Abstract
According to the European AIDS Clinical Society, tenofovir disoproxil fumarate can be used in HIV-infected pregnant women if started prior to pregnancy, although no data are available on the pharmacokinetics of tenofovir (TFV) during pregnancy. The aim of this study was to describe TFV pharmacokinetics in HIV-infected women and to evaluate the effect of pregnancy on TFV disposition. Samples were collected according to a therapeutic drug monitoring in 186 women, including 46 pregnant women treated with TFV and retrospectively analyzed by a population approach. TFV pharmacokinetics were ascribed to an open two-compartment model with linear absorption and elimination. The mean population parameter estimates (between-subject variability) were as follows: absorption rate constant, 0.56 h−1; elimination clearance, 59.9 liters h−1 (0.436); central volume of distribution, 552 liters (1.96); intercompartmental clearance, 172 liters/h; and peripheral volume of distribution, 1,390 liters. Pregnant women had a 39% higher apparent clearance compared to nonpregnant women. Apparent clearance significantly decreased with age. In order to obtain an exposure similar to the known exposure in adults and guarantee similar trough concentrations (Cmin) as observed in adults, an increase in the TFV dose should be considered for women from the second trimester to delivery.
INTRODUCTION
According to the latest United Nations Joint Programme on HIV/AIDS estimates (9a), 15.7 million women are infected with human immunodeficiency virus (HIV) around the world. In 2008, 45% of HIV-infected pregnant women received antiretroviral drugs to prevent transmission to their newborns, compared with 9% in 2004.
Because of the lack of data on the use of tenofovir disoproxil fumarate (TDF) in pregnancy and concerns over possible bone toxicity, U.S. guidelines recommend that TDF-based highly active antiretroviral therapy (HAART) should be used only after careful considerations of alternatives (8). However, for women who were already treated by TDF prior to pregnancy, European AIDS Clinical Society guidelines recommend to continue the TDF treatment during pregnancy. Thus, in these therapeutic drug monitoring data, some women were taking TDF during their pregnancy in the first, second, and third trimester.
TDF is already taken during pregnancy at the same dose as in adults although physiological changes associated with pregnancy can lead to significant variations in pharmacokinetics (modified absorption, distribution, and elimination). No pharmacokinetic data on the use of TDF before the 38th week of pregnancy are available. Two studies restricted to late pregnancy and labor suggest that tenofovir (TFV) exposure is lower than nonpregnant adult exposure (9, 16).
We performed here a population pharmacokinetic study of women during pregnancy, at delivery, and out of pregnancy in order to investigate TFV pharmacokinetics throughout pregnancy.
MATERIALS AND METHODS
Patients and treatments.
The population included nonpregnant women, pregnant women, and women on the day of delivery receiving oral TFV for treatment of HIV infection and whose antiretroviral drug plasma concentrations were monitored on a routine basis. TFV was administered chronically using a 300-mg once-daily regimen. For each woman, the time elapsed between administration and sampling times, the time of dosing, the body weight, the subject's age in years, and the weeks of gestation were carefully recorded. Ethics committee approval and patient consent are not compulsory in France in order to use therapeutic drug monitoring data, and thus they were not obtained.
Analytical method.
The TFV assay was performed according to a previously published method (10), with a limit of quantification (LOQ) of 0.01 mg/liter, an interassay precision of 9.6%, and a bias of 11.4%.
Population pharmacokinetic analysis.
