Abstract
In preclinical testing of antituberculosis drugs, laboratory-adapted strains of Mycobacterium tuberculosis are usually used both for in vitro and in vivo studies. However, it is unknown whether the heterogeneity of M. tuberculosis stocks used by various laboratories can result in different outcomes in tests of antituberculosis drug regimens in animal infection models. In head-to-head studies, we investigated whether bactericidal efficacy results in BALB/c mice infected by inhalation with the laboratory-adapted strains H37Rv and Erdman differ from each other and from those obtained with clinical tuberculosis strains. Treatment of mice consisted of dual and triple drug combinations of isoniazid (H), rifampin (R), and pyrazinamide (Z). The results showed that not all strains gave the same in vivo efficacy results for the drug combinations tested. Moreover, the ranking of HRZ and RZ efficacy results was not the same for the two H37Rv strains evaluated. The magnitude of this strain difference also varied between experiments, emphasizing the risk of drawing firm conclusions for human trials based on single animal studies. The results also confirmed that the antagonism seen within the standard HRZ regimen by some investigators appears to be an M. tuberculosis strain-specific phenomenon. In conclusion, the specific identity of M. tuberculosis strain used was found to be an important variable that can change the apparent outcome of in vivo efficacy studies in mice. We highly recommend confirmation of efficacy results in late preclinical testing against a different M. tuberculosis strain than the one used in the initial mouse efficacy study, thereby increasing confidence to advance potent drug regimens to clinical trials.
INTRODUCTION
With nearly 10 million new cases in 2010, tuberculosis (TB) continues to be a global health issue of the greatest magnitude (11). There is a critical need to discover new, more potent drugs to combat TB, the control of which is further complicated due to the increasing incidence of drug-resistant forms of Mycobacterium tuberculosis, now thought to exceed half a million new cases a year (48, 49). In addition, with new sensitive diagnostic tests for drug resistance, there will certainly be an upsurge in reported cases of drug-resistant TB (4). Equally troubling is the newly emerging evidence suggesting that a number of drug-resistant clinical isolates of M. tuberculosis can be of high virulence and infectivity, thereby spreading readily (17, 42–45). Therefore, there is a need to increase drug discovery efforts as well as to improve testing methods to identify new active compounds against clinically relevant M. tuberculosis strains. Typically, a series of preclinical studies with new chemical entities must be completed before a drug can advance to clinical trials. A sequence of in vitro assays followed by in vivo testing in validated animal models to assess the activity against M. tuberculosis, the pharmacology, and the toxicity is generally used for advancing compounds in a preclinical stage (31, 41). Generally, these preclinical studies are performed with historic laboratory-adapted M. tuberculosis strains such as H37Rv (ATCC 25618 and 27294) (22, 29) or Erdman (ATCC 35801) (9). M. tuberculosis is a member of the M. tuberculosis complex, and genetic diversity has recently been linked to clinical, pathogenic, and immunologic heterogeneity in disease progression and outcomes (5, 12, 30). Strains are currently being classified into six major phylogenic clades: East African, East Asian, Euro-American, Indo-Oceanic, and two West African (M. africanum) types (13, 14). Several studies have reported different biological, clinical, or epidemiological behaviors of the various strains, defined by their geographical location or strain type (7, 19, 21, 40). In addition, strains have also been shown to vary significantly in virulence in animal infection models, as measured by survival, bacterial loads in target organs, histopathology (3, 43), and the severity of pulmonary and extrapulmonary lesions, as well as the immune response engendered after infection (35). Various mycobacterial strains have shown a significant difference in expression of virulence genes involved in key cell wall macromolecules [such as phthiocerol dimycocerosate (PDIMS)] (22). Differences have also been observed in the various mannooligosaccharide units of lipoarabinomannans, which may translate to an altered pathobiology in the host (6). All these recent reports show that M. tuberculosis strains are more genetically diverse than was previously recognized (18, 23–26). There are major genetic regions of difference between strains, including mutations in regions encoding enzymes involved in the biosynthesis of surface PDIMS, and this occurs even within strains with the designation H37Rv. Furthermore, there are variations now described within region of difference 6 between Erdman and H37Rv, which highlights a divergence among TB strains that extends beyond those previously recognized (22, 34).
At least 10 new anti-TB drugs are currently in the pipeline for clinical evaluation (47); therefore, it is more crucial than ever to have reliable, validated testing animal models in order to build confidence that new drug combination regimens will perform better than the standard regimen in humans as it does in the animal model. Methods for preclinical animal model testing of drugs against M. tuberculosis vary from laboratory to laboratory; however, it was unknown until recently whether these variations can result in different outcomes. Our earlier work demonstrated that certain experimental variables in preclinical evaluations (such as the mouse strain, route of infection, drug formulations for rifampin, and dosing schedule) did not necessarily affect the result of mouse bactericidal efficacy trials for the TB drug combinations evaluated (9). By performing these head-to-head mouse studies, we were still unable to resolve the discrepancy seen among laboratories regarding the reported antagonism between the standard drugs isoniazid (H), rifampin (R), and pyrazinamide (Z), which has been observed in some (1, 37, 38) but not all (9, 20) laboratories. In our published head-to-head studies, the combination HRZ was significantly more effective than RZ dual therapy in all three mouse models evaluated (9), and we did not observe antagonism of H in the HRZ combination in the three infection models evaluated. Antagonism between the three standard drugs has been shown by other groups (1, 15), who demonstrated that the dual regimen of RZ after removal of H performed better than the standard three-drug regimen HRZ. The significance of the antagonism becomes apparent when a “new” drug regimen is being compared to a “less-effective” drug regimen prior to clinical trials. An example of the latter is mouse studies where moxifloxacin (M), substituted for isoniazid (INH), showed significant improvement in efficacy over standard therapy (HRZ), and most of this benefit was in fact attributed to removal of the antagonism between H and R plus Z (41). Our animal data showed that the activity of the moxifloxacin-containing regimen (MRZ) was similar to that of the standard drug regimen (HRZ), which more accurately recapitulates the outcome of the human trial TBTC 28 (TB Trials Consortium Study 28) (10).
