Abstract
The clinical Klebsiella pneumoniae INSRA6884 strain exhibited nonsusceptibility to all penicillins tested (MICs of 64 to >2,048 μg/ml). The MICs of penicillins were weakly reduced by clavulanate (from 2,048 to 512 μg/ml), and tazobactam restored piperacillin susceptibility. Molecular characterization identified the genes blaGES-7 and a new β-lactamase gene, blaSHV-107, which encoded an enzyme that differed from SHV-1 by the amino acid substitutions Leu35Gln and Thr235Ala. The SHV-107-producing Escherichia coli strain exhibited only a β-lactam resistance phenotype with respect to amoxicillin, ticarcillin, and amoxicillin-clavulanate combination. The kinetic parameters of the purified SHV-107 enzyme revealed a high affinity for penicillins. However, catalytic efficiency for these antibiotics was lower for SHV-107 than for SHV-1. No hydrolysis was detected against oxyimino-β-lactams. The 50% inhibitory concentration (IC50) for clavulanic acid was 9-fold higher for SHV-107 than for SHV-1, but the inhibitory effects of tazobactam were unchanged. Molecular dynamics simulation suggested that the Thr235Ala substitution affects the accommodation of clavulanate in the binding site and therefore its inhibitory activity.
INTRODUCTION
β-Lactamases are the most important mechanism of β-lactam drug resistance in Gram-negative bacteria. They hydrolyze and inactivate β-lactams and are divided into four major classes (A to D) on the basis of their sequence (1). Class B is composed of metalloenzymes which require the presence of zinc cations for activity; classes A, C, and D are hydrolases with an active-site serine (1, 5). Class A enzymes comprise several enzyme families, including the clinically relevant β-lactamases TEM-1 and SHV-1 (35). These enzymes confer resistance to penicillins and to narrow-spectrum cephalosporins but are inhibited by β-lactamase inhibitors such as clavulanate, which have been developed to evade the activity of these β-lactamases (17, 38).
However, the broad-spectrum β-lactamases TEM-1, TEM-2, and SHV-1 have acquired point mutations in key positions, often near the active site, that have given rise to (i) TEM/SHV-type extended-spectrum β-lactamases (ESBLs), which hydrolyze oxyimino-β-lactams; (ii) TEM/SHV-type inhibitor-resistant β-lactamases; and (iii) complex mutant TEMs (CMTs), which can combine resistance to β-lactam inhibitors and activity against oxyimino-β-lactams (4, 11, 12, 23, 34). In addition, many recent reports have described the emergence of class A ESBLs belonging to other families, such as GES, VEB, PER, and the worldwide-disseminated CTX-M family (2, 21). The GES family, specifically, GES-1, has also been reported in many areas including Portugal (28).
Since 1992, about 35 inhibitor-resistant TEMs (IRTs) and 11 CMTs have been found (http://www.lahey.org/Studies/). However, only six inhibitor-resistant SHVs (IRSs) have been detected clinically so far, mainly in Klebsiella pneumoniae strains, in different countries (8, 13, 14, 26, 31, 37) (Table 1). No complex mutant SHV has been encountered, which is surprising since SHV enzymes are usually more susceptible to inactivation by clavulanate than TEM, due to differences in the enzyme active sites (4, 11). In the SHV family, naturally occurring inhibitor-resistant phenotypes harbor substitutions at positions Met69 (SHV-49), Ser130 (SHV-10), Arg187 (SHV-26), and Lys234 (SHV-56, SHV-72, and SHV-84) (numbering according to the Ambler classification) (1, 11) (Table 1). Further investigations of most enzymes and the corresponding substitutions have been performed. However, the recently reported enzyme SHV-107 (AM941848) (28), which is suspected to belong to the IRS group, has not been characterized.
Table 1.