Data were analyzed using the nonlinear mixed effect modeling software program Monolix version 3.1s (11, 12) (http://wfn.software.monolix.org). Parameters were estimated by computing the maximum-likelihood estimator of the parameters without any approximation of the model (no linearization) using the stochastic approximation expectation maximization (SAEM) algorithm combined with a Markov chain Monte Carlo (MCMC) procedure. The number of MCMC chains was fixed to 10 for all estimations. Likelihood ratio tests, including the log likelihood, the Akaike information criterion (AIC), and the Bayesian information criterion (BIC), were used to test different hypotheses regarding the final model, the covariate effect(s) on pharmacokinetic parameter(s), the residual variability model (proportional versus proportional plus additive error model), and the structure of the variance-covariance matrix for the between-subject variability (BSV) parameters. Diagnostic graphics and other statistics were obtained using the R program (http://wfn.sourceforge.net/). Simulated TFV profiles and observed data were compared using a visual predictive check in order to validate the model. The vector of pharmacokinetic parameters from 1,000 patients was simulated using the final model. The 5th, 50th, and 95th percentiles of the simulated concentrations at each time point were then overlaid on the observed concentration data using the R program, and a visual inspection was performed.
Population pharmacokinetic modeling.
One- and two-compartment models were tested. TFV concentrations below the LOQ were set to half of the LOQ (2). Several error models were investigated (i.e., multiplicative and additive error models) to describe residual variability. Exponential model was used for BSV. Only significant BSVs on pharmacokinetic parameters were retained. The covariates tested were the woman's age and body weight on the day of sampling, labor status, and pregnancy status.
The effect of each patient covariate was systematically tested via generalized additive modeling on the basic model.
(i) Continuous covariates.
The continuous covariates (CO), age and body weight, were tested according to the following equation, using CL, for example:
where θCL is the typical value of clearance for a patient with the median covariate value and is the estimated influential factor for the continuous covariate. When a covariate was missing, it was set to the median value from all of the other women.
(ii) Delivery.
Delivery was considered a binary covariate, and its influence was tested as follows:
where DEL = 1 if delivery and DEL = 0 otherwise, and is the estimated influential factor for delivery.
(iii) Influence of pregnancy.
The influence of pregnancy on TFV pharmacokinetic parameters was investigated using two different approaches. In the first approach, with a continuous relation between clearance and gestational age, the influence of pregnancy was calculated as follows:
| (1) |
where Preg = 1 if pregnant or parturient and Preg = 0 otherwise, and is the estimated influential factor for pregnancy.
| (2) |
where Preg = 1 if pregnant and 0 otherwise, γ and GA50 are the estimated parameters of the Hill equation.
In the second approach, the influence of pregnancy was calculated by splitting the clearances according to gestational age into different classes based on the first (TR1), second (TR2), and third (TR3) trimesters of pregnancy. The classes were grouped together at each step when nonsignificant.
A covariate was kept if its effect was biologically plausible; it produced a reduction in AIC/BIC criteria (verified by a likelihood ratio test) and a reduction in the variability of the pharmacokinetic parameter, as assessed by the associated BSV. An intermediate model with all significant covariates was obtained. A backward elimination phase was finally performed by deleting each covariate from the intermediate model to obtain the final model.
RESULTS
Demographic data.
Data from 186 women (156 nonpregnant women and 46 pregnant women) were available for TFV pharmacokinetic evaluation. Table 1 summarizes the patient characteristics. At all sampling times, of all nonpregnant women, 66% were cotreated with a protease inhibitor (including 49.1% lopinavir-ritonavir cotreatments), 76.7% were cotreated with a nucleoside reverse transcriptase inhibitor (NRTI), and 14.9% were cotreated with non-nucleoside reverse transcriptase inhibitor (NNRTI). Of the pregnant women, 85% were cotreated with a protease inhibitor (including 66% lopinavir-ritonavir cotreatments), 89.6% were cotreated with NRTI, and 12.3% were cotreated with NNRTI.
Table 1.
Characteristics of the HIV-infected women
| Population | No. of patients | No. of samples | Median (range) |
||
|---|---|---|---|---|---|
| Age (yr) | Body wt (kg) | Gestational age (wk) | |||
| Total | 186 | 326 | 37 (16–62) | 64 (36–130) | 30 (2.3–41) |
| Pregnant women | 46 | 77 | 35 (17–43) | 71 (40–122) | 30 (2.3–41) |
| Nonpregnant women | 156 | 249 | 38 (16–62) | 64 (36–130) | |
Population pharmacokinetics.