The discrepancy between laboratories in observing antagonism between drugs in the HRZ regimen formed the premise of these studies. As several other parameters had already been evaluated in earlier studies, the use of different M. tuberculosis strains for the infection of mice by aerosol was addressed. We focused on two of the most widely used laboratory-adapted M. tuberculosis strains (H37Rv and Erdman), as well as two clinical strains (CDC1551 and HN878). The results described herein make the importance of the M. tuberculosis strain (and the stocks from which it arose) used in drug efficacy trials apparent.
MATERIALS AND METHODS
Mice.
Female BALB/c (Charles River Laboratories, Wilmington, MA) between the ages of 6 and 8 weeks were housed at five animals per cage in HEPA (high-efficiency particulate air)-filtered racks (Thoren Caging Systems Inc., Hazleton, PA) in certified animal biosafety level 3 (ABSL-3) laboratories and rested for at least 2 weeks before infection with M. tuberculosis.
Bacterial strains.
The M. tuberculosis strains used were provided by the following institutions: H37Rv is the main strain used at Johns Hopkins University (JHU); H37Rv was from Colorado State University (CSU) stocks; Erdman (TMC 107; ATCC 35801) is the main strain used at CSU; and CDC 1551 and HN878 were kindly provided by K. Dobos and A. Izzo at CSU (contract HHSN26620040091C). The M. tuberculosis H37Rv strain derived from JHU is referred to here as the JHU H37Rv stock, and the H37Rv strain derived from CSU is referred to as the CSU H37Rv stock.
The original culture for CSU H37Rv came from the Trudeau Institute, Saranac Lake, NY (TMC 102). The JHU H37Rv (kindly provided by E. Nuermberger) originated from the laboratory of the late Frank Collins at the Trudeau Institute and has been passaged at JHU through mice in order to maintain virulence. The Erdman strain (TMC 107; ATCC 35801) has been maintained at CSU and was derived from the original as a very-low-passage seed lot. CDC1551 was provided to CSU by Thomas Shinnick (from the U.S. Centers for Disease Control), and HN878 was provided to CSU by Barry Kreiswirth (Public Health Research Institute).
All strains, unless otherwise specified, were grown at CSU from original bacterial stock as a pellicle, as described previously (33) but with the following modifications. Briefly, the vial was resuspended in Proskauer-Beck (PB) medium and cultured as a pellicle for three passages. The final pellicle was harvested, dispersed into PB medium with Tween 80 (Sigma Chemical Co., St. Louis, MO), and snap-frozen as 1-ml seed stock cultures. Working stocks (maximum of 3 passages) were expanded into PB medium to generate the infectivity stocks from seed stocks and grow them to mid-log phase from the seed stocks. Infectivity stock vials were recovered from frozen storage immediately prior to use in animal model studies. Drug susceptibility testing of the five M. tuberculosis strains was performed for INH, rifampin (RIF), and pyrazinamide (PZA) at the Mycobacteriology Laboratory at National Jewish Health, Denver, CO, using the Bactec 460 method. The pncA gene was sequenced by the U.S. Centers for Disease Control and Prevention (CDC, Atlanta, GA). Drug susceptibility testing by agar proportion or Bactec MGIT 960 system and pncA sequencing were performed by the CDC. Additional MIC testing was performed in our laboratory as previously described (16) to obtain exact MIC endpoints. Spoligotyping revealed the following spoligotypes: HN878, 000000000003771; CSU H37Rv, 777777475760771; JHU H37Rv, 777777475760771; Erdman, 777777774020771; and CDC 1551, 700076757760771.
Antimicrobial agents, dosing, and formulations.
INH, PZA, and RIF were purchased from Sigma Chemical Co. (St. Louis, MO). RIF was administered at 10 mg/kg, PZA at 150 mg/kg, and INH at 25 mg/kg. All antimicrobial compounds were administered by oral gavage 5 days per week (at 0.2 ml per mouse). All drugs were prepared in water. RIF was ground in a clean mortar and pestle to a small particle size prior to the addition of sterile distilled water. RIF was dosed at least 1 h before the other drug(s). Organs were harvested at least 48 h after the last drug administration for the group to allow clearance of the drugs.
Infection.
Six- to eight-week-old female BALB/c mice were exposed to a high-dose aerosol infection in a GlasCol aerosol chamber with the virulent M. tuberculosis strains in the Colorado State University animal biosafety level 3 laboratories (ABSL-3), as previously described (9). A high-dose aerosol infection is defined here as an inoculum that will result in all animals succumbing to disease within 1 month after infection. All animal studies had received written approval after institutional review and approval by the Animal Care and Use Committee at CSU.
Fresh bacterial cultures for the high-dose aerosol infections of mice were grown in 7H9 media (Difco) supplemented with albumin-dextrose-catalase and Tween 80 and propagated at 37°C to log phase with an optical density at 600 nm (OD600) of 0.8 to 1.0 on the day of infection. The M. tuberculosis inoculum was enumerated by plating on 7H11 agar plates generating CFU, as described before (9). The actual bacterial load implanted in the lungs was determined from three mice per group on the day of or the day after aerosol infection. At the start of treatment, the bacterial load was determined in lungs and spleens of five mice each. For determination of the emergence of RIF-resistant colonies, tissue homogenates were plated on 7H11 plates containing 4 μg/ml of RIF for the first study and 2 μg/ml for the second study in order to ensure that all resistant mutants were recovered.