Comparison of amino acid substitutions, inhibitory properties, and epidemiology of IRS β-lactamases
| β-Lactamase | Amino acid at position no.:a |
IC50 (μM) (CLA/TAZ)b | Strainc | Country (yr)d | Reference(s) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 8 | 35 | 54 | 69 | 130 | 140 | 146 | 187 | 192 | 193 | 234 | 235 | 238 | 240 | |||||
| SHV-1 | I | L | G | M | S | A | A | A | K | L | K | T | G | E | 0.17/0.11 | K. pneumoniae | Switzerland (1972) | 36 |
| SHV-10 | Del | G | R | N | V | S | K | 6.9/1.3 | E. coli | Greece (1997) | 37 | |||||||
| SHV-26 | T | 0.48/NDe | K. pneumoniae | Taiwan (2001) | 8 | |||||||||||||
| SHV-49 | I | 1.5/2.5 | K. pneumoniae | France (2004) | 13 | |||||||||||||
| SHV-56 | Q | R | 2.5/0.75 | K. pneumoniae | France (2008) | 14 | ||||||||||||
| SHV-72 | F | V | R | 1.72/0.08 | K. pneumoniae | Portugal (2008) | 31 | |||||||||||
| SHV-84 | R | 2.21/0.03 | E. coli | Portugal (2010) | 26 | |||||||||||||
| SHV-107 | Q | A | 1.53/0.11 | K. pneumoniae | Portugal (2009) | 28; this study | ||||||||||||
Numbering according to Ambler et al. (1).
Values of clavulanate (CLA) and tazobactam (TAZ) for the different IRS β-lactamases. SHV-1, SHV-72, and SHV-84, SHV-10, and SHV-107 values were determined using 200 μM ticarcillin, 50 mM nitrocefin, and 200 μM penicillin, respectively, which were incubated with the inhibitor for 5 min at 37°C at pH 7; SHV-49 and SHV-56 values were determined by benzylpenicillin (100 μM) hydrolysis after 3 min of preincubation at 30°C; and SHV-26 values were determined after 10 min of preincubation of nitrocefin with the clavulanate at room temperature. IC50, 50% inhibitory concentration.
Strain producing IRS β-lactamase, in the first report.
Country that first reported the β-lactamase, and year (in parentheses).
ND, not determined.
In this study, we report in a clinical K. pneumoniae strain the coexpression of the ESBL GES-7 and a new IRS-type enzyme called SHV-107, which, compared to SHV-1, harbors the substitutions Leu35Gln and Thr235Ala. The kinetic constants of SHV-107 were determined, and molecular modeling was undertaken to investigate the role of Thr235Ala in the resistance to clavulanate.
MATERIALS AND METHODS
Bacterial strains and plasmid.
K. pneumoniae INSRA6884 was isolated in 2006 from the sputum of a 92-year-old woman in an internal medicine service at the Centro Hospitalar in Coimbra, Portugal (28). The Escherichia coli DH5α ΔampC strain and E. coli BL21(DE3) were used for initial cloning experiments and overexpression of the β-lactamase-encoding gene, respectively. E. coli C600 was used for mating-out assays. Plasmid pBK-CMV (Stratagene, Amsterdam, The Netherlands) was used for cloning experiments (9).
Antimicrobial susceptibility testing and ESBL detection.
An agar dilution method and criteria according to the French Society of Microbiology (SFM) (7) were used to determine the MICs of the antibiotics listed in Table 2.
Table 2.
MICs of β-lactam antibiotics for the K. pneumoniae clinical strain producing the SHV-107 enzyme, recipient, and transformanta
| Antimicrobial drug | MIC (μg/ml) for strain: |
||||
|---|---|---|---|---|---|
| K. pneumoniae INSRA6884 (SHV-107 + GES-7) | E. coli BL21(DE3) | E. coli BL21(DE3)(pBK-SHV-107) | E. coli C600 | E. coli C600 (GES-7) | |
| Amoxicillin | 2,048 | ≤2 | 256 | ≤2 | 256 |
| Amoxicillin + CLAb | 512 | ≤2 | 64 | ≤2 | 8 |
| Ticarcillin | >2,048 | ≤2 | 128 | ≤2 | 2,048 |
| Piperacillin | 64 | 1 | 8 | 0.5 | 4 |
| Piperacillin + TAZc | 4 | 1 | 1 | 0.5 | 0.5 |