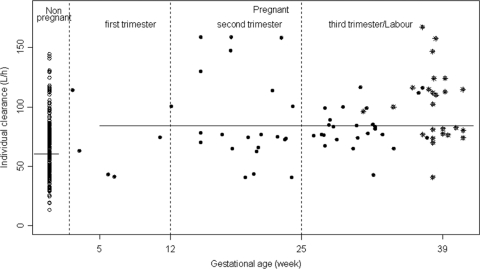
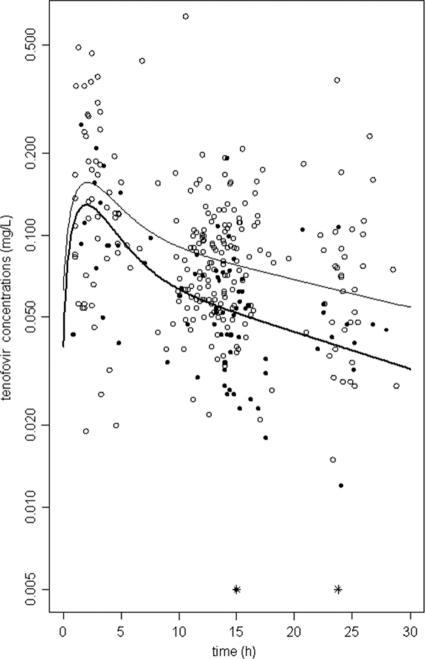
A total of 326 TFV concentrations were used for the pharmacokinetic analysis; among these concentrations, 52 samples were obtained during pregnancy at different gestational ages (6 concentrations in the first trimester of pregnancy, 18 in the second trimester of pregnancy, and 28 in the third trimester of pregnancy) and 25 were obtained on the day of delivery (70 women had more than one sample, and the maximum number of samples per woman was 7). The range of sampling times was 0.8 to 28.8 h after the dose was administered. Two TFV concentrations were lower than the LOQ, so they were set to half of the LOQ. All plasma samples were collected at steady state. A two-compartment model with first-order absorption and elimination best described the data. The parameters of the model were the absorption rate constant (ka), the elimination clearance (CL), the central volume of distribution (Vc), the intercompartmental clearance (Q), and the peripheral volume of distribution (Vp). Since TFV was orally administered, CL/F, Vc/F, Q/F, and Vp/F were apparent parameters, and F is the unknown bioavailability. The available data were not sufficient to estimate the BSV for ka, Q/F, and Vp/F and fixing the variance of these random effects to zero had no influence on the goodness-of-fit criteria. The variabilities were thus estimated for CL/F and Vc/F. A significant covariance of 0.92 was found between CL/F and Vc/F. The residual variability was best described by a proportional-error model. Pregnancy had a significant effect on CL/F: pregnant women and women on the day of delivery had a 39% higher apparent clearance compared to nonpregnant women. Adding pregnancy as a covariate resulted in a 9.3-U decrease in the objective function (OF) value, the BSV on CL/F decreased from 0.50 to 0.46, and it also improved the goodness of fit. Age had a significant effect on the CL/F; indeed, the apparent clearance decreased slightly but significantly with age, and adding age as a covariate resulted in a 8.2-U decrease in the OF value and the BSV on CL/F decreased from 0.46 to 0.43. Figure 1 represents individual clearances of TFV as a function of gestational age, showing the increase of clearance in pregnant versus nonpregnant women. A more precise relationship between gestational age and clearance could not be established, probably because of a lack of plasma samples performed in the first trimester of pregnancy. Figure 2 shows the TFV observed and predicted plasma concentrations as a function of time for nonpregnant and pregnant women.
Fig 1.
Individual TFV clearances (liters/h) as a function of gestational age (weeks). *, During labor.
Fig 2.
TFV concentration-time courses in a semilog scale. Observed (circles) and predicted (lines) TFV concentrations versus time for nonpregnant women (open circles and thin line) and for pregnant women (filled circles and thick line). *, Concentrations lower than the LOQ.
