Abstract
The aim of this study was to describe lamivudine (3TC) pharmacokinetics (PK) in HIV-infected nonpregnant and pregnant women and their fetuses. Samples were collected according to therapeutic drug monitoring from 228 women treated with lamivudine and retrospectively analyzed by a population approach. The samples were also collected from cord blood and amniotic fluid at birth. Lamivudine pharmacokinetics were ascribed to an open two-compartment model with linear absorption and elimination. Mean population parameter estimates (intersubject variability) for women were an absorption rate constant of 1.04 h−1, an elimination clearance rate of 23.6 (0.266) liters · h−1, a central volume of distribution of 109 (0.897) liters, an intercompartmental clearance rate of 6.7 liters/h, and a peripheral volume of distribution of 129 liters. A fetal compartment was linked to maternal circulation by mother-to-cord (or fetus) and cord-to-mother rate constants of 0.463 h−1 and 0.538 h−1, respectively. The amniotic fluid compartment was connected to the fetal compartment with an elimination rate constant of 0.163 h−1 and a fixed-constant swallowing flow. The placental transfer expressed as fetal-to-maternal area under the concentration-time curve (AUC) ratio was 0.86, and the lamivudine amniotic fluid accumulation, expressed as the amniotic fluid-to-fetal AUC ratio, was 2.9. Pregnant women had a 22% higher apparent clearance than nonpregnant and parturient women; however, this increase did not lead to subexposure and should not require a dosage adjustment.
INTRODUCTION
According to the latest United Nations Joint Programme on HIV/AIDS estimates (8a), 15.7 million women are infected with human immunodeficiency virus (HIV) around the world. In 2008, 45% of HIV-infected pregnant women received antiretroviral drugs to prevent transmission to their newborns, compared with 9% in 2004 (8a). After zidovudine, the nucleoside reverse transcriptase inhibitor used most frequently during pregnancy is lamivudine (3TC), due to its favorable safety, tolerability, and convenience (10, 13). However, lamivudine is classified as an FDA pregnancy category C drug.
Physiological changes associated with pregnancy can lead to significant variations in pharmacokinetics (PK) (modified absorption, distribution, and elimination) (2). Two studies on 3TC PK in HIV-infected pregnant women have been reported: one on the day of delivery (12) and the other at the 38th week of pregnancy and 1 week postpartum (14). Those studies concluded that there was a lack of an effect of pregnancy on 3TC PK after 38 weeks of pregnancy. However, lamivudine is eliminated predominantly in urine as unchanged drug (8), and physiological changes in renal plasma flow and glomerular filtration occur shortly after conception and persist throughout the second trimester (6). The impact of pregnancy on 3TC PK before the 38th week of gestation has not been investigated.
Drug studies during delivery are restricted to single maternal plasma samples with umbilical blood samples obtained at time of delivery. Thus, the cord-to-maternal concentration ratio, which is used as an index of relative fetal drug exposure, is always very high because it does not take into account the delay between the last drug administration and the sampling time. Indeed, the placental transfer of 3TC has been described in one study by the ratio between cord blood and maternal concentrations at delivery, and it varied from a negligible value, due to fetal concentrations below the limit of quantification (LOQ), up to 742%, with a median of 106% (12). That same study investigated 3TC amniotic fluid concentrations and estimated 3TC amniotic fluid accumulation by a fluid-to-mother plasma concentration ratio at delivery ranging from 0.26 to 133.3.
In this work, a population pharmacokinetic study was performed on maternal plasma, cord plasma, and amniotic fluid samples in order to (i) estimate the effect of pregnancy on lamivudine pharmacokinetics, (ii) to describe the transfer of lamivudine from mother to fetus using a fetus-to-mother exposure ratio, and (iii) estimate the accumulation of 3TC into the amniotic fluid and estimate the fetal elimination of 3TC into this compartment.
MATERIALS AND METHODS
Patients.
The population included nonpregnant women, pregnant women, and women on the day of delivery receiving oral lamivudine for the treatment of HIV infection and whose antiretroviral drug plasma concentrations were monitored on a routine basis. A lamivudine tablet was administered as a 150-mg twice-daily (BID) (in combination with zidovudine) or a 300-mg once-daily (OAD) (in combination with abacavir) formulation. For each woman, the time that elapsed between administration and samplings, time of dosing, body weight (BW), age, and weeks of gestation were carefully recorded.
