Abstract
With the emergence of Plasmodium falciparum infections exhibiting increased parasite clearance times in response to treatment with artemisinin-based combination therapies, the need for new therapeutic agents is urgent. Solithromycin, a potent new fluoroketolide currently in development, has been shown to be an effective, broad-spectrum antimicrobial agent. Malarial parasites possess an unusual organelle, termed the apicoplast, which carries a cryptic genome of prokaryotic origin that encodes its own translation and transcription machinery. Given the similarity of apicoplast and bacterial ribosomes, we have examined solithromycin for antimalarial activity. Other antibiotics known to target the apicoplast, such as the macrolide azithromycin, demonstrate a delayed-death effect, whereby treated asexual blood-stage parasites die in the second generation of drug exposure. Solithromycin demonstrated potent in vitro activity against the NF54 strain of P. falciparum, as well as against two multidrug-resistant strains, Dd2 and 7G8. The dramatic increase in potency observed after two generations of exposure suggests that it targets the apicoplast. Solithromycin also retained potency against azithromycin-resistant parasites derived from Dd2 and 7G8, although these lines did demonstrate a degree of cross-resistance. In an in vivo model of P. berghei infection in mice, solithromycin demonstrated a 100% cure rate when administered as a dosage regimen of four doses of 100 mg/kg of body weight, the same dose required for artesunate or chloroquine to achieve 100% cure rates in this rodent malaria model. These promising in vitro and in vivo data support further investigations into the development of solithromycin as an antimalarial agent.
INTRODUCTION
Artemisinin-based combination therapies have been broadly adopted as the first-line antimalarials of choice as Plasmodium parasites resistant to chloroquine and sulfadoxine/pyrimethamine have spread globally. The semisynthetic artemisinins artemether and artesunate rapidly reduce parasite burdens, have good therapeutic indices, and provide for successful treatment outcomes (32). Recently, however, there has been increasing concern regarding the development of resistance to the artemisinins (5, 21, 23, 24), emphasizing the need for new antimalarial agents with different mechanisms of action.
A number of antibiotics, including azithromycin, doxycycline, and clindamycin, have demonstrated efficacy against malarial parasites and act by specifically targeting the Plasmodium apicoplast organelle (3, 30). This organelle is believed to be a relic chloroplast, acquired by the apicomplexans through an endosymbiotic event with an alga (14). While the apicoplast has lost its photosynthetic function, it still retains a number of biological features in common with chloroplasts. It encodes its own genome, of cyanobacterial origin, and participates in biosynthetic pathways of prokaryotic origin, including the fatty acid synthase type II (FAS-II) pathway for fatty acid synthesis and the nonmevalonate pathway for isoprenoid synthesis (14, 33). Antibiotics such as azithromycin and doxycycline interfere specifically with the apicoplast-encoded ribosomal machinery. Interestingly, these compounds kill the progeny of the treated parasites rather than the treated parasites themselves. This phenomenon, referred to as delayed death, means that the antibiotics exhibit increased potency after two intraerythrocytic cycles (96 h) compared to that after just one (48 h) (14).
Solithromycin, a novel fluoroketolide, belongs to the well-known class of macrolide antibiotics that also includes azithromycin. Macrolides have broad therapeutic applications in infectious diseases and have a more than half-century history of safety and efficacy. Solithromycin has been shown to be active against bacteria resistant to other macrolides, such as azithromycin (11, 19, 27), and recently completed in a phase 2 clinical trial for community-acquired bacterial pneumonia (NCT01168713; Clinicaltrials.gov). Here, we describe a series of in vitro and in vivo experiments designed to characterize the activity of solithromycin against malarial parasites.
MATERIALS AND METHODS
Strains and culturing conditions.
