Abstract
Aspergillus fumigatus is the most frequent fungus found in the sputum of cystic fibrosis (CF) subjects. Itraconazole is prescribed for allergic bronchopulmonary aspergillosis (ABPA) or Aspergillus bronchitis in CF subjects. We hypothesized that A. fumigatus isolates in the sputum of CF subjects with previous exposure to itraconazole was associated with higher prevalence of azole resistance. From June 2010 to April 2011, sputum samples from adult CF subjects at Cochin University Hospital (France) were examined systematically for the detection of A. fumigatus. MICs of A. fumigatus isolates against azoles were screened using Etest, and reduced susceptibility to azoles was confirmed using the CLSI broth microdilution method. A. fumigatus was isolated from the sputum of 131/249 (52.6%) adult CF subjects, and 47/131 (35.9%) subjects had received previous treatment with itraconazole. Reduced A. fumigatus susceptibility to itraconazole (MIC, ≥2 mg/liter) was confirmed in 6/131 (4.6%) subjects. All 6 isolates also had reduced susceptibility to posaconazole (MIC, ≥0.5 mg/liter), and 3/6 isolates had reduced susceptibility to voriconazole (MIC, ≥2 mg/liter). Mutations in the cyp51A gene were detected at positions previously implicated to cause resistance in 5 isolates. Azole-resistant A. fumigatus isolates were found in 5/25 (20%) subjects exposed to itraconazole within the previous 3 years. High rates of azole-resistant A. fumigatus isolates were present in adult CF subjects and were associated with recent itraconazole exposure. Although the clinical implications of these findings will require further studies, the cautious use of itraconazole in adult CF subjects can be recommended.
INTRODUCTION
Aspergillus fumigatus is the most frequently found fungus in the sputum of cystic fibrosis (CF) subjects (18, 19). It has been isolated from sputum samples from 56% of patients at our CF center (19). A. fumigatus colonization has been associated with allergic bronchopulmonary aspergillosis (ABPA) that occurred in up to 15% of CF subjects (24). Furthermore, selected CF subjects with Aspergillus colonization showed respiratory exacerbations, which improved with antifungal therapy (22). Because the latter subjects had no ABPA, this condition was referred to as Aspergillus bronchitis (22). Although A. fumigatus colonization has not consistently been associated with clinical manifestations in all CF subjects (10), a recent report suggested that persistent A. fumigatus infection was an independent risk factor for hospital admissions (4). Thus, the interest on the impact of A. fumigatus on respiratory manifestations and use of antifungal therapy in CF subjects is growing (13).
Azoles (including itraconazole, voriconazole, and posaconazole) are the only orally active therapies against A. fumigatus. These antifungal agents have been proposed as an adjunctive treatment to oral steroids in CF subjects with ABPA (12, 24), and their use also was suggested for CF subjects with Aspergillus bronchitis (22). During the past 10 years, azole resistance has emerged in A. fumigatus isolates (14) and has been associated with treatment failure in subjects with aspergillosis (15). This increase in azole-resistant A. fumigatus isolates was suggested to occur as a consequence of increasing therapeutic or environmental exposure to azoles (27). The frequency of azole-resistant A. fumigatus isolates varied considerably among countries and diseases. For example, studies in the United Kingdom (15) and the Netherlands (23) have described high frequencies (5 to 6%) of azole resistance, especially in subjects with chronic aspergillosis (15). However, a recent study in France identified a very low prevalence of resistance to azoles in A. fumigatus collected from patients with invasive aspergillosis who had been treated for hematological malignancies (2).
Only limited data are available regarding the prevalence of azole-resistant A. fumigatus isolates in CF subjects and their relationship to previous azole exposure. In a study performed in Portugal, the authors tested 159 A. fumigatus isolates cultured from the sputum of 11 CF patients (7 who never received antifungal treatment and 4 who received azoles) and found no azole-resistant isolates, leading the authors to conclude that antifungal resistance appeared to be rare among CF patients (5). Another recent study, which was performed in a large cohort of Danish CF subjects (n = 133), reported that 4.5% of A. fumigatus isolates were azole resistant (18). The authors underlined that all 6 of these patients previously had been exposed to azoles, and they concluded that the emergence of azole resistance in the A. fumigatus isolates of CF patients was of concern (18). Thus, there is conflicting evidence on the prevalence of azole-resistant A. fumigatus in CF subjects. Furthermore, the impact of azole therapies on A. fumigatus resistance to azole has not been established in CF subjects.