Statistical analysis.
The numbers of CFU were converted to logarithms (log10 CFU), which were then evaluated by multiple comparison analyses of variance (ANOVAs), including a one-way ANOVA followed by a two-way ANOVA. For early treatment data, an ANOVA F test was done to compare all treatments, followed by comparisons of specific pairs of means. When the counts for a treatment group in general were low (e.g., <2 log10 CFU), a nonparametric test, the Wilcoxon rank sum test, was used.
RESULTS
Drug susceptibility testing of the M. tuberculosis strains used in vivo.
All M. tuberculosis strains used in the mouse studies were evaluated for drug susceptibility in standardized reference laboratories. All were found to be drug susceptible, defined as antimicrobial susceptibility below critical concentrations for INH, RIF, and PZA and exact endpoint MIC for RIF and INH (Table 1). No mutations in the pncA gene were detected in any of the five strains.
Table 1.
In vitro susceptibility and typing data of TB strains used in these studiesa
| Strain | Spoligotype | MIC (μg/ml) |
|
|---|---|---|---|
| INH | RIF | ||
| Erdman | 777777774020771 | 0.023 | 0.063 |
| CSU H37Rv | 777777475760771 | 0.023 | 0.049 |
| JHU H37Rv | 777777475760771 | 0.023 | 0.063 |
| CDC 1551 | 700076757760771 | 0.023 | 0.063 |
| HN878 | 000000000003771 | 0.049 | 0.031 |
No pncA mutations were found in any of the strains. Drug susceptibility testing done at critical concentrations for PZA, INH, and RIF at National Jewish Health Advanced Diagnostic Laboratories and drug susceptibility testing done at critical concentrations with a Bactec MGIT 960 system for PZA, INH, and RIF by agar dilution at Center for Disease Control TB Laboratories showed that all strains were pansusceptible based on predetermined critical concentration breakpoints.
The JHU H37Rv and Erdman M. tuberculosis strains have different responses to standard anti-TB drug therapy in the high-dose aerosol BALB/c model.
The purpose of this experiment was to compare the efficacy results of drug regimens after mice had been infected with either the JHU H37Rv or CSU Erdman strain, which are the standard reference M. tuberculosis strains used in mouse models by both laboratories. Table 2 presents the treatment groups and numbers of animals used. The bacterial loads at the start of treatment were statistically similar in the lungs of the control mouse groups infected with Erdman and with JHU H37Rv, 7.18 log10 CFU and 6.99 log10 CFU, respectively (P = 0.42). For the spleens at the start of treatment, the bacterial loads were significantly higher for Erdman than JHU H37Rv, 3.8 and 3.0 log10 CFU, respectively (P = 0.045).
Table 2.
Animal numbers per time point for the entire triala
| M. tuberculosis strain | Drug regimenb | No. of animals |
|||||
|---|---|---|---|---|---|---|---|
| Day −12 | Day 0 | Day 28 | Day 56 | Day 84 | Total | ||
| Erdman | 2HRZ, 1HR | 3 | 5 | 6 | 6 | 6 | 26 |
| Erdman | 3RZ | 6 | 6 | 7 | 19 | ||
| H37Rv | 2HRZ, 1HR | 3 | 5 | 6 | 6 | 6 | 26 |
| H37Rv | 3RZ | 6 | 6 | 6 | 18 | ||
Treatment started 12 days after infection.
Numbers indicate months (2 months HRZ, 1 month HR, 3 months RZ).
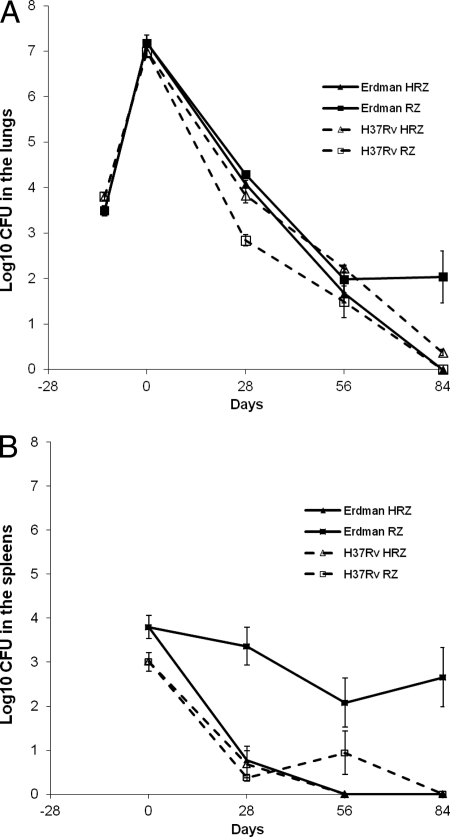
After 1 month of treatment with either the three-drug combination of INH, RIF, and PZA (HRZ) or the two-drug combination RIF and PZA (RZ), the lungs of infected animals showed a significant difference in bacterial load between the H37Rv and Erdman infections when analyzed overall by treatment (Fig. 1A). In the lungs of mice infected with JHU H37Rv, HRZ was far less efficacious than RZ [log10 CFU of 2.84 versus 3.82, respectively (P < 0.0001)] at the 1-month time point. In the groups of mice infected with Erdman, treatment with HRZ and RZ showed statistically similar efficacies [log10 CFU of 4.08 and 4.29, respectively (P = 0.22)]. When the treatment groups were compared according to the bacterial strain used for infection, the HRZ treatment groups showed statistically similar results for H37Rv and Erdman (P = 0.15). However, a highly significant difference was noted between the log10 CFU in the lungs of mice infected with the different strains and treated with RZ (P < 0.0001).
Fig 1.