Plasmodium falciparum NF54, Dd2, and 7G8 are commonly used reference strains that are available through MR4 (Malaria Research and Reference Reagent Resource Center, ATCC, Manassas, VA). NF54 is sensitive to most antimalarials; Dd2 exhibits resistance to chloroquine, quinine, pyrimethamine, and sulfadoxine; and 7G8 exhibits resistance to chloroquine and pyrimethamine. Laboratory-generated lines of Dd2 and 7G8 resistant to the macrolide azithromycin, AZ-RDd2 and AZ-R7G8, have been described previously (30). Plasmodium berghei causes a virulent infection in mice that is rapidly lethal. The P. berghei green fluorescent protein (GFP)-positive ANKA malaria strain (MRA-865; MR4) (12) was a kind donation from Andy Waters (Glasgow University, Scotland) and Chris Janse (Leiden University, The Netherlands).
In vitro drug susceptibility assays.
Compounds were tested against asynchronous intraerythrocytic forms of NF54 at the Swiss Tropical and Public Health Institute (Basel, Switzerland) using a previously described semiautomated microdilution assay (4, 18). The culture medium consisted of RPMI 1640 supplemented with 0.5% Albumax II, 25 mM HEPES, 25 mM NaHCO3 (pH 7.3), 0.36 mM hypoxanthine, and 100 μg/ml neomycin. Human erythrocytes served as host cells. The cultures were kept at 37°C in an atmosphere of 3% O2, 4% CO2, and 93% N2 in humidified modular chambers. Drug testing was carried out in 96-well microtiter plates. Chloroquine diphosphate (molecular weight [MW], 516), artesunate (MW, 384), and clindamycin hydrochloride (MW, 461) were purchased from Sigma. Solithromycin (MW, 845) was provided by Cempra Pharmaceuticals. The compounds were dissolved at 10 mg/ml by sonication in dimethylsulfoxide (DMSO), prediluted in hypoxanthine-free culture medium, and titrated in 100-μl duplicates in a 64-fold range. After the addition of an equal volume of parasite culture with an initial parasitemia of 0.3% in a 2.5% erythrocyte suspension, the test plates were incubated under the conditions described above for 72 (classical approach) or 120 h. Parasite growth was measured by the incorporation of radiolabeled [3H]hypoxanthine (0.5 μCi in a volume of 50 μl hypoxanthine-free culture medium) added 24 h prior to the termination of the test. Cultures were harvested onto glass fiber filters and washed with distilled water. The radioactivity was counted using a Betaplate liquid scintillation counter (Wallac, Zürich), and the results were recorded as counts per minute (cpm) per well at each drug concentration and expressed as a percentage of the untreated controls. Fifty percent inhibitory concentrations (IC50) were estimated by linear interpolation (13).
Drug assays with Dd2, 7G8, AZ-RDd2, and AZ-R7G8 were performed at Columbia University (New York, NY) using essentially the same conditions with the following exceptions: parasites were harvested after 96 rather than 120 h; assays were set up with an initial parasitemia of 0.1% synchronized ring-stage parasites and 1.0% hematocrit in complete RPMI 1640 medium; and drugs were diluted 2-fold across 10 wells in a 96-well plate, for a 512-fold range. Assays were harvested at 96 h, and parasitemias were ascertained by flow cytometry as described previously (10). IC50s were estimated by nonlinear regression analysis using Prism Software (Graphpad).
In vivo assays.
