Abstract
Vaginal microbicides may play an important role in protecting women from HIV infection. A strong synergy between HSV and HIV has been observed, and epidemiological studies demonstrate that HSV infection increases the risk of HIV acquisition. Incorporation of the antiretroviral tenofovir (TFV) along with the antiherpetic acyclovir (ACV) into combination intravaginal rings (IVRs) for sustained mucosal delivery of both compounds could lead to increased microbicide product adherence and efficacy compared with conventional vaginal formulations. A novel, dual-protection “pod IVR” platform developed in-house and delivering ACV and TFV was evaluated in rabbit and sheep models. The devices were safe and exhibited sustained release of both drugs independently and at controlled rates over the 28-day studies. Daily release rates were estimated based on residual drug content of the used devices: rabbits, 343 ± 335 μg day−1 (ACV) and 321 ± 207 μg day−1 (TFV); sheep, 174 ± 14 μg day−1 (ACV) and 185 ± 34 μg day−1 (TFV). Mean drug levels in sheep vaginal samples were as follows: secretions, 5.25 ± 7.31 μg ml−1 (ACV) and 20.6 ± 16.2 μg ml−1 (TFV); cervicovaginal lavage fluid, 118 ± 113 ng ml−1 (ACV) and 191 ± 125 ng ml−1 (TFV); tissue, 173 ng g−1 (ACV) and 93 ng g−1 (TFV). An in vitro-in vivo correlation was established for both drugs and will allow the development of future formulations delivering target levels for prophylaxis and therapy. These data suggest that the IVR based on the pod design has potential in the prevention of transmission of HIV-1 and other sexually transmitted pathogens.
INTRODUCTION
Significant progress has been achieved in providing increased access to HIV/AIDS services in several low- and middle-income countries (44). Despite these encouraging findings, women in the developing world remain disproportionately at risk of HIV infection. Seventy-six percent of new HIV infections in sub-Saharan Africa occur in women aged 15 to 24 years (42). The proportion is as high as 90% in South Africa (38). In the absence of a preventative vaccine, effective prophylactic biomedical technologies are urgently required. Campaigns aimed at encouraging monogamy and condom use have had limited success in areas where marriage has been identified as the major risk factor for HIV acquisition in women (3, 15, 16, 21, 25).
Human immunodeficiency virus type 1 (HIV-1) and herpes simplex virus type 2 (HSV-2), the serotype most commonly associated with genital herpes, are responsible for two intersecting epidemics, where the disease caused by one virus facilitates the transmission of and pathogenesis by the other. A strong synergy between HSV and HIV has been observed, and epidemiological studies demonstrate that HSV infection increases the risk of HIV-1 acquisition (33, 34, 43). A meta-analysis found that prevalent HSV-2 infection is associated with a threefold-increased risk of HIV acquisition among both men and women. These results suggest that, in areas of high HSV-2 prevalence, a substantial proportion of HIV infection is linked to HSV-2 infection (19). Forty-five million Americans are infected with HSV-2 (17), and studies in developing countries reveal seroprevalence rates ranging from 60% to 80% in young adults (27). In the United States, HSV-2 seropositivity is higher among women (23.1%) than men (11.2%) (45). These studies suggest that interventions against HSV-2 may have a key role in HIV prevention (18).
The nucleotide analog reverse transcriptase inhibitor (nRTI) tenofovir (TFV) is a promising topical microbicide under active investigation. It has demonstrated the ability to inhibit retroviral replication in animals and humans and was well tolerated when administered orally as its disoproxil fumarate prodrug (4, 14, 24). Parikh et al. evaluated a topical gel containing two long-acting antiretroviral drugs, TFV and emtricitabine (FTC), in a repeat challenge macaque model (31). These authors showed that a pre-exposure vaginal application of gel with 1% TFV alone or in combination with 5% FTC fully protected macaques from a total of 20 exposures to simian-human immunodeficiency virus SF162p3. The Centre for the AIDS Program of Research in South Africa (CAPRISA) 004 trial recently assessed the effectiveness and safety of a 1% vaginal gel formulation of TFV for the prevention of HIV acquisition in 889 women (22a). In the double-blind, randomized controlled trial, the TFV gel reduced HIV incidence by 39% compared to the placebo. This effect was unexpectedly associated with a significant (51%) decrease in HSV-2 acquisition, which may be explained by the high local concentrations of TFV in vivo. In high adherers (gel adherence > 80%), HIV incidence was 54% lower in the TFV gel arm. Adherence to the intervention reported by participants may not always accurately reflect actual practices. In the recent Carraguard microbicide trial run by the Population Council, 96% of women claimed to be using the candidate gel formulation correctly, but when applicators were tested it was found that only 44% had been used (6). Since intravaginal rings (IVRs) are believed to increase adherence, the development of ring formulations of antiviral drugs is an urgent global priority (20, 32, 35, 37).
The formulation of pharmaceutical agents into IVRs represents an attractive approach to achieving sustained release of compounds to the vaginal cavity for local or systemic delivery (40), thereby increasing efficacy and adherence to therapy while potentially decreasing toxic side effects compared to daily oral administration. This strategy has become popular in contraception and in estrogen replacement therapy (46), and several products are available commercially (26). Sustained vaginal delivery of antiviral agents from IVRs constitutes a potential route to HIV pre-exposure prophylaxis in women, particularly in the developing world (29, 36).
We have developed IVRs that deliver TFV and the guanosine analog antiherpetic drug acyclovir (ACV) at controlled rates for over 1 month (J. Moss, presented at Microbicides 2010, Pittsburgh, PA, 22 to 26 May 2010), with the long-term goal of formulating a safe and effective intravaginal device for the protection of women from HIV and other sexually transmitted infections. In the present study, the pharmacokinetic and safety profiles of these devices were investigated in female New Zealand White rabbits and in female Merino sheep.
