deep tissue involvement) including, for example, septic arthritis, deep tissue abscess, or infection involving prosthetic material including intravascular foreign material not previously removed. Patients not considered for inclusion were those with end-stage renal disease, patients on hemodialysis, patients with active malignancy (receiving antineoplastic agents), HIV infection, known active osteomyelitis, polymicrobial bacteremia, or catheter related bacteremia (except as noted above), or patients with an absolute neutrophil count of <1,000 cells/mm3.
Data collection included patient specific clinical characteristics, each patient's microbiological serum sample, and acuity and treatment information. Crude mortality was defined as death from any cause during hospitalization. Attributable mortality was defined as deaths where complicated bacteremia or infective endocarditis was documented as the primary cause of death or where complicated bacteremia or infective endocarditis was mentioned as the main diagnosis in the discharge abstract. Each specific patient isolate was tested in duplicate using Etest to determine the MIC of the MRSA isolate to vancomycin.
Vancomycin AUC24 calculations were performed if the patient received >48 h of vancomycin therapy and had one vancomycin level collected within 96 h of vancomycin therapy. Individual pharmacokinetic parameters were estimated for each patient by use of ADAPT II software. Vancomycin serum concentrations were fitted to a two-compartment volume-clearance model using the maximum a posteriori probability (MAP)-Bayesian approach to each individual patient's pharmacokinetic profile (13, 14, 24, 25, 32). The averages of the daily predicted AUC24/MIC values were used for the analysis in situations where renal function was changing. Once a patient's pharmacokinetic parameters had been estimated based on his or her vancomycin serum concentrations, the steady-state AUC24/MIC ratio was computed based on the patient's 24-h daily dose, fitted vancomycin clearance (calculated from the patient's creatinine clearance and body weight), and the MIC of the infecting pathogen.
Independent variables (including age, sex, concomitant antibiotics, concomitant infections, intensive care unit duration, other disease states, and acuity index) associated with the probability of a dichotomous categorical outcomes were evaluated using logistic regression with forward and backward stepping. Individual variables were included in the final model if they had a P of <0.02 on univariate analysis. Multivariable analyses, with a continuous, interval or ratio-scaled dependent variable, were accomplished using linear-mixed-effects analysis. Classification and regression tree analysis (CART) was used to identify the AUC24 of vancomycin associated with attributable mortality. Significance was defined as an alpha probability of <0.05. Statistical analyses were performed using Systat (version 13; Systat Software, Inc., Chicago, IL) and STATA (version 9; Stata Corp., College Station, TX).
Vancomycin MICs were measured using the microdilution Etest (AB Biodisk, Solna, Sweden; bioMérieux, Durham, NC) according to the manufacturers' instructions and according to Clinical and Laboratory Standards Institute guidelines (2, 5). The Etest macrodilution method was used to determine whether the patient was infected with hVISA as described previously (31). We investigated agr dysfunction by using the level of δ-hemolysin production. Specifically, δ-hemolysin production was measured by streaking the S. aureus isolate adjacent to a β-hemolysin disk on a tryptic soy agar plate with 5% sheep blood, incubating the sample at 37°C overnight, and then evaluating it for synergistic hemolysis within the β-hemolysin zone produced by the disk containing bacterial growth. The presence of synergistic hemolysis within the β-hemolysin zone indicates the production of δ-hemolysin by the test organism and, therefore, a functional agr locus (27). agr dysfunction was defined as the complete absence of δ-hemolysin within the β-hemolysin zone, as evidenced by the lack of synergistic hemolysis. Multiplex PCR was used to determine the agr group genotype as described previously, with appropriate control strains for agr groups I, II, III, and IV (11).
RESULTS
During the study period from 1 July 2006 to 30 June 2008, we evaluated 61 patients treated with vancomycin for confirmed complicated bacteremia and infective endocarditis due to MRSA. Of these 61 patients, 3 were excluded for being on hemodialysis, 2 were excluded for having an active osteomyelitis, 2 were excluded for having polymicrobial bacteremia, and 1 was excluded for having an active malignancy. Of the remaining 53 patients, 50 patients had medical information available for review. Baseline patient characteristics are outlined in Table 1, and these included 32 patients with complicated bacteremia and 18 patients with infective endocarditis. The crude mortality and attributable mortality were 24 and 16%, respectively.
Table 1.