The animal experiments described here were carried out at the Swiss Tropical and Public Health Institute (Basel, Switzerland), adhering to local and national regulations of laboratory animal welfare in Switzerland. The P. berghei acute infection model in rodents is adapted from the Peters 4-day suppressive test, consisting of four daily doses with an evaluation of parasitemia 24 h after the fourth dose (26). Compounds were tested starting at day 0, with P. berghei strain GFP ANKA, essentially as described previously (2, 26, 28). From donor mice with approximately 30% parasitemia, heparinized blood was taken and diluted in physiological saline to 108 parasitized erythrocytes/ml. An aliquot (0.2 ml) of this suspension was injected intravenously into experimental and control groups of mice. Control mice in this model die between day 6 and 7 postinfection (2). In these experiments, however, control animals (n = 5) were euthanized on day 4 postinfection. Azithromycin dihydrate (MW, 85) and clindamycin hydrochloride (MW, 461) were purchased from Sigma. Compounds were prepared at the appropriate concentrations in 100% DMSO followed by a 10-fold dilution in water or HPMC (0.5% [wt/vol] hydroxypropylmethylcellulose, 0.5% [vol/vol] benzyl alcohol, 0.4% [vol/vol] Tween 80, and 0.9% [wt/vol] sodium chloride in water). They were administered to groups of 3 mice, either as a single dose (24 h postinfection) or as 4 doses (3, 24, 48, and 72 h postinfection). Doses were administered orally (p.o.) in a volume of 10 ml/kg of body weight. With the single-dose regimen, the degree of infection (parasitemia was expressed as the percent infected erythrocytes) was determined by flow cytometry on day 3 (72 h postinfection). The blood samples from the quadruple-dose regimens were collected and analyzed by flow cytometry on day 4. The difference between the mean infection rate of the control group (100%) and that of the test group was calculated and expressed as percent reduction. As an example, activity determination with a mean of 2% parasitemia in treated mice and a mean of 40% parasitemia in the control animals was calculated with the following equation: (40% − 2%)/40% × 100 = 95% activity. The survival time in days was recorded up to 30 days after infection. A compound was considered curative if the animal survived to day 30 after infection with no detectable parasites (as confirmed by light microscopy with a detection limit of 1 parasite per 10,000 erythrocytes).
RESULTS
Solithromycin demonstrates potent antimalarial activity in vitro.
Like other prokaryotic protein synthesis inhibitors, solithromycin induced a delayed-death effect in Plasmodium parasites. Solithromycin was much more effective against the P. falciparum NF54 strain in the 120-h assay (IC50 measured at 2.8 nM; Table 1) than in the 72-h assay (IC50 range, 1,088 to 1,818 nM; Table 2). A similar result was observed with clindamycin (72-h IC50, >21,692 nM; 120-h IC50 range, 11.5 to 17 nM). In contrast, the fast-acting control drugs chloroquine and artesunate demonstrated no shift in potency between the two time points (72-h chloroquine IC50 range, 8.5 to 10.5 nM; 120-h chloroquine IC50 range, 8.9 to 10.2 nM; 72-h artesunate IC50 range, 7.3 to 8.3 nM; 120-h artesunate IC50 range, 8.6 to 10.9 nM) (Tables 1 and 2).
Table 1.
In vitro P. falciparum activity in 120-h assay
| Compound | IC50 (nM) for strain NF54 in test no.: |
||
|---|---|---|---|
| 1 | 2 | 3 | |
| Solithromycin | <185a | <18a | 2.8 |
| Clindamycin | <17a | 11.5 | 11.5 |
| Chloroquine | 8.9 | 8.9 | 10.2 |
| Artesunate | 10.9 | 10.2 | 8.6 |
The result was less than the lowest concentration tested.
Table 2.
In vitro P. falciparum activity in 72-h assay
| Compound | IC50 (nM) for strain NF54 in test no.: |
|||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Solithromycin | 1,088 | 1,818 | 1,760 | 1,489 |
| Clindamycin | >21,692a | >21,692a | >21,692a | >21,692a |
| Chloroquine | 8.5 | 10.5 | 8.5 | 9.3 |
| Artesunate | 7.8 | 8.3 | 7.8 | 7.3 |
Result was greater than the highest concentration tested.
Solithromycin demonstrates activity against multidrug-resistant parasites.