Evaluation and validation.
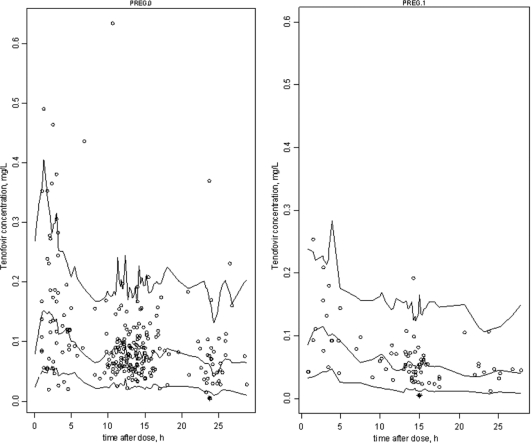
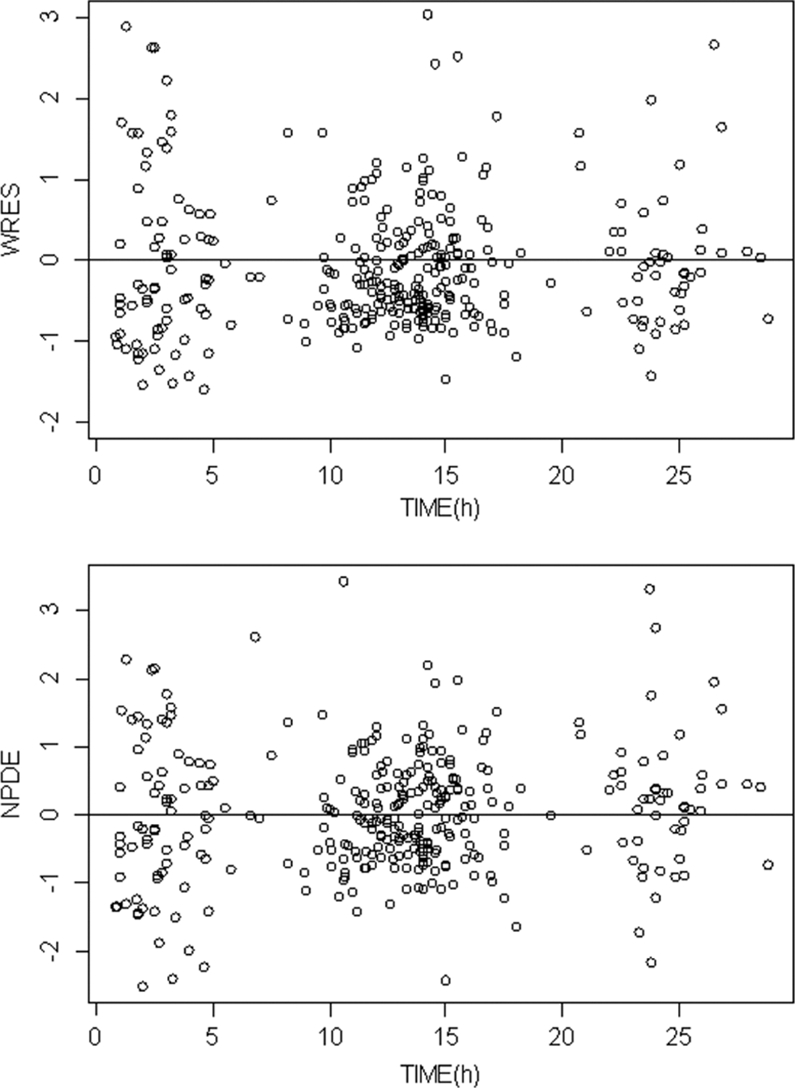
The final model performance was assessed by comparing population and individual predicted values to observed plasma concentrations and population weighted residuals versus predicted concentrations (not shown) and versus time (Fig. 3) for TFV. Figure 3 also shows the normalized predicted distribution errors versus time. As shown in Table 2, all of the parameters are well estimated with small relative standard errors (RSE %). The visual predictive check (Fig. 4) confirmed that the average prediction matched the observed concentrations, and the variability was well estimated.