| Amdinocillin | 1 | ≤0.015 | ≤0.015 | ≤0.015 | 0.25 |
| Cephalothin | 128 | 1 | 1 | 4 | 32 |
| Cefuroxime | 64 | 0.5 | 1 | 2 | 16 |
| Cefoperazone | 8 | ≤0.25 | ≤0.25 | ≤0.25 | 0.5 |
| Ceftazidime | 128 | 0.06 | 0.06 | 0.06 | 32 |
| Ceftazidime + CLA | 8 | 0.03 | 0.03 | ≤0.015 | 0.5 |
| Ceftriaxone | 4 | ≤0.015 | ≤0.015 | ≤0.015 | 0.5 |
| Ceftriaxone + CLA | 0.5 | ≤0.015 | ≤0.015 | ≤0.015 | ≤0.015 |
| Cefotaxime | 4 | ≤0.015 | ≤0.015 | 0.03 | 0.5 |
| Cefotaxime + CLA | 0.25 | ≤0.015 | ≤0.015 | ≤0.015 | 0.03 |
| Aztreonam | 2 | ≤0.015 | ≤0.015 | 0.06 | 1 |
| Aztreonam + CLA | 0.125 | ≤0.015 | ≤0.015 | 0.03 | 0.03 |
| Cefepime | 0.25 | ≤0.015 | ≤0.015 | ≤0.015 | 0.06 |
| Cefoxitin | 4 | 2 | 2 | 2 | 2 |
| Imipenem | 0.25 | 0.25 | 0.25 | ≤0.06 | ≤0.06 |
| Kanamycin | 4 | ≤0.125 | ≤0.125 | ≤0.125 | ≤0.125 |
| Nalidixic acid | 4 | ≤0.003 | ≤0.003 | 4 | 4 |
| Ciprofloxacin | 0.03 | ≤0.003 | ≤0.003 | 0.007 | 0.007 |
| Trimethoprim | 0.5 | ≤0.125 | ≤0.125 | ≤0.125 | ≤0.125 |
E. coli BL21(DE3)(pBK-SHV-107) and E. coli C600 (GES-7) were a transformant and conjugant, respectively, of K. pneumoniae INSRA6884 (harboring SHV-107 plus GES-7 enzyme).
CLA, clavulanate at a fixed concentration of 2 μg/ml.
TAZ, tazobactam at a fixed concentration of 4 μg/ml.
Analytical IEF.
β-Lactamases were characterized by isoelectric focusing (IEF) as previously described (6), with strains E. coli C600 (SHV-1, pI 7.6), K. pneumoniae INSRA5767 (TEM-24, pI 6.5, and SHV-1, pI 7.6), and E. coli INSRA7813 (GES-1, pI 5.8) as standards.
DNA amplification and sequencing of β-lactamase genes.
β-Lactamase genes blaSHV, blaOXA, blaTEM, blaCTX, and blaGES were detected by PCR amplification and then purified and sequenced as previously described (28).
blaGES-7 gene transfer.
Direct transfer of the β-lactam resistance phenotype and selection of transconjugants were performed as described previously (29).
Cloning.
The blaSHV-107 gene was cloned in the plasmid pBK-CMV and transferred as previously described (31). The recombinant pBK-SHV-107 plasmid was transformed by electroporation into the E. coli BL21(DE3) strain. Transformants were selected with 30 μg/ml of kanamycin and 50 μg/ml of amoxicillin. The orientation of the inserted genes was confirmed as described elsewhere (31).
Purification of β-lactamase.
SHV-107 was produced from E. coli BL21(DE3) in LB broth, supplemented with 30 μg/ml kanamycin, 16 μg/ml ticarcillin, and 0.1 mM IPTG (isopropyl-β-d-thiogalactopyranoside; Sigma Chemical Co., St. Louis, MO), overnight at 30°C. The enzyme was extracted by ultrasonic treatment, and the clarified supernatant was purified as described elsewhere (3, 31).
Determination of β-lactamase kinetic constants.
The Michaelis constant (Km) and catalytic activity (kcat) of SHV-107 and the concentrations of the inhibitors (clavulanate and tazobactam) required to inhibit enzyme activity by 50% (IC50s) were determined as previously described (3, 31). Specific activity and IC50s were monitored with penicillin G (200 μM) as the reporter substrate. The inhibitor kinetics with clavulanate were determined under steady-state conditions for SHV-1 and SHV-107 as previously described (18, 27). The enzyme activity was monitored with a UV-1800 spectrophotometer (Shimadzu, Champs sur Marne, France) in phosphate-buffered saline (PBS) 20 mM (pH 7.4) at 25°C by using 100 μM chromogenic substrate nitrocephin (Δε, 17,400 M−1 cm−1 at 482 nm) (Sigma-Aldrich, France) as the reporter substrate. The kinetic constants were determined three times for each subtract tested.
Molecular modeling.