MATERIALS AND METHODS
Manufacture of silicone intravaginal devices.
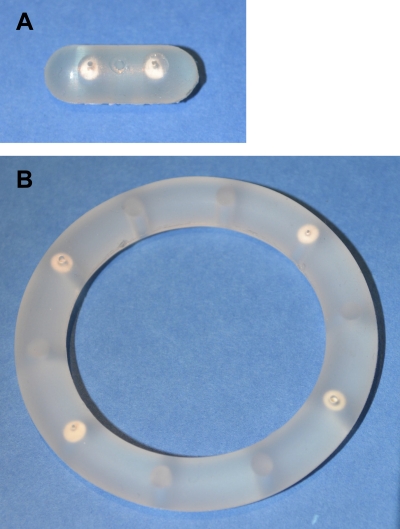
Silicone IVRs were prepared in a multistep process from Nusil MED-4840 liquid silicone elastomer (Nusil Silicone Technology, Carpinteria, CA) using an injection molding system developed in-house. The device specifications are provided in Table 1. The ring manufacture was accomplished in two separate injection molding steps. Pods consisted of compacted drug cores (16 mg each) consisting of either ({[(2R)-1-(6-amino-9H-purin-9-yl)propan-2-yl]oxy}methyl)phosphonic acid (tenofovir [TFV]; Sinoway International, China) or 2-amino-9-(propoxymethyl)-1H-purin-6(9H)-one (acyclovir [ACV]; Comfortcomms Group Co., China) were coated with poly-dl-lactide (PLA; molecular weight, ∼15,000) (Resomer R 202 S; Boehringer Ingelheim Pharma GmbH & Co. KG, Germany). The coated pods were placed in premolded, evenly spaced cavities. The second half of the ring then was injection molded onto the first. Images of the rabbit and sheep intravaginal devices are shown in Fig. 1.
Table 1.
Physical characteristics of intravaginal devices
| Device characteristic | New Zealand White rabbit | Merino sheep |
|---|---|---|
| Shape | Cylindrical | Torus |
| Dimensions (mm) | Diameter, 8; length, 20 | o.d., 56; i.d., 40; cross-sectional diameter, 8a |
| Number of pods | 1 (ACV), 1 (TFV) | 2 (ACV), 2 (TFV) |
| Drug reservoir (mg) | 16 (ACV), 16 (TFV) | 32 (ACV), 32 (TFV) |
| Delivery channel SAb (mm2) | 1.7 (ACV), 0.79 (TFV) | 1.7 (ACV), 0.79 (TFV) |
Nominally based on the dimensions of the commercial Estring device. o.d., outer diameter; i.d., inner diameter.
SA, surface area.
Fig 1.
Photographs of two-pod rabbit (A) and four-pod sheep (B) intravaginal devices.
In vitro studies.
Rings or ring segments were placed in 100 ml vaginal fluid simulant (VFS) consisting of 0.25 mM acetate buffer at pH 4.2 and 200 mosmol kg−1 NaCl, based on the recipe by Owen and Katz (30). The devices were incubated at 25°C ± 2°C and shaken at 110 rpm (S/P RotatorV, Baxter). Samples (1 ml) were removed at predetermined time points, were replaced with VFS (1 ml), and were stored at −30°C prior to analysis.
Quantification of TFV and ACV in VFS.
Samples were thawed to room temperature, filtered through 0.2-μm syringe filters, and analyzed by high-performance liquid chromatography with mass spectrometric detection (LC/MS) using an Agilent series 1100 system with the following features: YMC Pack-CN column (4.6 by 100 mm, 5 μm; Waters) controlled at 28°C; injection volume, 100 μl; mobile phase, ammonium citrate (0.01 M) buffered with formic acid (pH 3.0) run isocratically at a flow rate of 0.4 ml min−1; and single ion mode detection, m/z 288.1 (TFV, 4.97 min), 226.1 (ACV, 5.99 min). Linear calibration plots for TFV [y = (6.24 × 103)x + 51.2.103, R2 = 0.998] and ACV [y = (6.73 × 103)x + 93.7.103; R2 = 0.997] were obtained over the range from 1 to 1,000 ng ml−1.
In vivo rabbit study.
The in vivo rabbit study was carried out at MPI Research, Inc. (Mattawan, MI) and used six female New Zealand White rabbits (strain Hra:[NZW]SPF). At the onset of treatment (5 August 2010), the rabbits were approximately 5 to 8 months old and their mean body weight was 2.89 ± 0.10 kg. The protocol and any amendment(s) or procedures involving the care and use of animals in this study were reviewed and approved by MPI Research's Institutional Animal Care and Use Committee (IACUC). During the study, the care and use of animals were conducted in compliance with the U.S. Department of Agriculture's (USDA) Animal Welfare Act (9 CFR, parts 1, 2, and 3) (28). MPI Research's facility maintains an Animal Welfare Assurance statement with National Institutes of Health, Office of Laboratory Animal Welfare.
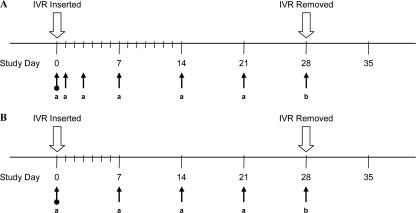
The complete study timeline is presented in Fig. 2A. On day 0 of the study, the intravaginal devices containing ACV and TFV were implanted aseptically in preanesthetized animals. The vaginal opening was infused with 2% liquid lidocaine, a midline laparotomy incision was made, and the vagina was isolated from surrounding soft tissues. The vaginal ring segment was lubricated with K-Y jelly, inserted through the exterior vaginal opening, and advanced cranially beyond the urethral opening through a speculum. Insertion was performed as a clean procedure by trained technical staff. Once visualized by the surgeon, the implant was anchored with a 5-0 Prolene suture to the vagina from the outer ventral wall. The laparotomy incision was closed with absorbable suture and skin staples.