High-dose aerosol infection models with M. tuberculosis in BALB/c mice using either JHU H37Rv or Erdman for aerosol infection. Bacterial numbers in lungs (A) or spleens (B) of BALB/c mice after a high-dose aerosol infection with M. tuberculosis and 3 months of treatment with H, Z, and R were determined.
After 1 month of treatment, most of the spleens were culture negative, and therefore, organs and treatments were analyzed using the Wilcoxon rank sum test. There was a significant difference in bacterial loads in the spleens of mice infected with the two bacterial strains when analyzed by treatment group (Fig. 1B). The HRZ treatment groups showed statistically similar results for H37Rv- and Erdman-infected mice, whereas for the RZ treatment, mice infected with H37Rv had far fewer CFU in the spleens than mice infected with Erdman (P = 0.003) (Fig. 1B).
After 2 months of treatment, the bacterial load in all lung homogenates was reduced to low numbers (<2 log). Statistically similar results were obtained for the different drug regimens for both TB strains. In addition, the HRZ and RZ treatments for both TB strains showed statistical similar efficacies in the lungs. However, this result this was barely not significant with the statistical methods used (P = 0.05), thereby showing a trend toward improved activity of RZ over HRZ for the JHU H37Rv-infected mouse groups.
No significant difference in the spleens was noted in the mice treated with RZ at 2 months and infected with the Erdman versus the JHU H37Rv strain, which showed bacterial loads of 2.08 log10 CFU and 0.94 log10 CFU, respectively (P = 0.37). Likewise, there were no differences between the mouse groups infected with Erdman and JHU H37Rv and then treated with HRZ (P = 1.0). However, most spleens at 2 months had no detectable CFU, which makes the analysis at this time point difficult.
By 3 months of therapy, most mice had reached negative cultures, and Wilcoxon analysis was used to assess statistical significance of treatment efficacy. Similar activity was seen in the lungs for the HRZ-treated groups infected with either Erdman or JHU H37Rv (P = 0.7). However, for the RZ treatment groups, the efficacy in lungs was significantly greater for the mice infected with JHU H37Rv (all culture negative) than for the mice infected with Erdman, which showed a mean average bacterial load of 2.03 log10 CFU (P = 0.005). Similarly, in the spleens after 3 months of treatment, the RZ treatment showed significantly improved efficacy for the mice infected with JHU H37Rv (all culture negative) compared to mice infected with Erdman [2.66 log10 CFU (P = 0.02)]. No RIF resistance was detected in bacteria from RZ groups plated at 1, 2, or 3 months on RIF-containing plates (4 μg/ml).
Clinical and laboratory-adapted M. tuberculosis strains have different responses to standard drug therapy in the high-dose aerosol BALB/c model.
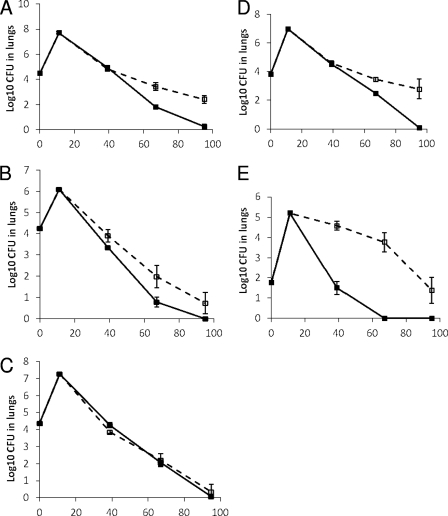
The purpose of this experiment was to compare the drug efficacy results after mice are infected with different laboratory-adapted strains of TB (CSU H37Rv, JHU H37Rv, or Erdman) or clinical strains of TB (CDC 1551, or HN878) after treatment with HRZ or RZ. Table 3 represents the treatment groups and the animal numbers per treatment group used. The starting inoculums used in the nebulizer were as follows: Erdman at 1.7 × 108 CFU/ml, CSU H37Rv at 2.5 × 109 CFU/ml, JHU H37Rv at 8.5 × 109 CFU/ml, CDC 1551 at 6.5 × 107 CFU/ml, and HN878 at 1.3 × 107 CFU/ml. On the day after high-dose aerosol infection, one whole lung each from three sacrificed mice was homogenized and plated, and the following bacterial numbers were obtained: for Erdman, 4.47 log10 CFU; for CSU H37Rv, 4.25 log10 CFU; for JHU H37Rv, 4.37 log10 CFU; for CDC 1551, 3.84 log10 CFU; and for HN878, 1.75 log10 CFU (Fig. 2). On the day when treatment was started, the bacterial loads in the lungs were 7.61, 6.11, 7.27, 6.97, and 5.2 log10 CFU for mice infected with Erdman, CSU H37Rv, JHU H37Rv, CDC1551, and HN878, respectively. Treatment was uneventful until one animal was lost on day 17 in the CDC 1551-infected RZ-treated group by technical error. One data point for lungs was discarded at day 28 in the CSU H37Rv HRZ group due to technical error.
Table 3.
Animal numbers per time point for the entire triala
| M. tuberculosis strain | Drug regimenb | No. of animals |
|||||
|---|---|---|---|---|---|---|---|
| Day −10–11 | Day 0 | Day 28 | Day 56 | Day 84 | Total | ||
| Erdman | 2HRZ, 1HR | 3 | 5 | 6 | 6 | 6 | 26 |
| Erdman | 3RZ | 6 | 6 | 7 | 19 | ||
| CSU H37Rv | 2HRZ, 1HR | 3 | 5 | 6 | 6 | 6 | 26 |
| CSU H37Rv | 3RZ | 6 | 6 | 6 | 18 | ||
| JHU H37Rv | 2HRZ, 1HR | 3 | 5 | 6 | 6 | 6 | 26 |
| JHU H37Rv | 3RZ | 6 | 6 | 7 | 19 | ||
| CDC 1551 | 2HRZ, 1HR | 3 | 5 | 6 | 6 | 6 | 26 |
| CDC 1551 | 3RZ | 6 | 6 | 7 | 19 | ||
| HN878 | 2HRZ, 1HR | 3 | 5 | 6 | 6 | 6 | 26 |
| HN878 | 3RZ | 6 | 6 | 7 | 19 | ||
Treatment started 10 to 11 days after infection.