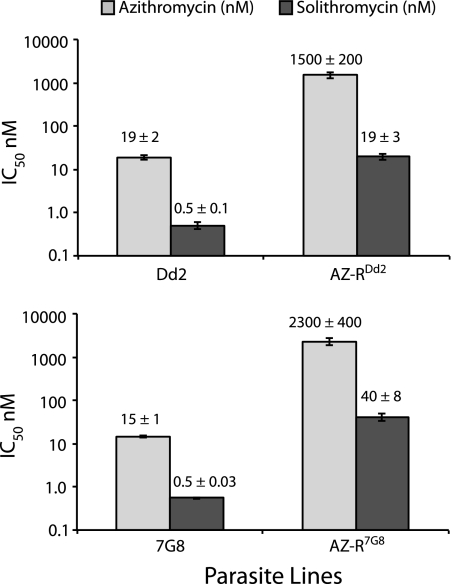
Solithromycin was tested in vitro against the P. falciparum lines Dd2 and 7G8, both of which are multidrug resistant, and against two azithromycin-resistant lines, AZ-RDd2 and AZ-R7G8, which were derived from parental lines Dd2 and 7G8. Azithromycin was used as a control. The compounds were tested in a 96-h assay, a dose-response curve was established, and IC50s were derived. Dd2 and 7G8 were found to be highly susceptible to solithromycin, with IC50s (means ± standard errors of the means [SEM]) of 0.5 ± 0.1 and 0.5 ± 0.003 nM, respectively (Fig. 1). The azithromycin-resistant lines exhibited statistically significant levels of cross-resistance to solithromycin (P < 0.03 by paired t test), but this effect was about 50% lower in magnitude than it was for azithromycin. As a result, the azithromycin-resistant lines were still quite susceptible to solithromycin, with IC50s in the 19 to 40 nM range, well below the ∼1.2 μM peak concentration measured in human plasma (31).
Fig 1.
Solithromycin exhibits activity against multidrug-resistant parasites. Two multidrug-resistant parasite lines, Dd2 and 7G8, and azithromycin-resistant clones derived from these parental lines, AZ-RDd2 and AZ-R7G8, were treated with the indicated compounds for 96 h. Solithromycin was significantly more potent than azithromycin for both the sensitive and resistant lines: Dd2, P = 0.004; AZ-RDd2, P = 0.007; 7G8, P = 0.0005; and AZ-R7G8, P = 0.01, all by paired t tests. Azithromycin-resistant parasites were significantly less susceptible to solithromycin than the parental strains (P < 0.05 for both lines), but this effect was only about 50% as great as the effect for azithromycin itself (for Dd2 versus AZ-RDd2 there was an 80- and 40-fold shift in potency for azithromycin and solithromycin, respectively; for 7G8 versus AZ-R7G8 there was a 160- and 70-fold shift in potency for azithromycin and solithromycin, respectively. IC50s (means ± SEM) for each line tested against azithromycin or solithromycin are shown above the individual bars (data were obtained from four independent experiments performed in duplicate).
In vivo antimalarial activity.
In vivo antimalarial activity was demonstrated in mice infected intravenously with P. berghei ANKA asexual blood-stage parasites. Control mice in this model survive an average of 6 to 7 days following infection (2). In our experiments, control animals were sacrificed after evaluation of parasitemia on day 4 postinfection and were euthanized. In two independent experiments, solithromycin treatment with four doses of 100 mg/kg resulted in a cure rate of 100% (9 of 9 mice), which was defined as survival on day 30 with no detectable parasites. An initial experiment compared solithromycin to clindamycin (both dissolved in DMSO), while a follow-up experiment compared solithromycin to azithromycin and clindamycin in two different vehicles (DMSO or HPMC). The treatment of mice with a single dose of 100 mg/kg solithromycin, clindamycin, or azithromycin resulted in mean survival times of 7 days for clindamycin, 14 days for azithromycin, and 13 to 16 days for solithromycin (Table 3). Treatment with clindamycin for 4 days (four doses of 100 mg/kg) resulted in an extended mean survival time of 15 to 20 days (Table 4). Four days of treatment with azithromycin (four doses of 100 mg/kg) extended mean survival to 24 to >30 days but was not considered curative, since two of the six mice died on days 17 and 25, respectively, and all four mice surviving to day 30 were parasitized. This contrasted with the complete cure of 9/9 mice with negative microscopic findings at day 30 following four doses of 100 mg/kg solithromycin (administered 3, 24, 48, and 72 h postinfection) (Table 4).