Fig 2.
Animal study timelines and biological sample collection points. (A) Rabbit study. Arrows indicate times of blood and vaginal secretion (Weck-Cel) collection (a) and blood, vaginal secretion (Weck-Cel), and tissue collection (b). There was one predose sample collection point (day 0). (B) Sheep study. Arrows indicate times of blood, vaginal secretion (Weck-Cel), and cervicovaginal lavage (CVL) fluid collection (a) and blood, vaginal secretion (Weck-Cel), CVL fluid, and tissue collection (b). There was one predose sample collection point (day 0).
Blood samples (approximately 3 ml each) were collected from the jugular vein, or another suitable vein, predose, at 24 h postdose, and on days 3, 7, 14, 21, and 28. Blood samples were transferred into tubes containing K3-EDTA anticoagulant and were immediately placed on wet ice prior to centrifugation. Plasma was immediately deep-frozen by placing on dry ice until storage at approximately −80°C.
A Weck-Cel sponge was placed into the vagina toward the lateral wall for approximately 1 min in order to absorb vaginal secretions. After sample collection, the sponge was returned to the labeled preweighed tube and the weight was recorded. The Weck-Cel sponges were kept on dry ice pending storage at approximately −80°C. Samples were collected predose, at 24 h postdose, and on days 3, 7, 14, 21, and 28.
All treated animals were euthanized on day 28 via intravenous overdose of sodium pentobarbital solution. Used intravaginal implants were individually packaged, labeled, stored at approximately −70°C, and sent to Oak Crest Institute for analysis. On completion of the necropsy of each animal, samples from the abdominal and urinary vagina (i.e., proximal and distal to the ring segment, respectively) as well as from the iliac lymph nodes were collected. Tissues were snap-frozen in liquid nitrogen and stored at approximately −80°C prior to analysis.
The animals were examined carefully for external abnormalities, including masses. The skin was reflected from a ventral midline incision, and any abnormalities were identified and correlated with antemortem findings. The abdominal, thoracic, and cranial cavities were examined for abnormalities, and the organs were removed and examined.
Used intravaginal implants were analyzed for residual drug content at Oak Crest Institute. The drug pods were excised from the devices, suspended individually in 50% methanol (20 ml), sonicated until a homogeneous solution was obtained, and analyzed by HPLC.
Weck-Cel, plasma, and tissue samples were processed and analyzed at MPI Research, Inc. (State College, PA) using validated methods. Details on these methods are included in the supplemental material. The lower limits of quantitation for these analytical methods are shown in Table 2, and the run-to-run coefficient of variation for all methods was less than 20%.
Table 2.
Analytical lower limits of quantitation (LLOQ) for biological samplesa
| Animal and sample | Units | LLOQ for: |
|
|---|---|---|---|
| ACV | TFV | ||
| Rabbit | |||
| Weck-Cel | ng sponge−1 | 1 | 5 |
| Tissue | ng g−1 | 2.4 | 20b |
| Plasma | ng ml−1 | 0.25 | 1 |
| Sheep | |||
| Weck-Celc | ng ml−1 | 0.5 | 5 |
| CVL fluid | ng ml−1 | 1 | 5 |
| Tissue | ng g−1 | 2 | 2 |
| Plasma | ng ml−1 | 5 | 5 |
The run-to-run coefficient of variation for all methods was less than 20%.
The total TFV LLOQ was 10 ng g−1; the intracellular TFV diphosphate LLOQ was 100 ng g−1.
In diluted extracts; the level of dilution varies between 5 and 100 depending on the sample.
In vivo sheep study.
The in vivo sheep study was carried out at the University of Texas Medical Branch at Galveston (UTMB) in June 2010 and used four yearling virginal female Merino sheep with a mean body weight of 36.5 ± 1.1 kg. The animals were housed and treated under a protocol approved by the Institutional Animal Care and Use Committee. Four sheep received IVRs containing four PLA-coated drug pods, two of ACV and two of TFV, as described above. The complete study timeline is presented in Fig. 2B. The IVRs were inserted on day 0 into the vaginal vault and retained for a period of 28 days. Vaginal colposcopy was used to confirm placement and retention of the vaginal rings. The animals were placed in a dorsal supine position on a V-tilt table after intubation and anesthesia with ketamine-diazepam and isoflurane. A pediatric speculum was used to access the vaginal vault to visualize the IVR placement and to obtain vaginal secretion samples. An Olympus colposcope with a digital camera attachment was used to assess the cervix and vagina by white-light low-power magnification (×7.5 to ×12). At the completion of the exam, the sheep were extubated and observed for resumption of activity before being returned to the vivarium.
Serum, local vaginal secretions, and cervicovaginal lavage samples were collected at predetermined time points (days 0, 7, 14, 21, and 28). Vaginal biopsy specimens were obtained on day 28 of the study. Blood was drawn from the external jugular vein and centrifuged to separate the plasma. Local vaginal secretions 0 to 2 cm from the ring were collected using Weck-Cel sponges placed at the vaginal wall for approximately 1 min. Samples from cervicovaginal lavages (CVLs) were collected by gently infusing phosphate-buffered saline solution (10 ml) into the vaginal vault via a sterile 10-ml syringe attached to a sterile pediatric Foley catheter (size 5 or 8 French) of adjusted length, and CVL fluid was drawn out with the same device.