Baseline patient characteristics
| Characteristic | Value |
|---|---|
| Avg age in yrs (SD) | 54.8 (16) |
| Male patients (%) | 50 |
| Patients with infective endocarditis (%) | 36 |
| Avg APACHE-II score (SD) | 9 (3) |
| Patients with an ICU admission (%) | 22 |
| Patients with hVISA (%) | 8 |
| Patients with agr dysfunction (%)a | 14 |
| Avg AUC24/MIC ratio (SD) | 488 (402) |
| Patients with concurrent gentamicin therapy (%) | 80 |
| Patients with concurrent rifampin therapy (%) | 36 |
| Patients receiving other concurrent antibiotics (%) | 0 |
| Patients receiving prior antibiotics within 30 days (%) | 36 |
| Avg no. of hospital days prior to culture positivity (SD) | 8.4 (8) |
That is, accessory gene regulator dysfunction.
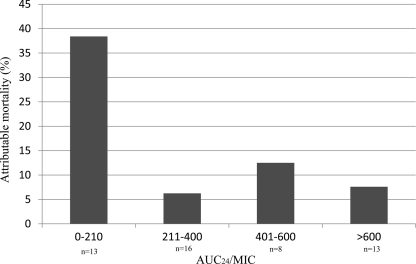
The CART AUC24/MIC breakpoint for attributable mortality was <211. Of these 38 patients, 76% received vancomycin doses that achieved an AUC24/MIC ratio of ≥211. Patients with an AUC24/MIC ratio of <211 had a >4-fold increase in attributable mortality compared to patients who received vancomycin doses that achieved an AUC24/MIC ratio of ≥211 (38 and 8%, respectively; P = 0.02). There was also a trend toward increased crude mortality among those with an AUC24/MIC ratio of <211 compared to those with an AUC24/MIC ratio of ≥211 (46% versus 16%, P = 0.06). The results of an examination of the different AUC24/MIC ratios achieved above and below the CART breakpoint are presented in Fig. 1. The attributable mortality was similar for all groups who exceeded the AUC24/MIC CART breakpoint.
Fig 1.
Attributable mortality stratified by the AUC24/MIC ratio.
The relationship between clinical features and attributable mortality is shown in Table 2. In the bivariate analysis, only the APACHE-II score and an AUC24/MIC ratio of <211 were significantly associated with attributable mortality. In the logistic regression analysis, each of the following factors were independently associated with attributable mortality: APACHE-II score (adjusted odds ratio [AOR] = 1.24; P = 0.04) and an AUC24/MIC ratio of <211 (AOR = 10.4; P = 0.01). The APACHE-II score was modeled continuously, so that the AOR was representative of a one-point increase (Table 3).
Table 2.
Bivariate analysis of relationship between clinical features and attributable mortality
| Independent variable | Attributable mortality (n = 8) | No attributable mortality (n = 42) | P |
|---|---|---|---|
| Avg age in yrs (SD) | 51 (20) | 60 (14) | 0.14 |
| Male patients (%) | 38 | 55 | 0.45 |
| Patients with infective endocarditis (%) | 50 | 50 | 0.43 |
| Avg APACHE-II score (SD) | 12 (3) | 9 (3) | 0.05 |
| Patients with an ICU admission (%) | 40 | 23.5 | 0.35 |
| Patients with hVISA (%) | 13 | 7 | 0.51 |
| Patients with agr dysfunction (%)a | 26 | 12 | 0.31 |
| Avg AUC24/MIC ratio (SD) | 267 (209) | 530 (446) | 0.12 |
| Patients with an AUC24/MIC ratio of <211 (%) | 63 | 19 | 0.02 |
| Patients with concurrent gentamicin therapy (%) | 100 | 76 | 0.18 |
| Patients with concurrent rifampin therapy (%) | 50 | 33 | 0.44 |
| Patients receiving prior antibiotics within 30 days (%) | 50 | 33 | 0.41 |
| Avg no. of hospital days prior to culture positivity (SD) | 10 (12) | 7 (7) | 0.31 |
That is, accessory gene regulator dysfunction.
Table 3.
Independent predictors of attributable mortality in logistic regression analysisa
| Predictor of attributable mortality | AOR | 95% CI | P |
|---|---|---|---|
| APACHE-II | 1.24 | 1.05–1.96 | 0.04 |
| AUC24/MIC ratio of <211 | 10.4 | 3.89–16.77 | 0.01 |
AOR, adjusted odds ratio; 95% CI, 95% confidence interval.