The structural model of SHV-107 was constructed from the crystal structure of SHV-1 using Chimera (24). Molecular dynamics simulations (MDSs) were performed as previously described with GROMACS software package, version 4.0 (40), using the parameters of the OPLSAA force field (22) in a periodic cubic box, as previously described (10). The AutoDock 4.0 package was used to perform molecular docking (33). Coordinates for clavulanate structure were obtained using CACTVS (http://www2.ccc.uni-erlangen.de/software/cactvs/) and the CORINA server (19). Structure optimization was calculated at the 6-31G** level using the Gamess quantum chemistry software (39). All the torsion angles in clavulanate were set free to perform flexible docking, and the Gasteiger-Hückel atomic charge was assigned. Kollman united atom partial charges were assigned for the receptor, which was kept rigid during the docking study. Water molecules, ions, and ligands were removed, except the catalytic water molecule. The empirical free-energy function and Lamarckian genetic algorithm were used for docking with the following settings: a maximum number of 25,000,000 energy evaluations, an initial population of 150 randomly placed individuals, a maximum number of 27,000 generations, a mutation rate of 0.02, a crossover rate of 0.80, and an elitism value (number of top individuals that automatically survive) of 1. For the local search, the Solis and Wets algorithm was used with a maximum of 300 iterations per search; 100 independent docking runs were performed for each docking experiment. Results were clustered according to default root mean square deviation (RMSD) criteria.
RESULTS AND DISCUSSION
General characteristics of K. pneumoniae INSRA6884.
In this study, we characterized a clinical K. pneumoniae strain isolated in a Portuguese hospital coexpressing two different β-lactamases, the IRS enzyme SHV-107 and the ESBL GES-7, of pIs 7.5 and 6.9, respectively.
Phenotypic characterization.
The transconjugant E. coli C600 (GES-7) acquired resistance only to amoxicillin, ticarcillin, cephalothin, cefuroxime, and ceftazidime, in contrast to the clinical strain, which was resistant to all penicillins and all cephalosporins tested except cefepime and cefoxitin (Table 2). The addition of clavulanate drastically reduced the MIC value of oxyimino-cephalosporins and aztreonam in both the transconjugant and the clinical strains. Like the clinical strain, E. coli BL21(DE3)(pBK-SHV-107) acquired resistance to amoxicillin and ticarcillin and exhibited a synergy of amoxicillin and piperacillin in combination with clavulanate (2-fold) and tazobactam (8-fold), respectively (Table 2).
β-lactamase analysis.
We report for the first time the GES-7 β-lactamase isolated in Portugal. Although not associated with class 1 integrons (data not shown), the gene encoding this β-lactamase was present in a conjugative plasmid. The deduced amino sequence of blaGES-7 differed from that of GES-1 (1) by Glu107Lys and Leu125Ala substitutions (Ambler numbering). SHV-107 enzyme, an IRS β-lactamase (IC50 ≥ 1 μM), is the seventh member of this subgroup, which is characterized by the expression of resistance to clavulanate (Table 1). This enzyme differed from SHV-1 by the amino acid substitutions Leu35Gln and Thr235Ala (Table 1) (28). The first substitution, Leu35Gln, frequently encountered and observed alone in the SHV-11 enzyme (30), is known to have no significant impact on kinetic constants (32). To our best knowledge, the second substitution is the first natural substitution reported at position 235 in class A enzymes (Table 1). In these enzymes, Thr235 residue is part of a conserved element (Lys234-Ser/Thr235-Gly236). It is situated on the β3 strand of a β-sheet in the α/β domain and forms a major wall of the catalytic cavity site (10). The hydroxyl function of Ser/Thr-235 contributes to the binding of the conserved C3/C4 carboxylate function of β-lactams (41).
Enzymatic parameters and properties of β-lactamase SHV-107.
After purification by ion exchange and gel filtration, the purity of β-lactamase SHV-107 was estimated to be ≥98% on a sodium dodecyl sulfate-polyacrylamide gel. The band of 28.8 kDa corresponds to the molecular mass deduced from the amino acid sequence (data not shown).