Vaginal tissue samples from two animals were collected for bioanalysis, and vaginal tissue samples from the other two animals were preserved for histology. Tissue samples were collected from the vagina (midvagina), vault (vagina near cervix), cervix, and uterus. The vagina samples were approximately 1.5 by 0.5 cm and were in the midvagina to lower vagina, within 1 to 2 cm of where the IVR was located. The vault samples were similar in size and were taken from a section of the vaginal tract that was adjacent to where the IVR was located. Cervix samples also were adjacent to the IVR and were approximately 1.5 cm in diameter, while the uterus samples were similar in size to the vaginal tissue samples. Samples were stored at −80°C and shipped overnight on dry ice to Oak Crest Institute for analysis. Biopsy specimens for histology of the midvagina, vault, and cervix were obtained using a 3.0- to 3.5-mm Keyes punch biopsy tool.
Used intravaginal implants were analyzed for residual drug content at Oak Crest Institute using the following methods. The drug pods were excised from the devices, suspended individually in 50% methanol (100 ml), sonicated until a homogeneous solution was obtained, diluted 1:10 in deionized water, and analyzed by HPLC.
The CVL fluid, plasma, and tissue samples were analyzed at Oak Crest Institute using the LC/MS method described for the in vitro studies. Sample preparation was carried out as follows: for Weck-Cel, see above; for CVL fluid, filtration through a 0.2-μm syringe filter; for plasma, protein precipitation with 4 volumes of acetonitrile containing formic acid (1% vol/vol) and internal standard (1 μg ml−1 lamivudine), evaporation to near dryness in a SpeedVac, and reconstitution in HPLC-grade water (50 μl), with an injection volume of 50 μl and lamivudine (m/z 230.0; retention time, 5.85 min) used as the internal standard; for tissue biopsy specimens, extraction of pulverized sample into internal standard solution (5 ml; 1 μg ml−1 lamivudine in 50% methanol), followed by incubation at 37°C for 30 min, sonication at 40°C for 30 min, protein precipitation with 4 volumes of acetonitrile, evaporation to near dryness in a SpeedVac, and reconstitution in HPLC-grade water (250 μl), with an injection volume of 50 μl. All bioanalyses were performed with at least 10 standards spanning the target concentration range. The standards were analyzed at the front and back ends of the runs along with quality controls, at least three per run. Standards and quality controls were prepared by spiking the appropriate matrix with known amounts of the target analytes. The lower limits of quantitation of these analytical methods are shown in Table 2, and the run-to-run coefficient of variation for all methods was less than 20%.
Statistical analysis.
Data were analyzed by using Microsoft Excel 2007. Statistical significance within experimental groups was determined by the two-tailed unpaired Student's t test (significance level [α] = 0.05).
RESULTS
In vitro studies.
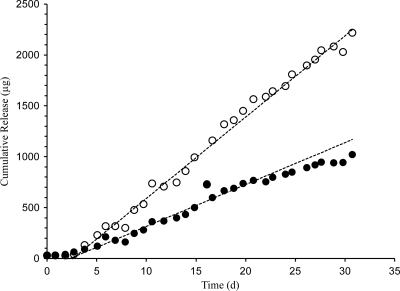
Profiles of in vitro drug release from the IVRs, shown in Fig. 3, exhibited pseudo-zero-order kinetics (i.e., linear cumulative drug release over time; R2 = 0.943 [ACV] and 0.959 [TFV]) and sustained release of ACV and TFV over the 30 days. The in vitro release rates calculated from the slopes of linear plots of the mean cumulative concentrations over this time period are presented in Table 3. Additional details on the multidrug IVR design and in vitro performance are presented elsewhere (26; Moss, presented at Microbicides 2010).
Fig 3.
In vitro cumulative release (means; n = 5) of ACV (solid circles) and TFV (open circles) from combination pod IVRs (16 mg loading for each drug) into VFS.
Table 3.
In vitro-in vivo correlation (IVIVC) of IVR data
| Animal | Release rate (μg day−1) |
IVIVCe |
||||
|---|---|---|---|---|---|---|
|
In vitroa,b,c |
In vivoa,d |
|||||
| ACV | TFV | ACV | TFV | ACV | TFV | |
| New Zealand White rabbits | 41 ± 11 | 80 ± 12 | 340 ± 340b | 320 ± 210b | 8.3 | 4.0 |
| Merino sheep | 82 ± 23 | 160 ± 24 | 170 ± 10f | 190 ± 30f | 2.1 | 1.2 |
Means ± SD.
Five replicates.
Full rings containing two pods of each drug were produced and cut into segments, each segment containing one ACV pod and one TFV pod, which were tested in vitro.
Determined from residual drug analyses.
Defined as in vivo release rate divided by in vitro release rate.
Four replicates.
In vivo studies. (i) Safety profiles.
No serious adverse effects were observed in either in vivo study. No test article-related macroscopic observations were made in female rabbits. In the sheep study, no toxicity or ring expulsions were noted by colposcopy or histology measures.
(ii) Drug profiles.
Weck-Cel sponge drug levels were below the lower limit of quantitation (LLOQ) for most samples in the 28-day rabbit study. Only 3 in 30 samples (10%) showed quantifiable ACV levels, and 1 in 30 samples (3%) showed quantifiable TFV levels. These results were surprising, since the tissue levels, even in distal vaginal tissue, and plasma levels (see below) are suggestive of drug release from the IVRs in vivo. A possible explanation for this observation is the small amount of vaginal secretions collected with the Weck-Cel sponges near the vaginal introitus. Moreover, these distally collected secretions are likely diluted by urination (see discussion of rabbit anatomy below).