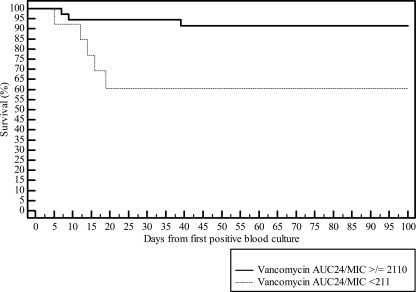
The overall vancomycin mean AUC24/MIC ratio (standard deviation [SD]) in the <211 AUC24/MIC group was 158 (SD = 48), and it was 590 (SD = 332) in the ≥211 AUC24/MIC group, with an average dose of 16 mg/kg/day in the <211 AUC24/MIC group compared to 22 mg/kg/day in the >211 AUC24/MIC group (Fig. 2). The average durationsof therapy in patients with an AUC24/MIC ratio of <211 was 11, and it was 18 days in those with an AUC24/MIC ratio of <211. Four patients went on to receive other anti-MRSA agents (daptomycin or linezolid), including three in the <211 AUC24/MIC group and one in the >211 AUC24/MIC group.
Fig 2.
Kaplan-Meier plot of patients with a vancomycin AUC24/MIC ratio of ≥211 or <211.
DISCUSSION
We sought here to identify the AUC24/MIC ratio associated with an increased attributable mortality among patients with MRSA-associated complicated bacteremia or infective endocarditis. Using CART, patients who received vancomycin treatment regimens that resulted in an AUC24/MIC ratio of <211 were at greatest risk for attributable mortality and had a 4-fold increase in attributable mortality compared to patients who had an AUC24/MIC ratio of ≥211. An AUC24/MIC ratio of <211 was independently associated with attributable mortality in the multivariate analysis. Furthermore, examination of the different AUC24/MIC ratios that were >211 showed that the attributable mortalities were similar across all groups (Fig. 1), and no statistically significant additional elevation in attributable mortality was noted when the AUC24/MIC ratio was ≥211. Collectively, these data strongly suggest that patients with a vancomycin AUC24/MIC ratio of <211 have a significantly increased risk for attributable mortality among patients with complicated bacteremia or infective endocarditis due to MRSA. Seven of the thirteen AUC/MIC values <211 were due to relatively low vancomycin trough levels (<10 mg/liter) as opposed to high MICs (≥2), suggesting that this was not an effect related to MIC but rather to exposure.
To date, several animal and human studies have identified the AUC24/MIC ratio to be the pharmacodynamic parameter best associated with vancomycin effectiveness (6–8, 22, 26). Descriptions of “effectiveness” in most of these studies are defined as a quantitative in vitro killing effect or clinical success. Unfortunately, the former is not routinely used in clinical practice and does not take into account important characteristics, such as the presence of agr dysfunction or hVISA phenotypes, that are commonly seen in clinical isolates. The latter may suffer from external validity issues, with practicing clinicians having different interpretations of successful treatment with vancomycin. Our study aim was to investigate an alternative pharmacodynamic outcome measure that has a direct and meaningful impact on the patients' infection-related outcome. Our definition of attributable mortality satisfies both of these since it includes the treating clinician's medical opinion regarding the cause of the patient's death.
A timely review of the peer-reviewed literature reveals that higher vancomycin MICs to S. aureus play a substantial role in determining a patient's mortality (15, 28, 30). In the attributable mortality cohort, 75% of the patients had vancomycin MICs of 2 μg/ml and 25% had MICs of 1 μg/ml compared to 24% with MICs of 2 μg/ml and 33% with MICs of 1 μg/ml in the non-attributable-mortality cohort. When performing multivariate analysis, it is important to have an understanding of the MIC distributions for each cohort, but this approach undoubtedly results in colinearity with the independent variables of interest, in this case the AUC24/MIC ratio. To avoid this colinearity, we chose not to include it in the parent analysis but rather to report these findings descriptively.
In the present study, we did not measure a vancomycin-free fraction in patients and, although the protein binding of this drug has been reported to vary between a low value of 29% and a high value of 71%, vancomycin has been considered for purposes of calculation to be ca. 60% protein bound (1, 29, 33). If the target AUC24/MIC ratio is corrected on the basis of a free fraction of 40%, then a total drug AUC24/MIC of 211 becomes a free level of ∼85. These values place vancomycin into the target AUC24/MIC ratio below that of other antibacterials, but for most of these agents the pharmacodynamically linked outcome parameter is clinical success rather than attributable mortality.