We observed that the substitution Thr235Ala altered SHV-107 kinetic constants against β-lactams without dramatic effects on the resistance to penicillins. Two-fold changes or less between the SHV-1 and SHV-107 β-lactamases were obtained for Km values for penicillins (11 to 31 μM and 11 to 42 μM, respectively) (Table 3). Catalytic efficiency against penicillins was 2- to 9-fold lower for SHV-107 (kcat/Km, 2.2 to 34.4 μM−1 · s−1) than for SHV-1 (kcat/Km, 20.0 to 84.2 μM−1 · s−1) (Table 3). Amoxicillin, with a kcat value of 657 s−1, was the best substrate for SHV-107. Likewise, previous enzymatic studies of TEM-1 and its isogenic mutant Ser235Ala showed similar results for penicillins, although the mutant had greatly reduced cephalosporinase activity (15, 20). In the SHV β-lactamase family, residue Thr235 is also critical for cephalosporinase activity, because its replacement by Ala235 in SHV-107 leads to severe impairment of the catalytic constant against cephalosporins, as observed in TEM-type enzyme (15, 20). Km values were 4-fold higher for cephalothin or not determinable for the other cephalosporins, because the hydrolysis rate was too low.
Table 3.
Comparative kinetic parameters for SHV-1 and SHV-107 β-lactamases
| Antibiotic | Kinetic parameters of enzyme |
|||||
|---|---|---|---|---|---|---|
| SHV-1b |
SHV-107c |
|||||
| Km (μM)a | kcat (s−1)a | kcat/Km (μM−1 · s−1) | Km (μM)a | kcat (s−1)a | kcat/Km (μM−1 · s−1) | |
| Penicillin G | 23 ± 0.4 | 1,937 ± 82 | 84 | 11 ± 0.9 | 378 ± 6 | 34 |
| Amoxicillin | 31 ± 1 | 1,044 ± 10 | 34 | 42 ± 4 | 657 ± 10 | 16 |
| Ticarcillin | 11 ± 3 | 220 ± 49 | 20 | 23 ± 12 | 51 ± 7 | 2 |
| Piperacillin | 24 ± 0.5 | 1,490 ± 96 | 62 | 31 ± 0.4 | 412 ± 87 | 13 |
| Cephalothin | 40 ± 1 | 128 ± 33 | 3 | 179 ± 9 | <0.1 | <0.001 |
| Ceftazidime | 142 ± 3 | <0.1 | NDd | ND | <0.1 | ND |
| Cefotaxime | 257 ± 21 | <0.1 | ND | ND | <0.1 | ND |
Values are means ± standard deviations.
The kinetic constants for SHV-1 are from reference 31.
E. coli BL21(DE3)(pBK-SHV-107) was the transformant producing SHV-107.
ND, not determinable, because the hydrolysis rates were too low.
It is noteworthy that amoxicillin, ticarcillin, and piperacillin have similar kinetic parameters, but production of SHV-107 β-lactamase increases the MIC by factors of >128, >64, and 8, respectively. In contrast, Ala235 residue, although it contributes to a loss of activity, seems to have no influence in the MIC of cephalothin. These discrepancies between phenotypic and biochemical values could result from the fact that the MIC of a β-lactam for β-lactamase-producing bacteria correlates not only the catalytic efficiency of the enzyme but also its concentration in the periplasm and the penetration of the antibiotic through the outer membrane, as noted before (25).
IC50 results showed that tazobactam was 14-fold more active than clavulanate against the SHV-107 enzyme, as it has a triazole group at the C2 β-methyl position, which led to the improvement of IC50s (11) (Table 1). IC50s of clavulanate were 9-fold higher for SHV-107 than for SHV-1, and no loss of sensitivity to tazobactam was observed. The Thr235Ala substitution affected the susceptibility to clavulanate and consequently the susceptibility to the combination of amoxicillin-clavulanate. Similarly, three IRS enzymes, SHV-56, SHV-72, and SHV-84, which presented an amino acid substitution in the vicinity of position 235, showed decreased susceptibility to clavulanate (14, 26, 31). Molecular dynamics simulations suggested that the Lys234Arg substitution observed in this mutant probably affects the positioning of the Ser130 side chain, a key element in the inhibition reaction mediated by clavulanate (31).
SHV-1 and SHV-107 inhibitor kinetics with clavulanate were further investigated (Table 4). Ki for clavulanate was almost 9-fold higher for SHV-107 than for SHV-1 (1.82 μM versus 0.21 μM). kinact (inactivation constant) and kcat values were closely similar for both enzymes. Consequently, the second-order rate constants, kinact/Ki and kcat/Ki, were 10-fold lower for SHV-107 than for SHV-1, because of the increase in Ki.
Table 4.