Tissue drug levels at day 28 of the rabbit study are summarized in Table 4. TFV and ACV levels in distal vaginal tissue were strongly correlated ([ACV] = (0.37 × [TFV]) + 1.7; R2 = 0.996), while levels in proximal vaginal tissue were uncorrelated. Distal vaginal tissue and lymph node tissue TFV levels also were strongly correlated (R2 = 0.928). Intracellular TFV diphosphate levels were below the 100-ng g−1 LLOQ for all samples.
Table 4.
Summary of drug levels measured in vaginal tissue samples on day 28 of the rabbit study (n = 5)a
| Tissue type | Concn (ng g−1) |
|||
|---|---|---|---|---|
| ACV |
TFVb |
|||
| Mean ± SD | Max | Mean ± SD | Max | |
| Distal vagina | 80 ± 101 | 208 | 209 ± 272 | 574 |
| Proximal vagina | 103 ± 96 | 261 | 904 ± 994 | 2,500 |
| Iliac lymph node | NAc | NA | 140 ± 200 | 461 |
Although six animals were used in the study, one animal was found to have no IVR segment at time of necropsy; therefore, data from that animal were removed from all analyses.
These levels correspond to free TFV.
Samples were not analyzed for ACV.
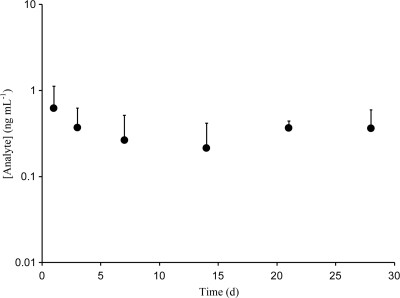
Rabbit ACV plasma levels are shown in Fig. 4. Mean concentrations were below 1 ng ml−1 but remained constant for the 28 days, suggesting sustained release from the IVR. Rabbit TFV plasma levels were around or below the LLOQ: mean ± standard deviation (SD), 0.8 ± 1.2 ng ml−1; maximum, 5.3 ng ml−1.
Fig 4.
Rabbit plasma ACV levels (means and standard deviations; n = 5) from the in vivo study. TFV levels mostly were below the LLOQ.
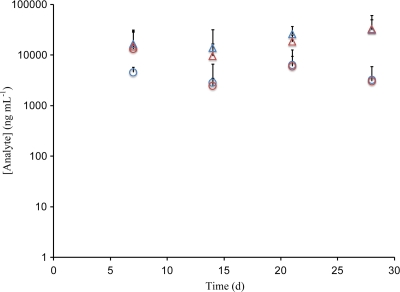
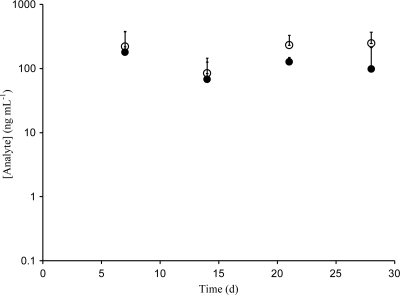
ACV and TFV levels in sheep vaginal secretions (n = 4), as determined from collected Weck-Cel sponges, are shown in Fig. 5. Samples were collected at the upper vagina (mean absorbed mass = 28 ± 17 mg, per sponge), but anteriorly (on the bladder side), and were the farthest from the IVR, while samples of pooled vaginal secretions were collected posteriorly, near the cervix and close to the IVR (mean absorbed mass = 66 ± 43 mg, per sponge). Drug levels at distal and proximal locations (ACV distal, 5.25 ± 7.31 μg ml−1; ACV proximal, 3.64 ± 9.75 μg ml−1; TFV distal, 22.2 ± 7.31 μg ml−1; TFV proximal, 18.9 ± 14.1 μg ml−1) in vaginal secretions were indistinguishable (P > 0.45, sample size = 16 for each population) (12) and constant over the course of the 28 days, suggesting that sustained release was achieved. On average, TFV levels in vaginal secretions were four times the ACV levels. ACV and TFV levels in sheep CVL samples (n = 4) are shown in Fig. 6. Mean levels were 118 ng ml−1 (ACV) and 196 ng ml−1 (TFV). Drug levels in CVL fluid represent a dilution of the levels present in the vaginal fluid, and the lavage was performed after the Weck-Cel sampling, which removed additional amounts of vaginal secretions and led to further dilution.
Fig 5.
Levels of ACV (circles) and TFV (triangles) in vaginal secretions (blue, distal; red, proximal) measured via Weck-Cel from sheep in vivo study. Data are means and standard deviations (n = 4).
Fig 6.
Levels of ACV (solid circles) and TFV (open circles) in cervicovaginal lavage fluid from the sheep in vivo study. Data are means and standard deviations (n = 4).
Day 28 sheep ACV and TFV tissue levels are provided in Table 5. While intracellular and extracellular TFV levels were measured in the rabbit study, only total tissue levels were measured in the sheep study. Ideally, drug levels are assayed only in the specific cell types where the antiviral activity is manifested (e.g., CD4 cells for TFV) to remove the signal noise introduced by contributions from other cell types and intracellular fluid. These assays, however, are highly resource intensive and were not warranted in the context of this preliminary report. As with the rabbit study, plasma drug levels in the sheep were mostly below the LLOQ for TFV. Plasma ACV levels were below the LLOQ on day 7, 2.1 ± 2.5 ng ml−1 on day 14, 1.2 ± 2.0 ng ml−1 on day 21, and 3.3 ± 6.4 ng ml−1 on day 28.
Table 5.