Numbers indicate months.
Fig 2.
High-dose aerosol infection models in BALB/c mice infected with five strains of M. tuberculosis and treated with either the three-drug combination (HRZ) or the two-drug combination (RZ). (A) Erdman; (B) CSU H37Rv; (C) JHU H37Rv; (D) CDC1551; (E) HN878. The x axes show days from experiment start. Drug treatment included 2 months of HZR, followed by HR for 1 month (solid lines) or ZR for 3 months (dashed lines).
After 1 month of therapy, there was a significant difference seen in treatment regimen efficacies in the lungs of infected mice based on the strain used to infect the mice (P < 0.0001). The bacterial loads indicated higher efficacy in the HRZ groups than in the RZ groups in the mice infected with CSU H37Rv [3.35 versus 3.90 log10 CFU, respectively (P = 0.014)] as well as for HN878 [1.49 versus 4.58 log10 CFU (P < 0.0001)] at 1 month of treatment. The efficacies for HRZ and RZ were similar for the JHU H37Rv strain (4.26 versus 3.86 log10 CFU, respectively [P = 0.063]), with a trend toward RZ being more effective than HRZ. Among mice infected with the other strains, bacterial loads in lungs of mice receiving RZ treatment were statistically similar to loads in mice receiving HRZ for those infected with CDC 1551 [4.51 versus 4.60 log10 CFU (P = 0.68)] as well as for those infected with Erdman [4.96 versus 4.83 log10 CFU (P = 0.53)].
After 2 months of therapy, there was a statistically significant difference in treatment efficacy based on the bacterial strain used for infecting the mice (P < 0.0001). There was no significant difference between HRZ and RZ treatment in mice infected with the JHU H37Rv strain, and this strain was the only one to show equivalent CFU burdens in HRZ- and RZ-treated mice [2.06 versus 2.21 log10 CFU, respectively (P = 0.67)]. For the groups of mice infected with the four other TB strains, the RZ treatment was in all cases statistically more effective than HRZ. The bacterial loads were significantly lower for the HRZ than the RZ treatment in mice infected with CSU H37Rv [0.78 versus 1.97, respectively (P = 0.0011)], HN878 [0 versus 3.77 (P < 0.0001)], CDC 1551 [2.47 versus 3.45 (P = 0.0068)], and Erdman [1.82 versus 3.43 (P < 0.0001)].
After 3 months of therapy, a sizable number of mice were culture negative, and for statistical analysis, the Wilcoxon rank sum exact P value was calculated. HRZ showed significantly higher efficacy than RZ in mice infected with CDC 1551 (P = 0.0013), Erdman (P = 0.022), and HN878 (P = 0.0070). The bacterial loads from the lungs of mice infected with strain CSU H37Rv and treated with HRZ versus those with RZ approached a statistically significant difference (P = 0.07), whereas loads from mice infected with JHU H37Rv showed a nonsignificant difference between the HRZ and RZ treatment arms (P = 0.73). Only one RIF-resistant colony was detected (from a single spleen from the RZ group plated at 3 months). There were no differences in PDIMs between the strains (data not shown).
DISCUSSION
Given the number of new drugs in the TB drug development pipeline (46, 47), it is more crucial than ever to have validated preclinical testing methods that are predictive of clinical outcome and that provide the highest confidence that the most potent drug regimens are being advanced into clinical trials. This is especially important in light of a recent initiative by the Gates Foundation (Critical Path to New TB Regimens [CPTR]) that will stimulate development of new combination regimens (of new investigational drugs along with existing TB drugs) to avoid developing each drug sequentially over decades to come (47). Although there is no doubt that the CPTR approach will lead to more effective drug regimens, it will become important to select the most promising combinations from preclinical studies for both bactericidal and sterilizing activity to advance and be tested clinically.
Our previously published studies focused on a multitude of variables in the methods used in preclinical drug testing in mice (9), and these ultimately revealed that the strain used in animal infection models deserved greater investigation. To our knowledge, there are no in vivo studies that have evaluated combination anti-TB drug regimens against various strains of M. tuberculosis in a single head-to-head experiment. In the studies presented here, we evaluated the bactericidal activity of the drug combinations HRZ and RZ in the BALB/c mouse model, infected via aerosol using five different strains of M. tuberculosis (JHU H37Rv, CSU H37Rv, Erdman, CDC1551, and HN878). The results showed that the in vivo efficacy of the tested drug combinations was not always the same for the different M. tuberculosis evaluated. In fact, the degrees of in vivo bactericidal activity of RZ for two different H37Rv strains were different, with the efficacy in the lungs being significantly better in JHU H37Rv-infected mice than in CSU H37Rv-infected mice. Moreover, the rankings of the HRZ and RZ regimens were not the same for both H37Rv strains. Antagonism in the HRZ combination was seen only with the JHU H37Rv strain, not with any of the four other strains evaluated.