Table 3.
In vivo antimalarial activity against P. berghei (single dose of 100 mg/kg given orally)
| Compound | Vehicle | Antimalarial activity (%) | Mean survival of mice (days) |
|---|---|---|---|
| Untreated control | 4a | ||
| Solithromycin | DMSO | 80 | 15.7 |
| HPMC | 81 | 12.7 | |
| Azithromycin | DMSO | 70 | 14.0 |
| HPMC | 65 | 14.0 | |
| Clindamycin | DMSO | 79 | 7.0 |
| HPMC | 79 | 7.3 |
0.4% (vol/vol) Tween 80 and 0.9% (wt/vol) sodium chloride in water.
Table 4.
In vivo antimalarial activity against P. berghei (four doses of 100 mg/kg given orally)
| Compound | Vehicle | Activity (%) | Mean survivala (days) | Parasitized RBC on day 30 for mouse no.e: |
% Cured | ||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | |||||
| Solithromycin | DMSOb | 99 | >30.0 | 0c | 0c | 0c | 100 |
| DMSO | 99.8 | >30.0 | 0c | 0c | 0c | 100 | |
| HPMC | 99.8 | >30.0 | 0c | 0c | 0c | 100 | |
| Azithromycin | DMSO | 99.1 | >30.0 | 34.3d | 2.20d | 35.3d | 0 |
| HPMC | 99.1 | 24.0 | NA | 74.0d | NA | 0 | |
| Clindamycin | DMSOb | 99 | 15.0 | NA | NA | NA | 0 |
| DMSO | 99.8 | 18.3 | NA | NA | NA | 0 | |
| HPMC | 99.6 | 20.0 | NA | NA | NA | 0 | |
Surviving mice were sacrificed on day 30 to determine the presence or absence of parasites.
Data are from an initial experiment conducted in 2008.
Parasite count by light microscopy (detection limit, 1 parasite per 10,000 erythrocytes; 0.01%).
Parasitemia on day 30 as measured by flow cytometry (expressed as the percentage of infected red blood cells).
NA, not applicable; these mice did not survive to 30 days. RBC, red blood cells.
DISCUSSION
Drug resistance remains a major obstacle in the treatment of malaria worldwide. For decades, chloroquine was the most widely used first-line antimalarial drug. However, the global spread of resistance led to its gradual replacement by other first-line drugs, such as sulfadoxine-pyrimethamine and mefloquine, which also succumbed to resistance (9). During the past 5 years, artemisinin-based combination therapies have emerged as the globally recommended first-line antimalarials (8). Recent reports of emerging resistance emphasize the need for new antimalarials with diverse chemotypes for which there are not preexisting resistance mechanisms in the field (5, 21, 23, 24).
While the antimalarial activity of various antibiotics is well recognized, none has been used as extensively as drugs such as chloroquine and sulfadoxine-pyrimethamine, and there are no reports in the literature of malarial parasite resistance to antibiotics such as doxycycline or azithromycin. Moreover, the advantages of antibiotic-based combination therapies have recently been promoted because of their ability to treat not only malaria but also bacterial coinfections and bacterial fevers that can be misdiagnosed as severe malaria (22, 29). Solithromycin has promising characteristics as a partner drug in an antibiotic-based combination therapy given the dramatic efficacy we demonstrate against malarial parasites and its well-documented antibacterial properties. Solithromycin, unlike telithromycin, does not contain a pyridine moiety in its side chain. This moiety has been shown to inhibit nicotinic acetylcholine receptors and is a potential cause of the severe adverse events, or Ketek effects, that were seen with telithromycin use. Solithromycin, like older macrolides, does not show significant inhibition of these receptors (1).