Summary of drug levels measured in vaginal tissue samples on day 28 of the sheep study (n = 2)a
| Tissue typeb | Concn (ng g−1) |
|||
|---|---|---|---|---|
| ACV |
TFV |
|||
| Subject S484 | Subject S536 | Subject S484 | Subject S536 | |
| Vagina | 203 | 142 | 137 | 49 |
| Vault | 52 | 46 | 23 | 28 |
| Cervix | 70 | 67 | 14 | 29 |
| Uterus | 170 | 81 | 18 | 11 |
Four animals were used in the study; vaginal tissue from two was used for bioanalysis and vaginal tissue from the remaining two was preserved for histology.
See Materials and Methods for details on sampling sites.
In vitro-in vivo correlation (IVIVC).
The pharmaceutical industry primarily uses IVIVC for quality control, and most IVIVCs seek to mimic the dissolution of oral formulations in the gastrointestinal tract (22). In the present study, however, IVIVC was investigated in an attempt to establish a framework minimizing the number of in vivo studies required to reach the target drug levels. The goal is to have in vitro experiments guide parameter selection in the development of sustained-release formulations. The calculated IVIVC for ACV and TFV delivered via IVRs in rabbits and sheep is given in Table 3, and these results may be explained by the anatomical and physiological differences between the two models described above.
DISCUSSION
Conventional IVR designs consist of devices that contain the solid drug homogeneously dispersed throughout the polymeric matrix, so-called “matrix IVRs,” and IVRs with a drug-loaded polymer layer below the surface of the ring, positioned between nonmedicated polymer layers, so-called “sandwich” or “reservoir rings” (26). The “pod IVR” design described here has been termed novel (26) because it consists of polymer-coated drug cores, referred to as pods, positioned in the unmedicated ring. Multiple pods, up to 40 mg each, have been loaded into a single IVR, up to 10 with the current design, enabling total drug loadings of up to 400 mg. The drug release rates from the IVRs are determined by the pods' biocompatible polymer membrane(s) and by the characteristics (e.g., number, geometry, and cross-sectional area) of the delivery channels in the impermeable IVR structure (Moss, presented at Microbicides 2010). These parameters allow sustained drug release from the IVR to be controlled with pseudo-zero-order kinetics (Fig. 3) for drugs spanning a range of aqueous solubilities. The features of the modular design hold three important implications: (i) up to 10 different drugs theoretically can be released from a single IVR, each with different, controlled linear cumulative release rates; (ii) drugs with high water solubility (e.g., TFV and ACV) can be released with constant daily rates (i.e., no initial burst), something that has proven to be challenging using matrix designs (26); and (iii) the release rate from each pod can be titrated to the requirements of the application over a wide dynamic range, by a factor of 10 and 40 for ACV and TFV, respectively (Moss, presented at Microbicides 2010). Finally, controlled and sustained release is independent of the ring material, which offers flexibility in polymer choice that could be important for future large-scale production.
The above in vivo data demonstrate that sustained-release TFV and ACV in both species over the course of the study was achieved. This finding represents the first report of a combination IVR releasing these antiviral agents in vivo in a controlled fashion. The ability to release multiple drugs independently from a single IVR provides important flexibility in drug choice using a platform that is likely to increase compliance, a key recommendation of the recent CAPRISA 004 trial (22a). Low systemic levels were obtained for both drugs in both animal models, a benefit of the topical delivery system, as it may reduce the risk of the emergence of resistance. The significantly higher corresponding drug concentrations measured in genital tissues are desirable, as this biological compartment is the relevant site of action for both drugs. Future pharmacokinetic and pharmacodynamic (PK-PD) studies will need to address how the cervicovaginal levels in these animal models relate to efficacious pre-exposure prophylaxis in humans (23).
The rabbit model data were more variable than those obtained with sheep. This finding could be due to the surgical implantation of the intravaginal device. The observed variability in the in vivo release rates in rabbits makes the IVIVC spurious, because of the large range in corresponding geometric mean ratios. While the in vivo release rate is twice that of the in vitro rate for sheep, the in vivo release rate in rabbits cannot be said to be 4 to 8 times higher than the in vitro rate. This observation, along with the undetectable ACV and TFV levels in rabbit vaginal secretions, point to a superiority of the sheep model in the present study. The rabbit model has been used previously to test the IVIVC and plasma levels of hormonal contraceptives from silicone devices (9) and was employed recently to compare polymer matrices and drug loadings in terms of in vivo release of an antiviral agent delivered from IVR segments (10). The sheep model has been developed as a cost-effective large-mammal animal model for study of the human reproductive system. To our knowledge, the current report is the first (according to a Medline search) to describe a sheep study examining the multicompartment concentration profiles of antiviral agents delivered from IVRs.
There are several physiological differences between the animal models and humans that may impact the pharmacokinetics of drugs delivered from intravaginal devices. The sheep vaginal toxicity model has been described as a good model based on gross and microscopic anatomical similarities between the sheep and human vaginal tract (41). Like the human, the sheep vaginal epithelium is stratified squamous tissue, but it is thinner, providing a more sensitive model of toxicity. The vaginal cavity is slightly smaller than that of a human but can accommodate human-sized IVRs, an advantage over other models, such as the macaque and rabbit. The rabbit model also has the disadvantage that intra-abdominal surgery is required for suturing the ring segment in place. The rabbit vagina is longer than the human vagina, with a urovaginal sphincter separating the lower urovagina and upper two thirds of the vagina, the cervicovagina (8). In addition, the epithelium of the upper cervicovagina is a columnar cell monolayer, similar to the human endocervix, whereas the epithelium of the lower urovagina is stratified squamous (5, 7). It is not practical to place the drug-containing rod in the urovagina since it will continually be exposed to urine, flushing out the drug and affecting pharmacokinetics studies. The drug-containing rod is placed in the rabbit cervicovagina, which requires drug absorption through the single layer of columnar cells. In humans and sheep absorption of drug from the IVR is through stratified squamous epithelium.