Recently, it has become clear that even among the H37Rv strains across different laboratories there is a significant genetic heterogeneity (22). Loerger et al. urge investigators to use caution in referring to H37Rv as a standard reference strain, as experimental results derived with “H37Rv” may depend on the laboratory in which it is maintained and its associated genetic characteristics. The whole-genome sequencing results obtained by the same authors showed that differences among H37Rv strains have arisen over time, and while they are relatively few (on the order of 5 to 10 polymorphisms per strain), they still could be functionally relevant. Which genetic differences are responsible for the strain-based differences in efficacy presented here remains unclear and would have to be investigated further. Ongoing genetic evolution has been observed in vitro via the accumulation of genetic differences during serial passaging of cultures (39); therefore, keeping the passage number low has been shown to be a definite requirement. The methods of propagation, storage, and passaging of strains are highly variable across different laboratories. Some investigators passage M. tuberculosis strains through animals to maintain virulence (8), while others grow the strain in a pellicle for the same purpose (32). In both cases, there is no published evidence that in fact shows that either protocol increases or maintains the virulence of the strain. In contrast, Converse et al. published results indicating that animal passaging of M. tuberculosis strains prior to quantitative virulence testing in mouse and guinea pig models did not enhance or restore potency to strains that may have lost virulence due to in vitro passaging (8). The authors mention that it is critical to verify virulence of parental strains before any manipulation is undertaken. For the studies described here, there was a clear difference in the origins of the H37Rv strain used by both groups, as well as the method of strain propagation: the JHU H37Rv strain was serially passaged in mice and individual bacteria were isolated and propagated, whereas the CSU H37Rv was grown as a pellicle, seed lots were frozen, and the passage number of the working stocks never exceeded six. Identifying the most important variable which can influence the virulence and drug responses of the strains used in animal models (source/origin, method of maintaining virulence, protocol for propagation, etc.) in TB drug evaluation mouse studies would require a careful stepwise investigation.
The chain of custody of different M. tuberculosis strains can become more uncertain over time and is often not reported in published works. For instance, in regard to the history of the strains, it is known that the parent strain of H37Rv (TMC102) was isolated by E. R. Baldwin from a human lung in 1905 and dissociated from this parent (H37) in 1934 at the Trudeau Institute, Saranac Lake, NY (27, 28). H37RV was continually passaged in Proskauer-Beck medium until 1970, when it was frozen at −70°C, and it is the neo-type strain from which ATCC 27294 was derived. M. tuberculosis Erdman was originally isolated from human sputum by W. Feldman at the Mayo Clinic in 1945 (personal communication, Trudeau Medical Library). The following year, in 1946, it was transferred to the Trudeau Institute, where it was maintained in Proskauer-Beck medium until 1970, when it was frozen at −70°C. We were unable to find documentation of the exact details of the years of additional propagation for both the H37Rv and Erdman strains.
The results here also shed some light on the question of whether the antagonism seen within the standard HRZ regimen by some investigators (15) but not others (2, 9) is a TB strain-specific phenomenon. Previous studies have often had widely differing experimental variables and endpoints, and therefore a direct comparison was not feasible. In the direct head-to-head-comparison studies described in this work, the combination of HRZ was as effective as or significantly more effective than RZ dual therapy for four of five TB strains tested, excepting only the JHU H37Rv strain, for which the efficacy of RZ under most conditions was better than that of HRZ. In none of the other four TB strains did we ever observe antagonism with the HRZ combination. Even in our laboratory, the antagonism in HRZ for JHU H37Rv is not seen to the same extent every time, and this may be dependent on the INH dose or drug exposure, as was previously reported (1). Another older study reported that the antagonism observed is dependent on certain timing of the infection and administration of drugs (36). Although the basis of the observed antagonism remains poorly understood, the results presented here make the point that the antagonism within the standard regimen appears to be a strain-specific phenomenon and is seen only under specific conditions. Murine models certainly make it possible to rank efficacies of drug regimens, if appropriate and sufficient control groups for each regimen are wisely chosen. If, for instance, the impact of a new compound is evaluated when it is added to the standard HRZ regimen against a particular TB strain, its activity should be compared to that of the standard regimen by itself. On the other hand, if the goal is to evaluate the efficacy of a new regimen where the new drug replaces a drug of the standard drug regimen (e.g., H) then the appropriate control (e.g., RZ) should be included alongside HRZ.
The studies presented here have some obvious limitations. Only a limited number of drug regimens were studied due to the labor-intensive nature of these comparative studies, and therefore the conclusions regarding the choice of M. tuberculosis strains can at this point be made only for the regimens tested. In addition, the responses of the various strains were evaluated only in terms of the bactericidal activity of certain drug regimens, not the sterilizing activity in long-term relapse trials. In relapse studies, the choice of M. tuberculosis strain might affect the outcome even more, as the presence of memory immunity for all strains is not equal. For instance, only clinical isolates of M. tuberculosis have been described to induce a regulatory T-cell population, which influences the degree of relapse of infection (7, 45). HN878, a member of the W-Beijing genotype, is known to be a potent inducer of regulatory T cells and is associated with increased virulence and pathology in lungs of mice (42). Additional mouse studies will be necessary to answer these important questions regarding the difference in responses of laboratory-adapted and clinical TB strains in relapse and for the assessment of other drug regimens. One limitation in our second study was that the inoculums for the various strains were not entirely identical, as they were in the first study. This was especially the case for HN878, which surprisingly grew less well than the comparator strains.
In conclusion, in the in vivo head-to-head experiments described here, the M. tuberculosis strain was found to be one of the most important variables that can change the outcome of the in vivo efficacy trial in mice. Therefore, we highly recommend confirmation of in vivo efficacy results in a late stage of preclinical testing against a second M. tuberculosis strain, thereby increasing the confidence to advance a potent drug regimen to clinical trials. Moreover, we suggest that these studies include current clinical M. tuberculosis strains (from one or more different clades based on their geographical locations) (14). Given the widespread dissemination of Beijing strains in the world and the coadaptation with humans over time, strong consideration should be given to including a member of the Beijing family of M. tuberculosis strains. In addition, it is highly recommended for all in vivo studies to use only M. tuberculosis strains that are fully characterized (genetically), have good growth characteristics and a limited passage number, have been propagated appropriately, and have a completely documented chain of custody.