In the present study, we prospectively evaluated the azole susceptibility of A. fumigatus isolates in our cohort of adult CF subjects. Our objectives were to evaluate the prevalence of A. fumigatus isolates with reduced susceptibility to azoles and to examine the impact of previous treatment with itraconazole on the azole susceptibility of A. fumigatus isolates.
MATERIALS AND METHODS
Patients.
From 1 June 2010 to 1 April 2011, all adult subjects attending the CF center at Cochin Hospital (Paris, France) were recruited in this prospective observational study. CF was diagnosed on the basis of clinical manifestations with a sweat chloride concentration exceeding 60 mM and/or two disease-causing mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene (11). Clinical information was obtained using our CF center database. Clinical data included age, gender, age at diagnosis of CF, CFTR mutations, pancreatic status, chronic airway colonization with Pseudomonas aeruginosa, body mass index, and spirometry. Chronic A. fumigatus colonization was defined as the presence of two or more positive sputum cultures during the time of the study (4). Intermittent colonization was defined as the presence of one positive sputum culture and at least one negative culture during the time of the study. ABPA was defined according to consensus criteria (24); subjects received itraconazole according to international guidelines. In brief, Aspergillus colonization was treated if it was symptomatic and no response was achieved with antibacterial therapy (22). For subjects with ABPA, azole therapy was prescribed in combination with systemic steroid therapy (24). Azole exposure was considered present when a subject was treated for more than 2 weeks with itraconazole, voriconazole, or posaconazole. We defined subjects with recent exposure to itraconazole as subjects who received this treatment (for at least 2 weeks) within the previous 3 years. Subjects who received itraconazole before the previous 3 years were defined as subjects with past itraconazole exposure. The study was approved by the Institutional Review Board on Medical Research (CCTIRS 08-370).
Culture and identification.
Respiratory samples were cultured on Sabouraud-chloramphenicol-gentamicin agar (bioMérieux, Marne la Coquette, France) and incubated for 10 days at 27 and 37°C to recover filamentous fungi, and cultures were inspected every day. A. fumigatus was identified on the basis of macroscopic and microscopic morphology. Aspergillus fumigatus colonies showed blue-green-gray surface pigmentation, and microscopic examination showed septate hyphae with conidial heads, which usually were columnar and uniseriate. Recently, novel Aspergillus species with reduced azole susceptibilities and morphological features resembling those of A. fumigatus have been described (3). To confirm that Aspergillus isolates with reduced azole susceptibilities had not been mistaken with other Aspergillus section Fumigati with primary resistance to itraconazole (e.g., A. lentulus or A. viridinutans), we further identified all isolates using matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry (1). This analysis confirmed that all isolates were A. fumigatus.
Susceptibility testing for azole resistance in A. fumigatus isolates.
Itraconazole, voriconazole, and posaconazole susceptibilities were determined initially using Etest strips according to the manufacturer's instructions (AB Biodisk, bioMérieux, France) after 24 h of incubation at 37°C. When more than one sputum samples had been tested for A. fumigatus azole susceptibility in a single patient, we described the sample with the highest itraconazole MIC. Isolates with itraconazole MICs of ≥2 mg/liter by Etest were further analyzed for MICs to itraconazole, voriconazole, and posaconazole using the CLSI M38-A2 broth microdilution method (8). We considered reduced susceptibilities to itraconazole and voriconazole for MICs of ≥2 mg/liter and to posaconazole for MICs of ≥0.5 mg/liter (20, 21, 26).
Mixed-format real-time PCR assay to detect A. fumigatus mutations.
All of the A. fumigatus isolates for which the CLSI itraconazole MIC was ≥2 mg/liter were subjected to a mixed-format real-time PCR assay, as described previously for the detection of mutations at positions in the cyp51A gene known to cause triazole resistance in A. fumigatus (17).
Statistics.
Continuous variables were reported as medians and interquartile ranges (IQR) and were compared using the Wilcoxon rank-sum or Kruskal-Wallis test, as appropriate. Categorical variables were reported as counts and percentages and were compared using Fisher's exact tests. Statistical analyses were two sided, and P < 0.05 was considered to have statistical significance. Analyses were performed using Stata 11.0 (StataCorp LP, TX).