Prior to the present study, Jeffres et al. sought to evaluate the use of targeted trough concentrations of 15 to 20 mg/liter in patients with MRSA pneumonia (17). Additional calculations were also performed to approximate the vancomycin AUCs, and no differences between survivors and nonsurvivors were observed. As previously outlined, that study had several severe limitations, such as the lack of MIC testing, which likely severely confounded the results. Our study overcomes these limitations and demonstrates the first AUC24/MIC pharmacodynamic relationship with attributable mortality among patient with complicated bacteremia or infective endocarditis due to MRSA.
There were several additional noteworthy observations in our analysis. Most surprising was the lack of a relationship between agr dysfunction or hVISA phenotypes and mortality, as demonstrated by other investigators who have studied this relationship in similar populations (27, 28). This is an interesting finding and suggests that, in our study sample, these are not significant contributors to poor overall outcomes such as mortality. Our findings, however, should be placed in the appropriate context. With our small sample size there is substantial risk of not being able to find these differences in agr dysfunction or hVISA phenotypes even if they truly do exist in this population under study (type II error).
In the present study, we have gained insight into to the AUC24/MIC threshold associated with an increased attributable mortality among patients with complicated bacteremia or infective endocarditis due to MRSA. It is important to note that despite our findings there are also limitations to our study. First, the sample size for the study is small. Although this did not affect our ability to find a statistical difference, it is not a sufficiently robust sample to quantify the magnitude of this effect. Second, the retrospective methods of this analysis open it up to confounding and bias that may well be avoided with prospective study methods. Third, due to the known colinearity of the vancomycin AUC24/MIC ratio and vancomycin MIC variables, the latter was not included in the multivariate model. Given this limitation, it is plausible that the observed relationship between an AUC24/MIC ratio of <211 and attributable mortality may be a surrogate for vancomycin MIC values. Lastly, these data are from a single institution, as opposed to multiple institutions, which leaves the question of the external validity of the present findings.
ACKNOWLEDGMENT
This study was supported in part by an investigator-initiated grant provided by Cubist.
Footnotes
Published ahead of print 28 November 2011
REFERENCES
- 1. Ackerman BH, Berg HG, Strate RG, Rotschafer JC. 1983. Comparison of radioimmunoassay and fluorescent polarization immunoassay for quantitative determination of vancomycin concentrations in serum. J. Clin. Microbiol. 18: 994–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Andrews JM. 2001. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 48 (Suppl. 1): 5–16 [DOI] [PubMed] [Google Scholar]
- 3. Anguera I, et al. 2005. Clinical characteristics and outcome of aortic endocarditis with periannular abscess in the International Collaboration on Endocarditis Merged Database. Am. J. Cardiol. 96: 976–981 [DOI] [PubMed] [Google Scholar]
- 4. Bayer AS. 1993. Infective endocarditis. Clin. Infect. Dis. 17: 313–322 [DOI] [PubMed] [Google Scholar]
- 5. Clinical and Laboratory Standards Institute 2008. Performance standards for antimicrobial susceptibility testing. Document M100–S19; 9th informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 6. Craig WA. 2003. Basic pharmacodynamics of antibacterials with clinical applications to the use of beta-lactams, glycopeptides, and linezolid. Infect. Dis. Clin. N. Am. 17: 479–501 [DOI] [PubMed] [Google Scholar]
- 7. Craig WA. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26: 1–12 [DOI] [PubMed] [Google Scholar]
- 8. Drusano GL. 2004. Antimicrobial pharmacodynamics: critical interactions of “bug and drug.” Nat. Rev. Microbiol. 2: 289–300 [DOI] [PubMed] [Google Scholar]
- 9. Engemann JJ, et al. 2005. Clinical outcomes and costs due to Staphylococcus aureus bacteremia among patients receiving long-term hemodialysis. Infect. Control Hosp. Epidemiol. 26: 534–539 [DOI] [PubMed] [Google Scholar]
- 10. Fowler VG, Jr, et al. 2005. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA 293: 3012–3021 [DOI] [PubMed] [Google Scholar]