The recommendation to evaluate and confirm anti-TB drug regimens against relevant M. tuberculosis strains in validated mouse models is of especially great importance given the CPTR effort to advance a combination of three novel drugs into human clinical trials in the next few years. In summary, we believe that all efficacy data generated with animal models should be confirmed in parallel mouse models in a second laboratory and should include a range of representative isolates.
ACKNOWLEDGMENTS
We thank Phillip Chapman (Statistics Department, CSU) for statistical assistance, Karen Dobos and Angelo Izzo for very helpful discussions of the TB strains, and members of the Mary Jackson laboratory for performing PDIM analysis. We are very grateful to Lois Diem, Beverly Metchock, and the team of the TB lab at the Centers for Disease Control and Prevention for performing the in vitro drug susceptibility testing, spoligotyping, and sequencing of the pncA gene. We also thank Courtney Hastings, Elizabeth Donegan, Elizabeth Brooks, and Laura Nold for technical assistance and the Laboratory Animal Resources staff for assistance with care and observation of the animals.
This project was supported by the Bill and Melinda Gates Foundation under Drug Accelerator grant ID number 42589, titled “Assay Standardization.”
Footnotes
Published ahead of print 5 December 2011
REFERENCES
- 1. Almeida D, et al. 2009. Paradoxical effect of isoniazid on the activity of rifampin-pyrazinamide combination in a mouse model of tuberculosis. Antimicrob. Agents Chemother. 53: 4178–4184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Andries K, Gevers T, Lounis N. 2010. Bactericidal potencies of new regimens are not predictive of their sterilizing potencies in a murine model of tuberculosis. Antimicrob. Agents Chemother. 54: 4540–4544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barczak AK, et al. 2005. In vivo phenotypic dominance in mouse mixed infections with Mycobacterium tuberculosis clinical isolates. J. Infect. Dis. 192: 600–606 [DOI] [PubMed] [Google Scholar]
- 4. Boehme CC, et al. 2011. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet 377: 1495–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Borrell S, Gagneux S. 2011. Strain diversity, epistasis and the evolution of drug resistance in Mycobacterium tuberculosis. Clin. Microbiol. Infect. 17: 815–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brown MC, Taffet SM. 1995. Lipoarabinomannans derived from different strains of Mycobacterium tuberculosis differentially stimulate the activation of NF-κB and KBF1 in murine macrophages. Infect. Immun. 63: 1960–1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burman WJ, et al. 2009. Relapse associated with active disease caused by Beijing strain of Mycobacterium tuberculosis. Emerg. Infect. Dis. 15: 1061–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Converse PJ, et al. 2010. The impact of mouse passaging of Mycobacterium tuberculosis strains prior to virulence testing in the mouse and guinea pig aerosol models. PLoS One 5: e10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. De Groote MA, et al. 2011. Comparative studies evaluating mouse models used for efficacy testing of experimental drugs against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 55: 1237–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dorman SE, et al. 2009. Substitution of moxifloxacin for isoniazid during intensive phase treatment of pulmonary tuberculosis. Am. j. Respir. Crit. Care Med. 180: 273–280 [DOI] [PubMed] [Google Scholar]
- 11. Dye C, Williams BG. 2010. The population dynamics and control of tuberculosis. Science 328: 856–861 [DOI] [PubMed] [Google Scholar]
- 12. Gagneux S, et al. 2006. Impact of bacterial genetics on the transmission of isoniazid-resistant Mycobacterium tuberculosis. PLoS Pathog. 2: e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gagneux S, et al. 2006. Variable host-pathogen compatibility in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 103: 2869–2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gagneux S, Small PM. 2007. Global phylogeography of Mycobacterium tuberculosis and implications for tuberculosis product development. Lancet 7: 328–337 [DOI] [PubMed] [Google Scholar]
- 15. Grosset J, Truffot-Pernot C, Lacroix C, Ji B. 1992. Antagonism between isoniazid and the combination pyrazinamide-rifampin against tuberculosis infection in mice. Antimicrob. Agents Chemother. 36: 548–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gruppo V, et al. 2006. Rapid microbiologic and pharmacologic evaluation of experimental compounds against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 50: 1245–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Henao-Tamayo M, Irwin SM, Shang S, Ordway D, Orme IM. 2011. T lymphocyte surface expression of exhaustion markers as biomarkers of the efficacy of chemotherapy for tuberculosis. Tuberculosis 91: 308–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hershberg R, et al. 2008. High functional diversity in Mycobacterium tuberculosis driven by genetic drift and human demography. PLoS Biol. 6: e311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Homolka S, Niemann S, Russell DG, Rohde KH. 2010. Functional genetic diversity among Mycobacterium tuberculosis complex clinical isolates: delineation of conserved core and lineage-specific transcriptomes during intracellular survival. PLoS Pathog. 6: e1000988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ibrahim M, et al. 2007. Synergistic activity of R207910 combined with pyrazinamide against murine tuberculosis. Antimicrob. Agents Chemother. 51: 1011–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ioerger TR, et al. 2010. The non-clonality of drug resistance in Beijing-genotype isolates of Mycobacterium tuberculosis from the Western Cape of South Africa. BMC Genomics 11: 670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ioerger TR, et al. 2010. Variation among genome sequences of H37Rv strains of Mycobacterium tuberculosis from multiple laboratories. J. Bacteriol. 192: 3645–3653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kato-Maeda M, Bifani PJ, Kreiswirth BN, Small PM. 2001. The nature and consequence of genetic variability within Mycobacterium tuberculosis. J. Clin. Invest. 107: 533–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kato-Maeda M, et al. 2011. Strain classification of Mycobacterium tuberculosis: congruence between large sequence polymorphisms and spoligotypes. Int. J. Tuberc. Lung Dis. 15: 131–133 [PMC free article] [PubMed] [Google Scholar]