Analytical method.
Lamivudine was measured in a 100-μl plasma sample by high-performance liquid chromatography. An internal standard was used. Lamivudine was extracted by solid-phase extraction on a Bond Elut C18 column and separated on a Satisfaction C8 Plus column (250 by 3 mm) with a gradient of solvent A (water with 0.01% trifluoroacetic acid, 2% methanol, and 3% acetonitrile) and solvent B (acetonitrile), as follows: 50% solvent A and 50% solvent B for 30 min, 90% solvent A and 10% solvent B for 30 min, and 98% solvent A and 2% solvent B for 30 min. The UV absorbance at 270 nm was used for the detection of 3TC. The limit of quantification (LOQ) was 0.02 mg/liter. The mean interassay precision for the low-quantity controls was 10%, and the rate of inaccuracies at the LOQ was 4.5%. The overall recovery rate was 65%.
Population pharmacokinetic modeling.
The pharmacokinetics of 3TC in women, cord blood, and amniotic fluid were studied sequentially as follows: (i) the pharmacokinetics of 3TC in adult women were investigated; (ii) the cord blood data were connected to the adult mother model, and the corresponding parameters were estimated, but the woman parameters were fixed; (iii) amniotic fluid was connected to the model, fixing woman and cord blood parameters; and (iv) all the parameters of the integrated model, woman plus cord blood plus amniotic fluid, were estimated simultaneously.
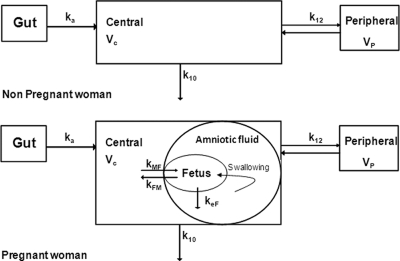
For the women's data, one- and two-compartment models were tested. For cord blood concentrations, two models were tested: an additional compartment linked to the mother compartment and an effect compartment linked by first-order processes to the maternal circulation (Fig. 1). For amniotic fluid concentrations, several models were investigated, such as an additional compartment linked to the fetal compartment and a more physiologic representation: a compartment linked to the fetus by a fixed swallowing flow constant (kSF) (calculated as kSF = 500 ml/24 h/amniotic fluid volume [1 liter]) (16) and an estimated first-order constant of the fetal rate of elimination into the amniotic compartment. Lamivudine concentrations below the LOQ were set to half of the LOQ (3). Several error models were investigated (i.e., multiplicative and additive error models) to describe residual variability. An exponential model was used for intersubject variability (ISV). Only significant ISVs in pharmacokinetic parameters were retained.
Fig 1.
Population pharmacokinetic model for the simultaneous prediction of lamivudine concentrations in the mother, cord, and amniotic fluid. A two-compartment model with first-order absorption and elimination best described maternal data. For concentrations in cord blood, an effect compartment is linked to the maternal plasma compartment by a first-order process. Amniotic fluid concentrations were linked to the fetal compartment via the fetal swallowing flow and fetal elimination rate constant. ka denotes the absorption rate constant, CL denotes the maternal elimination clearance from the central compartment, Vc denotes the volume of the central maternal compartment, Q denotes the maternal intercompartmental clearance, Vp denotes the volume of the peripheral maternal compartment, kMF denotes maternal-to-fetal rate constant, kFM denotes the fetal-to-maternal rate constant, and keF denotes the fetal elimination rate constant.
The covariates tested were women's body weight on the day of sampling, women's age, labor status, and pregnancy status.
The effect of each patient covariate was systematically tested via generalized additive modeling on the basic model.
Continuous covariates (CO), age and body weight, were tested according to the following equation using CL, for example: , where θCL is the typical value of clearance for a patient with the median covariate value and βCOCL is the estimated influential factor for the continuous covariate. When a covariate was missing, it was set to the median value from all the other women.
Delivery was considered a binary covariate, and its influence was tested as follows: , where DEL equals 1 for delivery and 0 is otherwise and where βDELCL is the estimated influential factor for delivery.