Fig 3.
Evaluation of the final model: comparison between the 5th, 50th, and 95th percentiles obtained from 1,000 simulations and the observed data (○) for TFV concentrations in nonpregnant women (left) and pregnant women (right). *, Concentrations lower than the LOQ.
Table 2.
Population pharmacokinetic parameters of TFV from the final modela
| Parameter | Estimate (RSE%) |
|---|---|
| Structural model | |
| ka (h−1) | 0.56 (49) |
| CL/F (liters/h) | 59.9 (5) |
| Vc/F (liters) | 552 (43) |
| Q/F (liters/h) | 172 (22) |
| Vp/F (liters) | 1390 (43) |
| θPreg on CL/F | 1.39 (6) |
| θAge on CL/F | 0.49 (22) |
| Statistical model | |
| ωCL/F | 0.43 (5) |
| ωV/F | 1.96 (10) |
| σ (proportional) | 0.34 (6) |
RSE%, percent relative standard error; ka, absorption rate constant; CL/F, maternal apparent elimination clearance from the central compartment; Vc/F, apparent central volume of distribution; Q/F, intercompartmental clearance; Vp/F, apparent peripheral volume of distribution; θPreg, effect of pregnancy on CL/F; θAge, effect of age on CL/F; σ, residual variability estimates (proportional error model); ω, between-subject variability estimates.
Fig 4.
Weighted residuals (WREs) versus time (upper panel) and normalized predicted distribution errors (NPDE) versus time (lower panel).
TFV concentrations in women after a 300-mg TDF administration.
Table 3 summarizes area-under-the-curve (AUC) values for TFV obtained after a 300-mg daily dose of TDF was given to nonpregnant and pregnant women compared to previous studies in adults (4, 3, 14).
Table 3.
Comparison of the concentration and AUC values for the present study versus other studiesa
| Parameter | Mean (95% CI) determined in the present study (n = 186) |
Adult mean according to: |
|||
|---|---|---|---|---|---|
| Nonpregnant subjects (n = 156) | Pregnant subjects (n = 46) | Boffito et al. (n = 29) | Blum et al. (n = 19) | Ramanathan et al. (n = 24) | |
| AUC (mg/liter · h) | 2.4 (1.1–5.2) | 1.6 (0.9–3.3) | 2.62 | 2.65 | 2.88 |
| Cmin (mg/liter) | 0.061 (0.031–0.164) | 0.039 (0.022–0.092) | 0.052 | 0.053 | 0.06 |
Simulations of doses in pregnant women.
AUCs and Cmin values in pregnant women were simulated following the administration of two tablets of TDF (600 mg of TDF, 272 mg of TFV) (Table 4).
Table 4.
Simulated AUC and Cmin values for pregnant women following daily administration of 136 or 272 mg of TFV (which corresponds to 300 and 600 mg of TDF) compared to values for nonpregnant women
| Parameter | Mean (95% CI) |
||
|---|---|---|---|
| Nonpregnant women (one tablet, 136 mg) | Pregnant women |
||
| One tablet (136 mg) | Two tablets (272 mg) | ||
| AUC (mg/liter · h) | 2.4 (1.1–5.2) | 1.6 (0.9–3.3) | 3.4 (1.7–6.5) |
| Cmin (mg/liter) | 0.061 (0.031–0.164) | 0.039 (0.022–0.092) | 0.078 (0.049–0.175) |
DISCUSSION
In the present work, the pharmacokinetics of TFV in women were satisfactorily described by a two-compartment model. The following observations support the validity of this model. (i) The population predicted concentrations correlated well with and observed concentrations, and (ii) the population model was validated by to the visual predictive check method. Moreover, the pharmacokinetic parameters obtained from our population model were close to the values reported in previous studies (Table 3).