Clavulanate kinetic parameters of SHV-1 and SHV-107 β-lactamases
| β-Lactamase | Ki (μM)a | kinact (s−1)a | kinact/Ki (μM−1 · s−1) | kcat (s−1)a | kcat/Ki (μM−1 · s−1) | kcat/kinact |
|---|---|---|---|---|---|---|
| SHV-1 | 0.21 ± 0.06 | 0.050 ± 0.007 | 0.23 | 0.94 ± 0.13 | 4.48 | 19 |
| SHV-107 | 1.82 ± 0.17 | 0.042 ± 0.009 | 0.02 | 0.75 ± 0.11 | 0.41 | 18 |
Values are means ± standard deviations.
Molecular modeling.
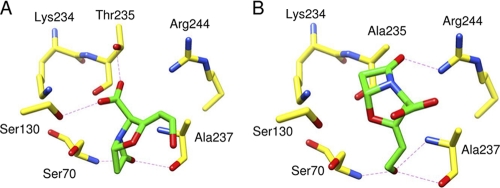
We investigated the recognition of clavulanate by the SHV-107 binding site using molecular modeling tools to explain the role of the Thr235Ala substitution in resistance against clavulanate. The introduction of the Thr235Ala substitution caused no overall or large-scale deviation of dynamic properties. Likewise, the survey of residues and water molecules located in the vicinity of position 235 revealed no dramatic change in this area (data not shown). For SHV-1, all docking experiments provided a similar conformation and positioning of clavulanate in the catalytic pocket (RMSD value for clavulanate atoms of <1 Å). The oxygen atom of the β-lactam carbonyl function was at a hydrogen bond distance of the Ser70 and Ala237 N atoms (2.9 and 2.8 Å, respectively), which form the oxyanion hole. The C3 carboxylate function of clavulanate established electrostatic interaction with Ser130 and Thr235 (2.7 to 2.9 Å). For SHV-107, 98% of clavulanate-docking experiments provided similar positionings of clavulanate in the SHV-107 catalytic pocket (RMSD value for clavulanate atoms of <1 Å). However, the positioning of clavulanate in SHV-107 was clearly different from that in SHV-1. Clavulanate was flipped (Fig. 1). Consequently, the oxygen atom of the β-lactam carbonyl function did not interact with the oxyanion hole. Instead, Ser70 and Ala237 N atoms and the Ala237 O atom shared hydrogen bonds with the C2 substituent of clavulanate (2.8 to 3.0 Å), and the β-lactam ring was accommodated in the vicinity of position 244. It emerges from these results that Thr235 favors the interaction of β-lactam carbonyl function with the oxyanion hole. This interaction is a key factor in active serine acylation by clavulanate and therefore in the inhibition process mediated by this inhibitor. The Thr235Ala substitution may therefore affect the correct orientation of clavulanate in the binding site and consequently the inhibitory activity of clavulanate. This possibility is supported by the increase in clavulanate Ki with SHV-107.
Fig 1.
Clavulanate positioning in SHV-1 (A) and SHV-107 (B) binding sites after docking experiments using AutoDock. Carbon atoms are in yellow for the protein or in green for clavulanate; oxygen atoms are red and nitrogen atoms blue. Hydrogen bonds are indicated by dashed lines.
Conclusion.
Our findings show that mutations at Thr235 of the SHV-1 β-lactamase contribute to decreased susceptibility to β-lactamase inhibitors. Given the increasing number of new enzymes conferring resistance to β-lactamase inhibitors, further investigations should be undertaken on IRS enzymes. This emergence may be the consequence of the widespread use of penicillins with β-lactamase inhibitors (J01CR) in different European countries, a practice that needs to be reassessed and modified (16).
ACKNOWLEDGMENTS
We thank Deolinda Louro for technical assistance.
This work was supported financially by the project POCTI/ESP/43037 from Fundação para a Ciência e a Tecnologia, Lisbon, Portugal, awarded to M. Caniça, and by a grant from INRA and Ministère de l'Education Nationale, de l'Enseignement Supérieur et de la Recherche (Paris, France), awarded to R. Bonnet. V. Manageiro was supported by grant SFRH/BD/32578/2006 from Fundação para a Ciência e a Tecnologia, Lisbon, Portugal.