The influence of pregnancy on 3TC PK parameters was investigated by using the following two different approaches: (i) by a continuous relation between clearance and gestational age,
where PREG equals 1 if pregnant and 0 is otherwise and βGACL is the estimated influential factor for pregnancy, and
where PREG equals 1 if pregnant and 0 is otherwise, GA is gestational age, and θ1 and θ2 are the estimated influential factors for pregnancy, and (ii) by splitting the clearances according to gestational age in different classes: first trimester of pregnancy (TR1), second trimester (TR2), and third trimester (TR3). The classes were grouped together at each step which was nonsignificant.
A covariate was retained if its effect was biologically plausible; it produced a reduction in the Bayesian information criterion (BIC) and a reduction in the variability of the pharmacokinetic parameter, assessed by the associated intersubject variability.
An intermediate model with all significant covariates was obtained.
A backward elimination phase was finally performed by deleting each covariate from the intermediate model to obtain the final model.
Population pharmacokinetic analysis.
Data were analyzed by using the nonlinear mixed-effect modeling software program Monolix, version 31s (http://wfn.software.monolix.org) (9, 11). Parameters were estimated by computing the maximum likelihood estimator of the parameters without any approximation of the model (no linearization) using the stochastic approximation expectation maximization (SAEM) algorithm combined with an MCMC (Markov chain Monte Carlo) procedure. The number of MCMC chains was fixed to 10 for all estimations. The BIC was used to test different hypotheses regarding the final model and covariate effect on the pharmacokinetic parameter(s). Diagnostic graphics and other statistics were obtained by using the R program. Simulated lamivudine profiles and observed data were compared by a visual predictive check in order to validate the model. The vector of pharmacokinetic parameters from 1,000 patients was simulated by using the final model. The 5th, 50th, and 95th percentiles of the simulated concentrations at each time were then overlaid onto the observed concentration data by using the R program, and a visual inspection was performed.
Maternal and fetal exposure to 3TC and placental transfer.
At delivery, the ratio between cord blood and maternal concentrations was calculated. Maternal and fetal 3TC areas under the curve (AUCs) were derived from the estimated individual PK parameters, and the ratio between fetal and maternal area under the concentration-time curve from 0 to 24 h (AUC0–24) values was calculated.
Amniotic fluid accumulation.
At delivery, the ratio between amniotic fluid and cord blood concentrations was calculated. Amniotic fluid and fetal 3TC areas under the curve were derived from the estimated individual PK parameters, and the ratio between amniotic fluid and fetal AUC0–24 values was calculated.
RESULTS
Demographic data.
Data from 228 women were available for the 3TC pharmacokinetic evaluation. Table 1 summarizes the patients' characteristics.
Table 1.
Characteristics of HIV-infected women (n = 228)
| Group | No. of patients (no. of samples) | Median age (yr) (range) | Median body wt (kg) (range) | Median gestational age (wk) (range) |
|---|---|---|---|---|
| Whole population | 228 (387) | 33 (16–43) | 73.5 (46–119) | 30 (6–41) |
| Pregnant women | 114 (171) | 32 (19–42) | 67.5 (51–115) | 29 (6–39) |
| Women in labor | 123 (128) | 33 (23–43) | 73.5 (51–119) | 38 (36–41) |
| Nonpregnant women | 47 (88) | 34 (16.5–43) | 57 (46–98) |
Population pharmacokinetics.
A total of 387 concentrations from women and 125 cord blood and 44 amniotic fluid concentrations were used for the pharmacokinetic analysis: a total of 171 samples were obtained during pregnancy (9 in the first trimester, 55 in the second trimester, and 105 in the third trimester), 128 were obtained on the day of delivery, and 88 were obtained after pregnancy. The range of sampling times was 0.2 to 37 h after the dose. Sampling times higher than 30 h were reported for women on the day of delivery. Eighteen maternal concentrations, two cord blood concentrations, and one amniotic fluid concentration were lower than the LOQ, so they were set to half of the LOQ. All plasma samples were collected at steady state. Eleven body weights were missing, so they were set to the median body weight value. A two-compartment model with first-order absorption and elimination best described the women's data. The effect compartment satisfactorily described cord blood concentrations. Amniotic fluid concentrations were best described as a compartment connected to the fetal compartment via fetal swallowing flow (16) and first-order fetal elimination; the differential equations used are presented in the appendix. Estimated parameters of the model were the maternal absorption rate constant (ka), maternal elimination clearance (CL), maternal central volume of distribution (Vc), maternal intercompartmental clearance (Q), maternal peripheral volume of distribution (Vp) maternal-to-fetal rate constant (kMF), fetal-to-maternal rate constant (kFM), and fetal elimination rate constant (keF). Since 3TC was administered orally, CL/F, Vc/F, Q/F, and Vp/F were apparent parameters, where F is the unknown bioavailability. The fetal swallowing flow constant was calculated as follows: 500 ml/24 h/amniotic fluid volume (1 liter) (16). The available data were not sufficient to estimate intersubject variability for ka, Q/F, Vp/F, kMF, kFM, and keF, and fixing the variance of these random effects to zero did not increase the BIC value. Variabilities were thus estimated for CL/F and Vc/F. The residual variabilities were best described by a proportional error model.