- 25. Kato-Maeda M, et al. 2010. Differences among sublineages of the East-Asian lineage of Mycobacterium tuberculosis in genotypic clustering. Int. J. Tuberc. Lung Dis. 14: 538–544 [PMC free article] [PubMed] [Google Scholar]
- 26. Kato-Maeda M, et al. 2001. Comparing genomes within the species Mycobacterium tuberculosis. Genome Res. 11: 547–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kilburn JO, Silcox VA, Kubica GP. 1969. Differential identification of Mycobacteria. V. The tellurite reduction test. Am. Rev. Respir. Dis. 99: 94–100 [DOI] [PubMed] [Google Scholar]
- 28. Kim TH, Kubica GP. 1972. Long-term preservation and storage of mycobacteria. Appl. Microbiol. 24: 311–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kubica GP, Gontijo-Filho PP, Kim T. 1977. Preservation of mycobacteria at −70°C: persistence of key differential features. J. Clin. Microbiol. 6: 149–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lari N, Bimbi N, Rindi L, Tortoli E, Garzelli C. 2011. Genetic diversity of human isolates of Mycobacterium bovis assessed by spoligotyping and variable number tandem repeat genotyping. Infect. Genet. Evol. 11: 175–180 [DOI] [PubMed] [Google Scholar]
- 31. Lenaerts AJ, Degroote MA, Orme IM. 2008. Preclinical testing of new drugs for tuberculosis: current challenges. Trends Microbiol. 16: 48–54 [DOI] [PubMed] [Google Scholar]
- 32. Lenaerts AJ, Gruppo V, Brooks JV, Orme IM. 2003. Rapid in vivo screening of experimental drugs for tuberculosis using gamma interferon gene-disrupted mice. Antimicrob. Agents Chemother. 47: 783–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lenaerts AJ, et al. 2005. Preclinical testing of the nitroimidazopyran PA-824 for activity against Mycobacterium tuberculosis in a series of in vitro and in vivo models. Antimicrob. Agents Chemother. 49: 2294–2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Manabe YC, et al. 2003. Different strains of Mycobacterium tuberculosis cause various spectrums of disease in the rabbit model of tuberculosis. Infect. Immun. 71: 6004–6011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Manca C, et al. 2005. Hypervirulent M. tuberculosis W/Beijing strains upregulate type I IFNs and increase expression of negative regulators of the Jak-Stat pathway. J. Interferon Cytokine Res. 25: 694–701 [DOI] [PubMed] [Google Scholar]
- 36. McCune RM, Feldmann FM, McDermott W. 1966. Microbial persistence. II. Characteristics of the sterile state of tubercle bacilli. J. Exp. Med. 123: 469–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McCune RM, Jr., McDermott W, Tompsett R. 1956. The fate of Mycobacterium tuberculosis in mouse tissues as determined by the microbial enumeration technique. II. The conversion of tuberculous infection to the latent state by the administration of pyrazinamide and a companion drug. J. Exp. Med. 104: 763–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McCune RM, Jr., Tompsett R. 1956. Fate of Mycobacterium tuberculosis in mouse tissues as determined by the microbial enumeration technique. I. The persistence of drug-susceptible tubercle bacilli in the tissues despite prolonged antimicrobial therapy. J. Exp. Med. 104: 737–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Molina-Torres CA, et al. 2010. Effect of serial subculturing on the genetic composition and cytotoxic activity of Mycobacterium tuberculosis. J. Med. Microbiol. 59: 384–391 [DOI] [PubMed] [Google Scholar]
- 40. Nahid P, et al. 2010. Influence of M. tuberculosis lineage variability within a clinical trial for pulmonary tuberculosis. PLoS One 5: e10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nuermberger E. 2008. Using animal models to develop new treatments for tuberculosis. Semin. Respir. Crit. Care Med. 29: 542–551 [DOI] [PubMed] [Google Scholar]
- 42. Ordway D, et al. 2007. The hypervirulent Mycobacterium tuberculosis strain HN878 induces a potent TH1 response followed by rapid down-regulation. J. Immunol. 179: 522–531 [DOI] [PubMed] [Google Scholar]
- 43. Palanisamy GS, et al. 2009. Clinical strains of Mycobacterium tuberculosis display a wide range of virulence in guinea pigs. Tuberculosis 89: 203–209 [DOI] [PubMed] [Google Scholar]
- 44. Palanisamy GS, et al. 2008. Disseminated disease severity as a measure of virulence of Mycobacterium tuberculosis in the guinea pig model. Tuberculosis 88: 295–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shang S, et al. 2011. Increased Foxp3 expression in guinea pigs infected with W-Beijing strains of M. tuberculosis. Tuberculosis 91: 378–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Spigelman M, Gillespie S. 2006. Tuberculosis drug development pipeline: progress and hope. Lancet 367: 945–947 [DOI] [PubMed] [Google Scholar]
- 47. Spigelman M, Woosley R, Gheuens J. 2010. New initiative speeds tuberculosis drug development: novel drug regimens become possible in years, not decades. Int. J. Tuberc. Lung Dis. 14: 663–664 [PubMed] [Google Scholar]
- 48. Vashishtha VM. 2009. WHO global tuberculosis control report 2009: tuberculosis elimination is a distant dream. Indian Pediatr. 46: 401–402 [PubMed] [Google Scholar]
- 49. World Health Organization 2010. Global tuberculosis control: key findings from the December 2009 WHO report. Wkly Epidemiol. Rec. 85: 69–80 [PubMed] [Google Scholar]