Footnotes
Published ahead of print 14 November 2011
REFERENCES
- 1. Ambler RP, et al. 1991. A standard numbering scheme for class A β-lactamases. Biochem. J. 276: 269–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bonnet R. 2004. Growing group of extended-spectrum β-lactamases: the CTX-M enzymes. Antimicrob. Agents Chemother. 48: 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bonnet R, et al. 2001. Novel cefotaximase (CTX-M-16) with increased catalytic efficiency due to substitution Asp-240→Gly. Antimicrob. Agents Chemother. 45: 2269–2275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bonomo RA, Rice LB. 1999. Inhibitor resistant class A β-lactamases. Front. Biosci. 4: e34–e41 [DOI] [PubMed] [Google Scholar]
- 5. Bush K, Jacoby JA. 2010. An updated functional classification of β-lactamases. Antimicrob. Agents Chemother. 54: 969–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Caniça MM, et al. 1997. Properties of IRT-14 (TEM-45), a newly characterized mutant of TEM-type β-lactamases. Antimicrob. Agents Chemother. 41: 374–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cavallo JD, et al. 2009. Recommandations. 2009. Comité de l'antibiogramme de la Société Française de Microbiologie, Paris, France [Google Scholar]
- 8. Chang F-Y, Siu LK, Fung C-P, Huang M-H, Ho M. 2001. Diversity of SHV and TEM β-lactamase in Klebsiella pneumoniae: gene evolution in northern Taiwan and two novel β-lactamases, SHV-25 and SHV-26. Antimicrob. Agents Chemother. 45: 2407–2413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Delmas J, Robin F, Carvalho F, Mongaret C, Bonnet R. 2006. Prediction of the evolution of ceftazidime resistance in extended-spectrum β-lactamase CTX-M-9. Antimicrob. Agents Chemother. 50: 731–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Delmas J, et al. 2008. Structure and dynamics of CTX-M enzymes reveal insights into substrate accommodation by extended-spectrum β-lactamases. J. Mol. Biol. 375: 192–201 [DOI] [PubMed] [Google Scholar]
- 11. Drawz SM, Bonomo RA. 2010. Three decades of β-lactamase inhibitors. Clin. Microbiol. Rev. 23: 160–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Du Bois SK, Marriott MS, Amyes SG. 1995. TEM- and SHV-derived extended-spectrum β-lactamases: relationship between selection, structure, and function. J. Antimicrob. Chemother. 35: 7–22 [DOI] [PubMed] [Google Scholar]
- 13. Dubois V, et al. 2004. SHV-49, a novel inhibitor resistant β-lactamase in a clinical isolate of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 48: 4466–4469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dubois V, et al. 2008. Molecular and biochemical characterization of SHV-56, a novel inhibitor-resistant β-lactamase from Klebsiella pneumoniae. Antimicrob. Agents Chemother. 52: 3792–3794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dubus A, Wilkin JM, Raquet X, Normark S, Frère JM. 1994. Catalytic mechanism of active-site serine β-lactamases: role of the conserved hydroxy group of the Lys-Thr(Ser)-Gly triad. Biochem. J. 301: 485–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ferech M, et al. 2006. European Surveillance of Antimicrobial Consumption (ESAC): outpatient antibiotic use in Europe. J. Antimicrob. Chemother. 58: 401–407 [DOI] [PubMed] [Google Scholar]
- 17. Finlay J, Miller L, Poupard JA. 2003. A review of the antimicrobial activity of clavulanate. J. Antimicrob. Chemother. 52: 18–23 [DOI] [PubMed] [Google Scholar]
- 18. Frère JM, Dormans C, Duyckaerts C, De Graeve J. 1982. Interaction of β-iodopenicillanate with the beta-lactamases of Streptomyces albus G and Actinomadura R39. Biochem. J. 207: 437–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gasteiger J, Rudolph C, Sadowski J. 1990. Automatic generation of 3D-atomic coordinates for organic molecules. Tetrahedron Comput. Methods 3: 537–547 [Google Scholar]
- 20. Imtiaz U, Manavathu EK, Lerner SA, Mobashery S. 1993. Critical hydrogen bonding by serine 235 for cephalosporinase activity of TEM-1 β-lactamase. Antimicrob. Agents Chemother. 37: 2438–2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jacoby GA, Munoz-Price LS. 2005. The new β-lactamases. N. Engl. J. Med. 352: 380–391 [DOI] [PubMed] [Google Scholar]