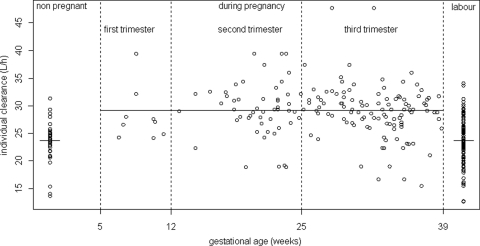
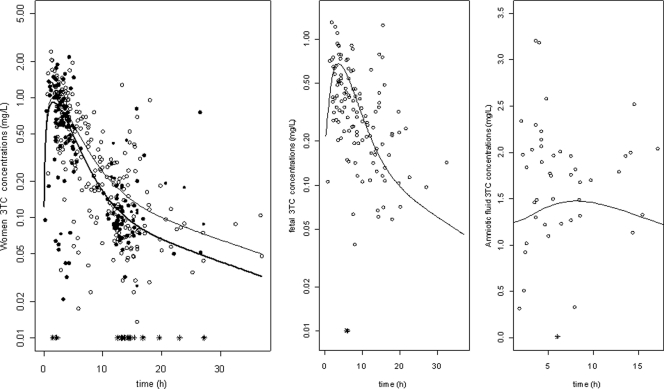
Body weight and age had no significant effect on the apparent clearance and apparent volume of distribution of 3TC. Delivery had no significant effect on CL/F, so parturient women had the same clearance as that of nonpregnant women. Pregnancy had a significant effect on CL/F considering pregnancy as a binary covariate: pregnant women had a 22% higher apparent clearance rate than nonpregnant women. Figure 2 represents individual clearances of lamivudine as a function of gestational age, showing the increase in the rate of clearance during pregnancy. A more precise relationship between gestational age and clearance could not be established, probably because of a lack of plasma samples obtained in the first trimester of pregnancy. Figure 3 displays observed and predicted plasma concentrations of 3TC as a function of time for the women, the cord blood, and the amniotic fluid compartments.
Fig 2.
Individual women's lamivudine clearances (liter/h) as a function of gestational age (weeks).
Fig 3.
(Left) Observed and model-predicted lamivudine concentrations versus time in nonpregnant women (open circles and thin line) and pregnant women (filled circles and thick line). (Middle) Observed (open circles) and predicted (line) concentrations in cord blood. (Right) Observed (open circles) and predicted (line) concentrations in amniotic fluid. Asterisks indicate concentrations lower than the LOQ.
Evaluation and validation.
The final model performance was appreciated by comparing population predicted and individual predicted concentrations to observed plasma concentrations and population weighted residuals versus predicted concentrations and versus time for 3TC (not shown).
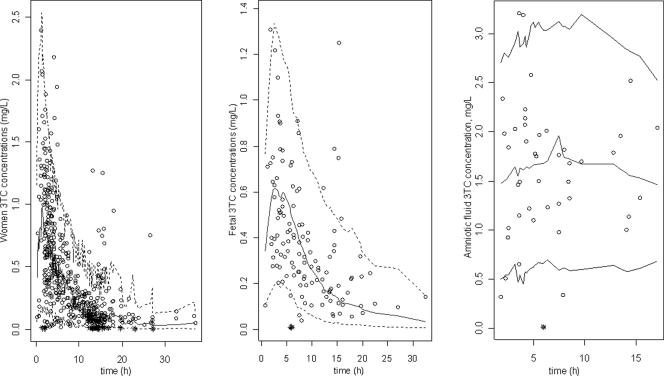
As shown in Table 2, all the parameters are well estimated, with small percent relative standard errors (RSE%). The visual predictive check (Fig. 4) confirmed that the average prediction matched the observed concentrations, and the variability was well estimated.