The sheep estrous cycle is 17 days, with cycles throughout most of the year. The rabbit is a reflex ovulator, and rabbits do not have a typical estrous cycle. They have a 16- to 18-day cycle with a 12- to 14-day period of receptivity, during which the doe will have ovulation induced by coitus (8). While ideally an animal model would emulate the human menstrual cycle, there exists great variation in ovulatory cycles among women, with 21% of American women using hormonal contraceptives that suppress ovulation (39) and 6 to 10% of reproductive-age women experiencing anovulation (2). A limitation in animal models is the fact that the vaginal pH of humans is acidic, with values reported to be 4.0 to 5.0 (13), while the pH of most animals, including rabbits (8), sheep (41), and macaques (11), is close to neutral for the majority of the cycle. Vaginal pH differences could affect the pharmacokinetics of drug delivered to the vagina, but this cannot be avoided with preclinical models.
A novel, dual-protection, pod IVR platform capable of delivering ACV and TFV was evaluated in rabbit and sheep models. The devices showed preliminary safety and exhibited sustained release of both drugs independently and in a controlled fashion over the 28-day studies. The data suggest that the IVR based on the pod design has potential in the prevention of transmission of HIV-1 and other sexually transmitted infections.
Supplementary Material
ACKNOWLEDGMENTS
We thank the National Institutes of Health (grants 5R21AI079791 and 5R21AI076136), CONRAD (service contracts PSA-08-10 and PPC-09-017), the International Partnership for Microbicides, and the U.S. Agency for International Development (cooperative agreement GPO-A-00-05-00041-00) for funding support. The support of Missy Peet and Devon Kyle of MPI Research, Inc., in coordination of the rabbit studies and sample bioanalytical analysis is gratefully acknowledged. The views expressed by the authors do not necessarily reflect those of USAID.
Footnotes
Published ahead of print 28 November 2011
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1. Reference deleted. [Google Scholar]
- 2. American Congress of Obstetricians and Gynecologists 2000. Practice bulletin, management of anovulatory bleeding, clinical management guidelines for obstetrician-gynecologists, p. 1049–1056 ACOG 2009 compendium, vol. 14 American Congress of Obstetricians and Gynecologists, Washington, DC [Google Scholar]
- 3. Anglewicz PA, Bignami-Van Assche S, Clark S, Mkandawire J. 2010. HIV risk among currently married couples in rural Malawi: what do spouses know about each other? AIDS Behav. 14:103–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Antoniou T, Park Wyllie LY, Tseng AL. 2003. Tenofovir: a nucleotide analog for the management of human immunodeficiency virus infection. Pharmacotherapy 23:29–43 [DOI] [PubMed] [Google Scholar]
- 5. Barberini F, Desantis F, Correr S, Motta PM. 1992. The mucosa of the rabbit vagina—a proposed experimental model for correlated morphofunctional studies in humans. Eur. J. Obstet. Gynecol. Reprod. Biol. 44:221–227 [DOI] [PubMed] [Google Scholar]
- 6. Cairns G. 26 February 2008, posting date. Microbicides 2008: accurate adherence reporting essential for microbicide trials. NAM Publications, London, United Kingdom: http://www.aidsmap.com/Microbicides-2008-Accurate-adherence-reporting-essential-for-microbicide-trials/page/1429709/ [Google Scholar]
- 7. Carr EB. 1953. The development of the rabbit vagina. J. Anat. 87:423–431 [PMC free article] [PubMed] [Google Scholar]
- 8. Castle PE, Hoen TE, Whaley KJ, Cone RA. 1998. Contraceptive testing of vaginal agents in rabbits. Contraception 58:51–60 [DOI] [PubMed] [Google Scholar]
- 9. Chien YW, et al. 1975. Controlled drug release from polymeric delivery devices. 3. In vitro-in vivo correlation for intravaginal release of ethynodiol diacetate from silicone devices in rabbits. J. Pharm. Sci. 64:1776–1781 [DOI] [PubMed] [Google Scholar]
- 10. Clark MR, et al. 2011. Pharmacokinetics of UC781-loaded intravaginal ring segments in rabbits: a comparison of polymer matrices. Drug Deliv. Trans. Res. 1:238–246 [DOI] [PubMed] [Google Scholar]
- 11. Cole AM, et al. 2010. The formulated microbicide RC-101 was safe and antivirally active following intravaginal application in pigtailed macaques. PLoS One 5:e15111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cumming G, Fidler F, Vaux DL. 2007. Error bars in experimental biology. J. Cell Biol. 177:7–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cunningham FG, et al. 1997. Williams obstetrics, 20th ed Appleton & Lange, Stamford, CT [Google Scholar]
- 14. De Clercq E. 2004. Antiviral drugs in current clinical use. J. Clin. Virol. 30:115–133 [DOI] [PubMed] [Google Scholar]
- 15. Dunkle KL, et al. 2008. New heterosexually transmitted HIV infections in married or cohabiting couples in urban Zambia and Rwanda: an analysis of survey and clinical data. Lancet 371:2183–2191 [DOI] [PubMed] [Google Scholar]
- 16. Eyawo O, et al. 2010. HIV status in discordant couples in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Infect. Dis. 10:770–777 [DOI] [PubMed] [Google Scholar]
- 17. Fleming DT, et al. 1997. Herpes simplex virus type 2 in the United States, 1976 to 1994. N. Engl. J. Med. 337:1105–1111 [DOI] [PubMed] [Google Scholar]
- 18. Freeman EE, et al. 2007. Proportion of new HIV infections attributable to herpes simplex 2 increases over time: simulations of the changing role of sexually transmitted infections in sub-Saharan African HIV epidemics. Sex. Transm. Infect. 83:I17–I24 [DOI] [PubMed] [Google Scholar]