Pharmacokinetics of TFV in pregnant women were also described in two previous studies. Hirt et al. (9) reported the TFV pharmacokinetics in women on the day of delivery and 1 week postpartum, and Rodman et al. (16) investigated the pharmacokinetics of TFV after a single dose at the start of labor, the two studies concluded that a maternal 600-mg TDF administration at the start of labor produces the same concentrations as a 300-mg administration in other adults (9, 16). A more recent study confirmed the necessity of an increased dose of TDF during labor (7) and noted that the administration of 600 mg and 900 mg of TDF was safe and well tolerated in HIV-infected women in active labor or prior to caesarean section. In our study, women were treated by TDF during their pregnancy and during delivery by the same 300-mg TDF dose, we confirmed the increase of clearance in parturient women and thus the necessity to increase TDF dose in these women.
Burchett et al. (5) observed a lower TDF exposition in the third trimester of pregnancy compared to postpartum. The magnitude of the AUC decrease in pregnancy was only ca. 15%, however. In our study, we could analyze TFV pharmacokinetics not only in late pregnancy but from weeks 2 to 41 of gestation, during labor, and after pregnancy. We observed that TFV clearance was ca. 39% higher in women during pregnancy compared to nonpregnant women. TFV is primarily excreted by the kidney by a combination of glomerular filtration and active tubular secretion system, with ca. 70 to 80% of the dose excreted unchanged in the urine (15). In pregnancy, the glomerular filtration rate (GFR) and effective renal plasma flow increase to levels 50 to 80% above nonpregnant levels (1, 6). This increase occurs shortly after conception and persists throughout the second trimester (6). In our study, the increased clearance observed during pregnancy could be explained by these physiological changes in GFR during pregnancy. However, no information was available about serum creatinin levels or GFR in our population to confirm it. In addition to the glomerular filtration, TFV undergoes active tubular secretion dependent on hOAT1, hOAT2, and MRP4 (15). Very little is known
regarding the effect of pregnancy on these transporters (1).
In our study, the ages of the woment ranged from 16 to 62 years, and this wide range allowed us to describe the age effect on TFV clearance. Indeed, TFV clearance seemed to decrease slightly but significantly with age, a finding in agreement with the pattern of the GFR decline with aging that has been described in the Kidney Disease Outcomes Quality Initiative (13). The subject age range was higher for nonpregnant women (62 years versus 43 years), but the effect of pregnancy was observed with or without the older nonpregnant women. We found that lopinavir-ritonavir cotreatment had no effect on the apparent clearance of TFV, in contrast to previous studies.
Since no relationship between TFV plasma concentration and virologic response has been established, a reasonable goal for TFV dosing during pregnancy is to achieve plasma exposure in pregnant women equivalent to that observed in nonpregnant adults treated with the standard 300-mg TDF dose. In our study, the Cmin values for nonpregnant women were similar to those reported in nonpregnant adults (Table 3), and their mean TFV exposures expressed as AUCs were slightly lower but not significantly different from nonpregnant adult values (2.4 versus 2.65, P = 0.3). Since TDF is only available in 300-mg tablets, we simulated pregnant women AUCs and Cmin values following the administration of two tablets of TDF (600 mg of TDF, 272 mg of TFV). It appears that the exposure of pregnant women to TFV is low after the administration of one tablet of TDF but may be too high after the administration of two tablets (Table 4).
In conclusion, TFV pharmacokinetics in women were accurately described by a two-compartment model. TFV's apparent clearance was increased by 39% in pregnant women. In order to obtain an exposure similar to the known exposure in adults and to guarantee similar trough concentrations as expected in adults, a TFV dose escalation should be considered for women from the second trimester to delivery. Since there is only limited clinical experience with doses higher than the therapeutic 300-mg TDF dose, further investigations are needed.