Table 2.
Population pharmacokinetic parameters of lamivudine from the final modela
| Model and parameter | Estimated value (RSE%) |
|---|---|
| Structural model | |
| ka | 1.04 (30) |
| CL/F (liters/h) | 23.6 (5) |
| Vc/F (liters) | 109 (15) |
| Q/F (liters/h) | 6.7 (22) |
| Vp/F (liters) | 129 (37) |
| θPREG on CL/F | 1.22 (5) |
| kMF (h−1) | 0.463 (20) |
| kFM (h−1) | 0.538 (17) |
| keF (h−1) | 0.163 (7) |
| Statistical model | |
| ωCL/F | 0.266 (14) |
| ωV/F | 0.897 (12) |
| σwomen | 0.498 (5) |
| σcord | 0.405 (9) |
| σamniotic fluid | 0.371 (14) |
RSE%, percent relative standard error; ka, absorption rate constant; CL/F, maternal apparent elimination clearance from the central compartment; Vc/F, apparent central volume of distribution; Q/F, intercompartmental clearance; Vc/F, apparent peripheral volume of distribution; kMF, maternal-to-fetal rate constant; kFM, fetal-to-maternal rate constant; keF, fetus elimination rate constant; θPREG, effect of pregnancy on CL/F; σ, residual variability estimates (proportional error model); ω, interindividual variability estimates.
Fig 4.
Evaluation of the final model by comparison between the 5th (dash line), 50th (solid line), and 95th (dashed line) percentiles obtained from 1,000 simulations and the observed data (open circles) for lamivudine concentrations in women (left) and cord blood (right). Asterisks indicate concentrations lower than the LOQ.
Maternal and fetal exposure to 3TC and placental transfer.
Table 3 summarizes the apparent clearance and area under the curve (AUC) obtained after a 300-mg daily dose of 3TC in this study with patients split into three groups, nonpregnant women, pregnant women, and parturient women, compared to results of previous studies with adults (7, 15, 17). On the day of delivery, the median delay between the administration of 3TC to the mothers and delivery was 7.5 h (minimum to maximum [min-max], 0.7 to 37 h). At delivery, the median observed cord blood and maternal concentrations were 0.342 mg/liter (min-max, 0.01 to 1.31 mg/liter) and 0.289 mg/liter (min-max, 0.01 to 1.66 mg/liter), respectively. The median observed cord blood concentration-to-maternal concentration ratio was 1.22 (range, 0.32 to 21.8). The predicted median maternal 3TC AUC0–24 was 12.50 mg · h/liter (min-max, 8.81 to 23.58 mg · h/liter), given that the maternal pharmacokinetic variability for the 0- to 24-h fetal exposure period varied from 7.59 to 20.30 mg · h/liter. Placental transfer was estimated by the ratio between fetal and maternal 3TC AUCs for 24 h (4). The predicted fetal-to-maternal AUC0–24 ratio was 0.86.
Table 3.
Median apparent clearances and areas under the curve after a 300-mg daily dose of 3TC
Amniotic fluid accumulation.
At delivery, the median observed amniotic fluid concentrations were 1.80 mg/liter (min-max, 0.01 to 3.21 mg/liter). The median observed amniotic fluid-to-fetal concentration ratio was 4 and ranged from 0.43 to 15. The median calculated ratio between amniotic fluid and fetal AUC0–24 values was 2.9.
DISCUSSION
In the present work, the pharmacokinetics of lamivudine in women, cord blood, and amniotic fluid were satisfactorily described by the proposed compartmental model. The following observations support the validity of this model: (i) in women, cord blood, and amniotic fluid, the population predicted concentrations were well correlated with the observed concentrations, and (ii) the population model was validated with the visual predictive checks (VPC) method. Moreover, pharmacokinetic parameters obtained from our population model were close to the values reported in previous studies (Table 3). The fetal elimination rate constant estimated with these amniotic fluid data is close to the elimination rate constant calculated with another PK model for neonates and children (5).