- 22. Jorgensen WL, Maxwell DS, Tirado-Rives J. 1996. Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc. 118: 11225–11236 [Google Scholar]
- 23. Knox JR. 1995. Extended-spectrum and inhibitor-resistant TEM-type β-lactamases: mutations, specificity, and three-dimensional structure. Antimicrob. Agents Chemother. 39: 2593–2601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kuzin AP, et al. 1999. Structure of the SHV-1 β-lactamase. Biochemistry 38: 5720–5727 [DOI] [PubMed] [Google Scholar]
- 25. Lakaye B, Dubus A, Lepage S, Groslambert S, Frère J-M. 1999. When drug inactivation renders the target irrelevant to antibiotic resistance: a case story with β-lactams. Mol. Microbiol. 31: 89–101 [DOI] [PubMed] [Google Scholar]
- 26. Manageiro V, Ferreira E, Albuquerque L, Bonnet R, Caniça M. 2010. Biochemical study of a new inhibitor-resistant β-lactamase, SHV-84, produced by a clinical Escherichia coli strain. Antimicrob. Agents Chemother. 54: 2271–2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Matagne A, et al. 1995. Kinetic study of interaction between BRL 42715, β-lactamases, and d-alanyl-d-alanine peptidases. Antimicrob. Agents Chemother. 39: 227–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mendonça N, Ferreira E, Louro D, ARSIP participants. Caniça M. 2009. Molecular epidemiology and antimicrobial susceptibility of extended- and broad-spectrum β-lactamase-producing Klebsiella pneumoniae isolated in Portugal. Int. J. Antimicrob. Agents 34: 29–37 [DOI] [PubMed] [Google Scholar]
- 29. Mendonça N, Ferreira E, Louro D, Caniça M. 2006. Occurrence of a novel SHV-type enzyme (SHV-55) among isolates of Klebsiella pneumoniae from Portuguese origin in a comparison study for extended-spectrum β-lactamase-producing evaluation. Diagn. Microbiol. Infect. Dis. 56: 415–420 [DOI] [PubMed] [Google Scholar]
- 30. Mendonça N, Nicolas-Chanoine M, Caniça M. 2009. Diversity of the blaSHV genes. Diagn. Microbiol. Infect. Dis. 65: 439–446 [DOI] [PubMed] [Google Scholar]
- 31. Mendonça N, et al. 2008. The Lys234Arg substitution in the enzyme SHV-72 is a determinant for resistance to clavulanate inhibition. Antimicrob. Agents Chemother. 52: 1806–1811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nuesch-Inderbinen MT, Kayser FH, Hachler H. 1997. Survey and molecular genetics of SHV β-lactamases in Enterobacteriaceae in Switzerland: two novel enzymes, SHV-11 and SHV-12. Antimicrob. Agents Chemother. 41: 943–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Osterberg F, Morris GM, Sanner MF, Olson AJ, Goodsell DS. 2002. Automated docking to multiple target structures: incorporation of protein mobility and structural water heterogeneity in AutoDock. Proteins 46: 34–40 [DOI] [PubMed] [Google Scholar]
- 34. Page MG. 2008. Extended-spectrum β-lactamases: structure and kinetic mechanism. Clin. Microbiol. Infect. 14: 63–74 [DOI] [PubMed] [Google Scholar]
- 35. Paterson DL, Bonomo RA. 2005. Extended-spectrum β-lactamases: a clinical update. Clin. Microbiol. Rev. 18: 657–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pitton JS. 1972. Mechanism of the bacterial resistance to antibiotics, p 15–93 In Adrian RH, et al. (ed), Reviews of physiology, vol 65 Springer, Berlin, Germany: [DOI] [PubMed] [Google Scholar]
- 37. Prinarakis EE, Miriagou V, Tzelepi E, Gazouli M, Tzouvelekis LS. 1997. Emergence of an inhibitor-resistant β-lactamase (SHV-10) derived from an SHV-5 variant. Antimicrob. Agents Chemother. 41: 838–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Reading C, Cole M. 1977. Clavulanate: a β-lactamase-inhibiting β-lactam from Streptomyces clavuligerus. Antimicrob. Agents Chemother. 11: 852–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schmidt MW, Jr, et al. 2004. General atomic and molecular electronic structure system. J. Comput. Chem. 14: 1347–1363 [Google Scholar]
- 40. Van Der Spoel D, et al. 2005. GROMACS: fast, flexible, and free. J. Comput. Chem. 26: 1701–1718 [DOI] [PubMed] [Google Scholar]
- 41. Zafaralla G, Manavathu EK, Lerner SA, Mobashery S. 1992. Elucidation of the role of arginine-244 in the turnover processes of class A β-lactamases. Biochemistry 31: 3847–3852 [DOI] [PubMed] [Google Scholar]