Footnotes
Published ahead of print 28 November 2011
REFERENCES
- 1. Anderson GD. 2005. Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin. Pharmacokinet. 44:989–1008 [DOI] [PubMed] [Google Scholar]
- 2. Beal SL. 2001. Ways to fit a PK model with some data below the quantification limit. J. Pharmacokinet. Pharmacodyn. 28:481–504 [DOI] [PubMed] [Google Scholar]
- 3. Blum MR, Chittick GE, Begley JA, Zong J. 2007. Steady-state pharmacokinetics of emtricitabine and tenofovir disoproxil fumarate administered alone and in combination in healthy volunteers. J. Clin. Pharmacol. 47:751–759 [DOI] [PubMed] [Google Scholar]
- 4. Boffito M, et al. 2005. Lack of pharmacokinetic drug interaction between tenofovir disoproxil fumarate and nelfinavir mesylate. Antimicrob. Agents Chemother. 49:4386–4389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burchett S, et al. 2007. Tenofovir pharmacokinetics during pregnancy, at delivery, and postpartum. 14th Conference on Retroviruses and Opportunistic Infections, Los Angeles, CA [Google Scholar]
- 6. Davison JM. 1987. Kidney function in pregnant women. Am. J. Kidney Dis. Apr. 9:248–252 [DOI] [PubMed] [Google Scholar]
- 7. Flynn P, et al. 2011. Pharmacokinetics and safety of single-dose tenofovir disoproxil fumarate and emtricitabine in HIV-1-infected pregnant women and their Infants. Antimicrob. Agents Chemother. 55:5914–5922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Foster C, et al. 2009. Tenofovir disoproxil fumarate in pregnancy and prevention of mother-to-child transmission of HIV-1: is it time to move on from zidovudine? HIV Med. 10:397–406 [DOI] [PubMed] [Google Scholar]
- 9. Hirt D, et al. 2009. Population pharmacokinetics of tenofovir in HIV-1-infected pregnant women and their neonates (ANRS 12109). Clin. Pharmacol. Ther. 85:182–189 [DOI] [PubMed] [Google Scholar]
- 9a. Joint United Nations Programme on HIV/AIDS and World Health Organization 2009. AIDS epidemic update. UNAIDS, Geneva, Switzerland: http://data.unaids.org/pub/Report/2009/JC1700_Epi_Update_2009_en.pdf [Google Scholar]
- 10. Jullien V, Treluyer JM, Pons G, Rey E. 2003. Determination of tenofovir in human plasma by high-performance liquid chromatography with spectrofluorimetric detection. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 785:377–381 [DOI] [PubMed] [Google Scholar]
- 11. Kuhn E, Lavielle M. 2005. Maximum likelihood estimation in nonlinear mixed effects models. Comput. Stat. Data Anal. 49:1020–1038 [Google Scholar]
- 12. Lavielle M, Mentré F. 2007. Estimation of population pharmacokinetic parameters of saquinavir in HIV patients with the MONOLIX software. J. Pharmacokinet. Pharmacodyn. 34:229–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. National Kidney Foundation 2002. Kidney Disease Outcomes Quality Initiative (KDOQI) clinical practice guidelines for chronic kidney disease. 4. Definition and classification of stages of chronic kidney disease, Fig. 9. National Kidney Foundation, New York, NY [Google Scholar]
- 14. Ramanathan S, Shen G, Cheng A, Kearney BP. 2007. Pharmacokinetics of emtricitabine, tenofovir, and GS-9137 following coadministration of emtricitabine/tenofovir disoproxil fumarate and ritonavir-boosted GS-9137. J. Acquir. Immune Defic. Syndr. 45:274–279 [DOI] [PubMed] [Google Scholar]
- 15. Ray AS, et al. 2006. Mechanism of active renal tubular efflux of tenofovir. Antimicrob. Agents Chemother. 50:3297–3304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rodman J, et al. 2006. Pharmacokinetics (PK) and safety of tenofovir disoproxil fumarate (TDF) in HIV-1-infected pregnant women and their infants, poster 155. 13th Conference on Retroviruses and Opportunistic Infections. [Google Scholar]