- 11. Gilot P, Lina G, Cochard T, Poutrel B. 2002. Analysis of the genetic variability of genes encoding the RNA III-activating components Agr and TRAP in a population of Staphylococcus aureus strains isolated from cows with mastitis. J. Clin. Microbiol. 40: 4060–4067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gold M. 1992. Cure rates and long-term prognosis, p 455–464 In Kaye D. (ed), Infective endocarditis. Raven Press, New York, NY [Google Scholar]
- 13. Golper TA, et al. 1988. Vancomycin pharmacokinetics, renal handling, and nonrenal clearances in normal human subjects. Clin. Pharmacol. Ther. 43: 565–570 [DOI] [PubMed] [Google Scholar]
- 14. Healy DP, Polk RE, Garson ML, Rock DT, Comstock TJ. 1987. Comparison of steady-state pharmacokinetics of two dosage regimens of vancomycin in normal volunteers. Antimicrob. Agents Chemother. 31: 393–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hidayat LK, Hsu DI, Quist R, Shriner KA, Wong-Beringer A. 2006. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Arch. Intern. Med. 166: 2138–2144 [DOI] [PubMed] [Google Scholar]
- 16. Hill EE, et al. 2007. Infective endocarditis: changing epidemiology and predictors of 6-month mortality: a prospective cohort study. Eur. Heart J. 28: 196–203 [DOI] [PubMed] [Google Scholar]
- 17. Jeffres MN, et al. 2006. Predictors of mortality for methicillin-resistant Staphylococcus aureus health-care-associated pneumonia: specific evaluation of vancomycin pharmacokinetic indices. Chest 130: 947–955 [DOI] [PubMed] [Google Scholar]
- 18. Li JS, et al. 2000. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin. Infect. Dis. 30: 633–638 [DOI] [PubMed] [Google Scholar]
- 19. Lodise TP, et al. 2008. Relationship between vancomycin MIC and failure among patients with methicillin-resistant Staphylococcus aureus bacteremia treated with vancomycin. Antimicrob. Agents Chemother. 52: 3315–3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maclayton DO, Suda KJ, Coval KA, York CB, Garey KW. 2006. Case-control study of the relationship between MRSA bacteremia with a vancomycin MIC of 2 μg/ml and risk factors, costs, and outcomes in inpatients undergoing hemodialysis. Clin. Ther. 28: 1208–1216 [DOI] [PubMed] [Google Scholar]
- 21. Mansur AJ, et al. 2001. Relapses, recurrences, valve replacements, and mortality during the long-term follow-up after infective endocarditis. Am. Heart J. 141: 78–86 [DOI] [PubMed] [Google Scholar]
- 22. Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. 2004. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin. Pharmacokinet. 43: 925–942 [DOI] [PubMed] [Google Scholar]
- 23. Patel N, et al. 2011. Vancomycin: we can't get there from here. Clin. Infect. Dis. 52: 969–974 [DOI] [PubMed] [Google Scholar]
- 24. Rodvold KA, et al. 1988. Vancomycin pharmacokinetics in patients with various degrees of renal function. Antimicrob. Agents Chemother. 32: 848–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rotschafer JC, et al. 1982. Pharmacokinetics of vancomycin: observations in 28 patients and dosage recommendations. Antimicrob. Agents Chemother. 22: 391–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rybak MJ. 2006. Pharmacodynamics: relation to antimicrobial resistance. Am. J. Med. 119: S37–S70 [DOI] [PubMed] [Google Scholar]
- 27. Sakoulas G, et al. 2002. Accessory gene regulator (agr) locus in geographically diverse Staphylococcus aureus isolates with reduced susceptibility to vancomycin. Antimicrob. Agents Chemother. 46: 1492–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sakoulas G, et al. 2004. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J. Clin. Microbiol. 42: 2398–2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schentag JJ. 2001. Antimicrobial management strategies for Gram-positive bacterial resistance in the intensive care unit. Crit. Care Med. 29: N100–N107 [DOI] [PubMed] [Google Scholar]
- 30. Tenover FC, Moellering RC., Jr 2007. The rationale for revising the Clinical and Laboratory Standards Institute vancomycin minimal inhibitory concentration interpretive criteria for Staphylococcus aureus. Clin. Infect. Dis. 44: 1208–1215 [DOI] [PubMed] [Google Scholar]
- 31. Walsh TR, et al. 2001. Evaluation of current methods for detection of staphylococci with reduced susceptibility to glycopeptides. J. Clin. Microbiol. 39: 2439–2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang JC, Moise-Broder PA. 2008. Predictions of vancomycin exposure based on sparse data or demographics alone. 110th Meeting of the American Society for Clinical Pharmacology and Therapeutics, Orlando, FL [Google Scholar]
- 33. Zokufa HZ, et al. 1989. The influence of serum albumin and α1-acid glycoprotein on vancomycin protein binding in patients with burn injuries. J. Burn Care Rehabil. 10: 425–428 [DOI] [PubMed] [Google Scholar]