Our studies show that solithromycin demonstrates a delayed-death effect against P. falciparum similarly to the antimalarial effect of bacterial protein synthesis inhibitors, such as tetracycline, clindamycin, and doxycycline (3, 15, 30, 34). This finding suggests that solithromycin targets the cyanobacterial-derived apicoplast organelle of Plasmodium parasites. Solithromycin exhibited efficacy against multiple strains of P. falciparum, including NF54 and the multidrug-resistant strains Dd2 and 7G8. As shown for various macrolide-resistant bacteria (6, 7, 19), solithromycin also retains moderate efficacy against azithromycin-resistant P. falciparum. This may be due to an increased number of ribosomal binding sites, as evidence suggests that solithromycin has three sites of interaction with the bacterial ribosome (17). Solithromycin might therefore still be effective as an antimalarial even if azithromycin resistance arose in the field.
Solithromycin was tested as a monotherapy in a murine model of infection with a virulent ANKA strain of P. berghei. The pharmacokinetics of solithromycin in mice differ substantially from those in humans. In mice, solithromycin is metabolized to a greater extent (25), exhibits a shorter half-life, and is less orally bioavailable (unpublished data from Cempra Pharmaceuticals). The efficacy of macrolides is driven primarily by the area under the concentration versus time curve (AUC), and a dose of 100 mg/kg once per day was required to achieve relevant AUCs in this model. After a single 100 mg/kg dose in mice, substantial (28- to 158-fold above the IC50s) plasma levels are observed 24 h postdose (unpublished data from Cempra Pharmaceuticals).
A single dose of solithromycin (100 mg/kg) was approximately as effective as a single dose of azithromycin (100 mg/kg), but treatment with four doses showed readily apparent differences between these two macrolide antibiotics. Azithromycin (four doses of 100 mg/kg) led to a 30-day survival in four out of six mice and was not curative, as parasitemia was detected in all of the surviving mice by flow cytometry. Solithromycin, under these conditions, was 100% curative in 9 mice in two independent experiments, with zero mice showing microscopically detectable parasite infections up to day 30. Clindamycin, when given as four doses, only increased survival to 15 to 20 days from 7 days as a single dose. Solithromycin thus compares quite favorably to other known antibiotics with antimalarial activity. Clinically, we propose that solithromycin needs to be combined with another agent that acts more rapidly to obtain maximum therapeutic benefit. This has been shown to be an effective therapy in human trials with azithromycin tested in combination with artesunate or quinine (20).
Solithromycin monotherapy has cure rates similar to that for artesunate, which also has demonstrated 30-day cures when given as four doses of 100 mg/kg (2). In addition, solithromycin is the most potent bacterial protein synthesis inhibitor that has been tested against P. berghei. Several phase 1 studies have shown solithromycin to be well tolerated (31), and this compound recently completed a successful phase 2 trial for the treatment of community-acquired bacterial pneumonia. Human plasma concentrations after the administration of oral solithromycin attain peak levels of ∼1 μg/ml (31), corresponding to ∼400- to 2,000-fold the IC50s observed after 96 to 120 h of drug exposure in vitro (Tables 1 and 2). Solithromycin also has been reported to achieve higher intracellular concentrations than other macrolides, such as azithromycin (16). These promising in vivo antimalarial data, combined with the in vitro activity against resistant strains of P. falciparum, warrant further investigation of solithromycin as an antimalarial candidate.
ACKNOWLEDGMENTS
This work was supported by Medicines for Malaria Venture and Cempra Pharmaceuticals.
We thank Brian D. Jamieson for his assistance in preparing the manuscript.
S.W., E.E., J.L., I.B., and D.A.F. declare no conflict of interest. JCC is a consultant to and P.F. is an employee of Cempra Pharmaceuticals.