- 19. Freeman EE, et al. 2006. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS 20:73–83 [DOI] [PubMed] [Google Scholar]
- 20. Geonnotti AR, Katz DF. 2010. Compartmental transport model of microbicide delivery by an intravaginal ring. J. Pharm. Sci. 99:3514–3521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guthrie BL, et al. 2009. Sexually transmitted infections among HIV-1-discordant couples. PLoS One 4:e8276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hayes S, Dunne A, Smart T, Davis J. 2004. Interpretation and optimization of the dissolution specifications for a modified release product with an in vivo-in vitro correlation (IVIVC). J. Pharm. Sci. 93:571–581 [DOI] [PubMed] [Google Scholar]
- 22a. Karim QA, et al. 2010. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 329:1168–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Karim SSA, Kashuba ADM, Werner L, Karim QA. 2011. Drug concentrations after topical and oral antiretroviral pre-exposure prophylaxis: implications for HIV prevention in women. Lancet 378:279–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lerbaek A, et al. 2004. Tenofovir treatment in an unselected cohort of highly antiretroviral experienced HIV positive patients. Scand. J. Infect. Dis. 36:280–286 [DOI] [PubMed] [Google Scholar]
- 25. London S. 2008. Most heterosexual HIV transmission in urban Rwanda and Zambia occurs in married or cohabiting couples. Int. Fam. Plan. Perspect. 34:146 [Google Scholar]
- 26. Malcolm RK, Edwards KL, Kiser P, Romano J, Smith TJ. 2010. Advances in microbicide vaginal rings. Antiviral Res. 88:S30–S39 [DOI] [PubMed] [Google Scholar]
- 27. Mbopi-Keou FX, et al. 2000. Interactions between herpes simplex virus type 2 and human immunodeficiency virus type 1 infection in African women: opportunities for intervention. J. Infect. Dis. 182:1090–1096 [DOI] [PubMed] [Google Scholar]
- 28. National Academy Press 1996. The guide for the care and use of laboratory animals. Institute of Laboratory Animal Resources, National Academy Press, Washington, DC [Google Scholar]
- 29. Nel A, et al. 2009. Safety and pharmacokinetics of dapivirine delivery from matrix and reservoir intravaginal rings to HIV-negative women. J. AIDS 51:416–423 [DOI] [PubMed] [Google Scholar]
- 30. Owen DH, Katz DF. 1999. A vaginal fluid simulant. Contraception 59:91–95 [DOI] [PubMed] [Google Scholar]
- 31. Parikh UM, et al. 2009. Complete protection from repeated vaginal simian-human Immunodeficiency virus exposures in macaques by a topical gel containing tenofovir alone or with emtricitabine. J. Virol. 83:10358–10365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Promadej-Lanier N, et al. 2009. Development and evaluation of a vaginal ring device for sustained delivery of HIV microbicides to non-human primates. J. Med. Primatol. 38:263–271 [DOI] [PubMed] [Google Scholar]
- 33. Renzi C, et al. 2003. Herpes simplex virus type 2 infection as a risk factor for human immunodeficiency virus acquisition in men who have sex with men. J. Infect. Dis. 187:19–25 [DOI] [PubMed] [Google Scholar]
- 34. Reynolds SJ, et al. 2003. Recent herpes simplex virus type 2 infection and the risk of human immunodeficiency virus type 1 acquisition in India. J. Infect. Dis. 187:1513–1521 [DOI] [PubMed] [Google Scholar]
- 35. Romano J, et al. 2009. Safety and availability of dapivirine (TMC120) delivered from an intravaginal ring. AIDS Res. Hum. Retrovir. 25:483–488 [DOI] [PubMed] [Google Scholar]
- 36. Saxena BB, et al. 2009. Sustained release of microbicides by newly engineered vaginal rings. AIDS 23:917–922 [DOI] [PubMed] [Google Scholar]
- 37. Smith DJ, et al. 2008. An evaluation of intravaginal rings as a potential HIV prevention device in urban Kenya: behaviors and attitudes that might influence uptake within a high-risk population. J. Women's Health 17:1025–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. UNAIDS 2008. 2008 report on the global AIDS epidemic. UNAIDS, Geneva, Switzerland [Google Scholar]
- 39. US Department of Health Human Services 2010. Use of contraception in the United States 1982-2008, vital and health statistics. U.S. Department of Health and Human Services, Washington, DC [Google Scholar]
- 40. Valenta C. 2005. The use of mucoadhesive polymers in vaginal delivery. Adv. Drug Deliv. Rev. 57:1692–1712 [DOI] [PubMed] [Google Scholar]
- 41. Vincent KL, et al. 2009. High resolution imaging of epithelial injury in the sheep cervicovaginal tract: a promising model for testing safety of candidate microbicides. Sex. Transm. Dis. 36:312–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Voelker R. 2005. Women shoulder growing HIV/AIDS burden. JAMA 293:281–282 [DOI] [PubMed] [Google Scholar]
- 43. Wald A, Link K. 2002. Risk of human immunodeficiency virus infection in herpes simplex virus type 2 seropositive persons: a meta-analysis. J. Infect. Dis. 185:45–52. [DOI] [PubMed] [Google Scholar]
- 44. World Health Organization Towards universal access: scaling up priority HIV/AIDS interventions in the health sector. HIV/AIDS Department, World Health Organization, Geneva, Switzerland [Google Scholar]
- 45. Xu FJ, et al. 2006. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA 296:964–973 [DOI] [PubMed] [Google Scholar]
- 46. Yoo JW, Lee CH. 2006. Drug delivery systems for hormone therapy. J. Control. Release 112:1–14 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.