Moodley et al. previously reported 3TC pharmacokinetics in women at week 38 of pregnancy and 1 week after delivery and concluded that there was a lack of an effect of pregnancy on lamivudine disposition (14). In our study, we analyzed 3TC pharmacokinetics not only in late pregnancy but also from week 6 to week 39 of gestation, during labor, and after pregnancy. We observed that the rate of lamivudine clearance was about 22% higher in women during pregnancy. Lamivudine is eliminated predominantly in the urine as unchanged drug (15). The rate of renal clearance of lamivudine is higher than the glomerular filtration rate (GFR), which implies that lamivudine is actively secreted into the renal tubule (8). In pregnancy, the GFR and effective renal plasma flow increase to levels 50% to 80% above levels for nonpregnant women (2, 6). This increase occurs shortly after conception and persists throughout the second trimester (6). A decrease in the GFR during the last 3 weeks of pregnancy was also reported previously, reaching postpartum values by the last week of pregnancy (2). In our study, the increased clearance in pregnant women can be explained by these physiological changes in the GFR during pregnancy. The description of clearance as a function of gestational age probably failed because of the lack of concentration data in the first trimester, when the increase in the GFR occurs. Tubular secretion is dependent on saturable membrane transport proteins. Very little is known regarding the effect of pregnancy on tubular secretion and/or reabsorption (2).
The level of 3TC exposure in pregnant women, although lower than the level of exposure in nonpregnant and parturient women, was relatively close to data reported previously for nonpregnant adults (Table 3), and no dosage adjustment seems to be necessary to reach the adult AUC value.
Few data on 3TC placental transfer have been reported. A previous study by Mandelbrot et al. described placental transfer by a simple cord blood-to-maternal concentration ratio, which is highly variable, from a negligible value up to 742% (12). In our study, from one sample at delivery (at various times after drug administration) for each mother-cord pair, we were able to assess the maternal and cord blood concentration profiles over time. Placental transfer could be estimated as a fetal-to-maternal drug exposure ratio, which was estimated to be 86%.
The high 3TC amniotic fluid concentration can be explained as follows: 3TC diffuses from the maternal blood to fetal blood through the placenta, the fetal kidney removes 3TC from fetal blood and concentrates it in urine, and fetal micturition causes the rise in the concentration of 3TC in amniotic fluid. 3TC returns from amniotic fluid to fetal blood mainly because of fetal swallowing, and the larger part of this 3TC will again be excreted by the kidney into the amniotic compartment. This mechanism is satisfactorily described by our model and seems to be applicable to other substances cleared mainly by kidney, as was described previously for para-amino-hippurate (1). This model allowed us to estimate the capacity for fetal elimination into the amniotic fluid compartment. We could also draw the 3TC PK profile for the amniotic fluid and thus express the accumulation into this compartment via an exposition constant ratio of 3. The high 3TC amniotic fluid concentrations may have clinical implications for the fetus, either beneficial or detrimental. Indeed, the lamivudine swallowed may account for an oral loading dose for the fetus; it can also be protective against concomitant exposure to infectious HIV by the oral route. On the other hand, the potential toxicity of perinatal exposure to nucleoside analogs may be of concern.
In conclusion, maternal, fetal, and amniotic fluid lamivudine pharmacokinetics were accurately described by the proposed model. The apparent rate of clearance of lamivudine was increased by 22% in pregnant women compared to nonpregnant or parturient women. As rates of exposure in pregnant women are close to values reported previously for nonpregnant adults, no dose adjustment should be needed. Maternal-to-fetal transfer was assessed by using an exposure ratio that was about 86%. The fetal elimination rate constant of 3TC for the amniotic fluid was estimated to be 0.181 h−1. The accumulation in the amniotic fluid compartment expressed as an AUC ratio was 3.
APPENDIX
The differential system connected with the model depicted in Fig. 1 is as follows:
where G = D at t = 0,
where A1 = 0 at t = 0,
where A2 = 0 at t = 0,
where A3 = 0 at t = 0, and
where A4 = 0 at t = 0.
G, A1, A2, A3, and A4 correspond to the amounts of 3TC in the gut compartment, the maternal central compartment, the maternal peripheral compartment, the fetal compartment, and the amniotic fluid compartment, respectively.
k10 equals CL/Vc, k12 equals Q/Vc, k21 equals Q/Vp, and kSF (swallowing flow constant) equals 500 ml/24 h/amniotic fluid volume (1 liter) (16). ka denotes the absorption rate constant; CL and Q denote the maternal elimination from the central compartment and intercompartmental clearance, respectively; Vc and Vp indicate the volume of the central and peripheral maternal compartments, respectively; kMF denotes the maternal-to-fetal rate constant; kFM indicates the fetal-to-maternal rate constant; and keF denotes the fetal elimination rate constant.