Footnotes
Published ahead of print 14 November 2011
We dedicate this article to the late Ian Bathurst, a wonderful scientist and tremendous advocate of antimalarial drug research.
REFERENCES
- 1. Bertrand D, Bertrand S, Neveu E, Fernandes P. 2010. Molecular characterization of off-target activities of telithromycin: a potential role for nicotinic acetylcholine receptors. Antimicrob. Agents Chemother. 54: 5399–5402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Charman SA, et al. 2011. Synthetic ozonide drug candidate OZ439 offers new hope for a single-dose cure of uncomplicated malaria. Proc. Natl. Acad. Sci. U. S. A. 108: 4400–4405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dahl EL, Rosenthal PJ. 2007. Multiple antibiotics exert delayed effects against the Plasmodium falciparum apicoplast. Antimicrob. Agents Chemother. 51: 3485–3490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Desjardins RE, Canfield CJ, Haynes JD, Chulay JD. 1979. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 16: 710–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dondorp AM, et al. 2009. Artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 361: 455–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dubois J, Fernandes P. 2010. CEM-101, a novel ketolide; in vitro activity against resistant strains of Streptococcus pneumoniae and Haemophilus influenzae, abstr p-904. Abstr. 20th Eur. Congr. Clin. Microb. Infect. Dis. [Google Scholar]
- 7. Dubois J, Fernandes P. 2009. In vitro activity of CEM-101 against resistant strains of Staphylococcus aureus, abstr F1-2037. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC [Google Scholar]
- 8. Eastman RT, Fidock DA. 2009. Artemisinin-based combination therapies: a vital tool in efforts to eliminate malaria. Nat. Rev. Microbiol. 7: 864–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ekland EH, Fidock DA. 2007. Advances in understanding the genetic basis of antimalarial drug resistance. Curr. Opin. Microbiol. 10: 363–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ekland EH, Schneider J, Fidock DA. 2011. Identifying apicoplast-targeting antimalarials using high-throughput compatible approaches. FASEB J. 25: 3583–3593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Farrell DJ, et al. 2010. Antimicrobial characterisation of CEM-101 activity against respiratory tract pathogens, including multidrug-resistant pneumococcal serogroup 19A isolates. Int. J. Antimicrob. Agents 35: 537–543 [DOI] [PubMed] [Google Scholar]
- 12. Franke-Fayard B, et al. 2004. A Plasmodium berghei reference line that constitutively expresses GFP at a high level throughout the complete life cycle. Mol. Biochem. Parasitol. 137: 23–33 [DOI] [PubMed] [Google Scholar]
- 13. Huber W, et al. 1993. Sensitivity of Plasmodium falciparum field-isolates from Tanzania to chloroquine, mefloquine and pyrimethamine during in vitro cultivation. Acta Trop. 52: 313–316 [DOI] [PubMed] [Google Scholar]
- 14. Kalanon M, McFadden GI. 2010. Malaria, Plasmodium falciparum and its apicoplast. Biochem. Soc. Trans. 38: 775–782 [DOI] [PubMed] [Google Scholar]
- 15. Lell B, Kremsner PG. 2002. Clindamycin as an antimalarial drug: review of clinical trials. Antimicrob. Agents Chemother. 46: 2315–2320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lemaire S, Van Bambeke F, Tulkens PM. 2009. Cellular accumulation and pharmacodynamic evaluation of the intracellular activity of CEM-101, a novel fluoroketolide, against Staphylococcus aureus, Listeria monocytogenes, and Legionella pneumophila in human THP-1 macrophages. Antimicrob. Agents Chemother. 53: 3734–3743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Llano-Sotelo B, et al. 2010. Binding and action of CEM-101, a new fluoroketolide antibiotic that inhibits protein synthesis. Antimicrob. Agents Chemother. 54: 4961–4970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Matile H, Pink JRL. 1990. Plasmodium falciparum malaria parasite cultures and their use in immunology, p. 221–234 In Pernis B. (ed.), Immunological methods, vol. 4 Academia Press, San Diego, CA [Google Scholar]