Footnotes
Published ahead of print 21 November 2011
REFERENCES
- 1. Althabe O, et al. 1976. Transference of para-amino-hippurate from the mother to the amniotic fluid. J. Perinat. Med. 4:227–233 [DOI] [PubMed] [Google Scholar]
- 2. Anderson GD. 2005. Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin. Pharmacokinet. 44:989–1008 [DOI] [PubMed] [Google Scholar]
- 3. Beal SL. 2001. Ways to fit a PK model with some data below the quantification limit. J. Pharmacokinet. Pharmacodyn. 28:481–504 [DOI] [PubMed] [Google Scholar]
- 4. Benaboud S, et al. 2010. Population pharmacokinetics of nevirapine in HIV-1-infected pregnant women and their neonates. Antimicrob. Agents Chemother. 55:331–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bouazza N, et al. 2011. Developmental pharmacokinetics of lamivudine in 580 pediatric patients from neonates to adolescents. Antimicrob. Agents Chemother. 55:3498–3504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Davison JM. 1987. Kidney function in pregnant women. Am. J. Kidney Dis. 9:248–252 [DOI] [PubMed] [Google Scholar]
- 7. Heald AE, et al. 1996. Pharmacokinetics of lamivudine in human immunodeficiency virus-infected patients with renal dysfunction. Antimicrob. Agents Chemother. 40:1514–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Johnson MA, Moore KH, Yuen GJ, Bye A, Pakes GE. 1999. Clinical pharmacokinetics of lamivudine. Clin. Pharmacokinet. 36:41–66 [DOI] [PubMed] [Google Scholar]
- 8a. Joint United Nations Programme on HIV/AIDS and World Health Organization 2009. AIDS apidemic update. UNAIDS, Geneva, Switzerland: http://data.unaids.org/pub/Report/2009/JC1700_Epi_Update_2009_en.pdf [Google Scholar]
- 9. Kuhn E, Lavielle M. 2005. Maximum likelihood estimation in nonlinear mixed effects models. Comput. Stat. Data Anal. 49:1020–1038 [Google Scholar]
- 10. Kumar PN, Patel P. 2010. Lamivudine for the treatment of HIV. Expert Opin. Drug Metab. Toxicol. 6:105–114 [DOI] [PubMed] [Google Scholar]
- 11. Lavielle M, Mentré F. 2007. Estimation of population pharmacokinetic parameters of saquinavir in HIV patients with the MONOLIX software. J. Pharmacokinet. Pharmacodyn. 34:229–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mandelbrot L, Peytavin G, Firtion G, Farinotti R. 2001. Maternal-fetal transfer and amniotic fluid accumulation of lamivudine in human immunodeficiency virus-infected pregnant women. Am. J. Obstet. Gynecol. 184:153–158 [DOI] [PubMed] [Google Scholar]
- 13. Mirochnick M, Capparelli E. 2004. Pharmacokinetics of antiretrovirals in pregnant women. Clin. Pharmacokinet. 43:1071–1087 [DOI] [PubMed] [Google Scholar]
- 14. Moodley J, et al. 1998. Pharmacokinetics and antiretroviral activity of lamivudine alone or when coadministered with zidovudine in human immunodeficiency virus type 1-infected pregnant women and their offspring. J. Infect. Dis. 178:1327–1333 [DOI] [PubMed] [Google Scholar]
- 15. Moore KH, et al. 1996. Pharmacokinetics of lamivudine administered alone and with trimethoprim-sulfamethoxazole. Clin. Pharmacol. Ther. 59:550–558 [DOI] [PubMed] [Google Scholar]
- 16. Pritchard JA. 1966. Fetal swallowing and amniotic fluid volume. Obstet. Gynecol. 28:606–610 [PubMed] [Google Scholar]
- 17. Yuen GJ, et al. 2004. Equivalent steady-state pharmacokinetics of lamivudine in plasma and lamivudine triphosphate within cells following administration of lamivudine at 300 milligrams once daily and 150 milligrams twice daily. Antimicrob. Agents Chemother. 48:176–182 [DOI] [PMC free article] [PubMed] [Google Scholar]