- 19. McGhee P, et al. 2010. In vitro activity of CEM-101 against Streptococcus pneumoniae and Streptococcus pyogenes with defined macrolide resistance mechanisms. Antimicrob. Agents Chemother. 54: 230–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Noedl H, et al. 2006. Azithromycin combination therapy with artesunate or quinine for the treatment of uncomplicated Plasmodium falciparum malaria in adults: a randomized, phase 2 clinical trial in Thailand. Clin. Infect. Dis. 43: 1264–1271 [DOI] [PubMed] [Google Scholar]
- 21. Noedl H, et al. 2008. Evidence of artemisinin-resistant malaria in western Cambodia. N. Engl. J. Med. 359: 2619–2620 [DOI] [PubMed] [Google Scholar]
- 22. Noedl H. 2009. ABC-antibiotics-based combinations for the treatment of severe malaria? Trends Parasitol. 25: 540–544 [DOI] [PubMed] [Google Scholar]
- 23. Noedl H, Socheat D, Satimai W. 2009. Artemisinin-resistant malaria in Asia. N. Engl. J. Med. 361: 540–541 [DOI] [PubMed] [Google Scholar]
- 24. O'Brien C, Henrich PP, Passi N, Fidock DA. 2011. Recent clinical and molecular insights into emerging artemisinin resistance in Plasmodium falciparum. Curr. Opin. Infect. Dis. 24: 570–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pereira DP, Degenhardt T, Fernandes P. 2010. Comparison of CEM-101 metabolism in mice, rats, monkeys and humans, abstr A-687. Abstr 50th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC [Google Scholar]
- 26. Peters W. 1987. Chemotherapy and drug resistance in malaria, 2nd ed. Academic Press, London, United Kingdom [Google Scholar]
- 27. Putnam SD, Sader HS, Farrell DJ, Biedenbach DJ, Castanheira M. 2011. Antimicrobial characterisation of solithromycin (CEM-101), a novel fluoroketolide: activity against staphylococci and enterococci. Int. J. Antimicrob. Agents 37: 39–45 [DOI] [PubMed] [Google Scholar]
- 28. Ridley RG, et al. 1997. Antimalarial activity of the bisquinoline trans-N1,N2-bis (7-chloroquinolin-4-yl)cyclohexane-1,2-diamine: comparison of two stereoisomers and detailed evaluation of the S,S enantiomer, Ro 47-7737. Antimicrob. Agents Chemother. 41: 677–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Scott JA, et al. 2011. Relation between falciparum malaria and bacteraemia in Kenyan children: a population-based, case-control study and a longitudinal study. Lancet 378: 1316–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sidhu AB, et al. 2007. In vitro efficacy, resistance selection, and structural modeling studies implicate the malarial parasite apicoplast as the target of azithromycin. J. Biol. Chem. 282: 2494–2504 [DOI] [PubMed] [Google Scholar]
- 31. Still JG, et al. 2011. Pharmacokinetics of solithromycin (CEM-101) after single or multiple oral doses and effects of food on single-dose bioavailability in healthy adult subjects. Antimicrob. Agents Chemother. 55: 1997–2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. White NJ. 1999. Delaying antimalarial drug resistance with combination chemotherapy. Parassitologia 41: 301–308 [PubMed] [Google Scholar]
- 33. Yeh E, Derisi JL. 2011. Chemical rescue of malaria parasites lacking an apicoplast defines organelle function in blood-stage Plasmodium falciparum. PLoS Biol. 9: e1001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yeo AE, Rieckmann KH. 1995. Increased antimalarial activity of azithromycin during prolonged exposure of Plasmodium falciparum in vitro. Int. J. Parasitol. 25: 531–532 [DOI] [PubMed] [Google Scholar]