RESULTS
From 1 June 2010 to 1 April 2011, 570 sputum samples were prospectively collected from 249 adult CF subjects. A. fumigatus was present in 285/570 (50.0%) samples, corresponding to 131/249 (52.6%) individual CF subjects. During the study period, 97/131 (74%) subjects met criteria for chronic Aspergillus colonization (≥2 A. fumigatus-positive sputum samples), and 19/131 (15%) subjects had intermittent colonization. The remaining 15/131 (11%) had only one (positive) sample processed during the study period.
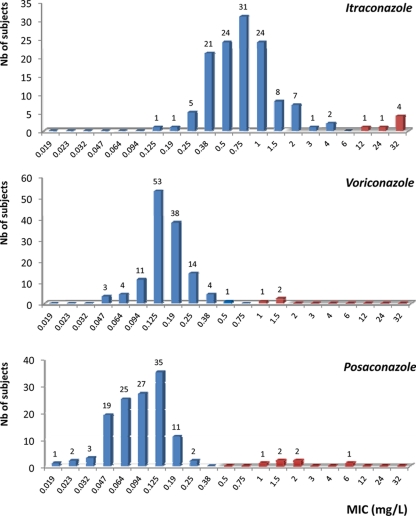
Distribution of azole MICs, as determined by Etest, in subjects with A. fumigatus isolates is provided in Fig. 1. A. fumigatus isolates with Etest itraconazole MICs of ≥2 mg/liter were found in 16/131 (12.2%) subjects. These 16 isolates were further analyzed using the CLSI reference broth microdilution, and reduced susceptibility to itraconazole was confirmed in 6/131 (4.6%) subjects. A comparison of azole MICs between Etest and CLSI methods is provided in Table 1. The 6 isolates with Etest MICs of >4 mg/liter also had elevated CLSI MICs to itraconazole, whereas isolates with Etest MICs of ≤4 mg/liter had CLSI MICs to itraconazole of <2 mg/liter. A. fumigatus isolates with reduced susceptibilities to posaconazole (Etest MICs of ≥0.5 mg/liter) were found in 6/131 (4.6%) subjects, and all of these isolates were confirmed to be resistant to posaconazole and itraconazole using the CLSI method. Furthermore, 3 of these 6 isolates had reduced susceptibility to voriconazole.
Fig 1.
MICs of itraconazole, voriconazole, and posaconazole against A. fumigatus isolates obtained from the sputum of 131 CF subjects. Blue bars indicate azole-susceptible isolates. Red bars indicate isolates in which resistance was confirmed by the CLSI broth dilution technique.
Table 1.
Comparison of Etest and CLSI methods for determination of azole MICs in 16 isolates with Etest itraconazole MICs of ≥2 mg/litera
| Patient no. | MIC (mg/liter) and test method |
cyp51A mutation | |||||
|---|---|---|---|---|---|---|---|
| Itraconazole |
Voriconazole |
Posaconazole |
|||||
| Etest | CLSI | Etest | CLSI | Etest | CLSI | ||
| 1 | >32 | >16 | 1.5 | >16 | 2 | 8 | Unknown |
| 2 | >32 | 16 | 0.047 | 0.25 | 1.5 | 1 | G54E |
| 3 | >32 | >16 | 0.5 | 0.5 | 6 | 0.5 | M220R |
| 4 | >32 | >16 | 0.25 | 2 | 2 | 0.5 | M220I |
| 5 | 24 | >16 | 1.5 | 8 | 1 | 1 | TR/L98H |
| 6 | 12 | >16 | 1 | 8 | 1.5 | 1 | TR/L98H |
| 7 | 4 | 1 | 0.38 | 1 | 0.125 | 0.25 | |
| 8 | 4 | 1 | 0.25 | 1 | 0.19 | 0.25 | |
| 9 | 3 | 0.25 | 0.38 | 0.5 | 0.25 | 0.125 | |
| 10 | 2 | 0.5 | 0.19 | 0.5 | 0.094 | 0.125 | |
| 11 | 2 | 0.5 | 0.125 | 0.5 | 0.19 | 0.25 | |
| 12 | 2 | 0.5 | 0.25 | 0.5 | 0.125 | 0.125 | |
| 13 | 2 | 0.5 | 0.19 | 0.5 | 0.125 | 0.125 | |
| 14 | 2 | 0.5 | 0.25 | 0.5 | 0.125 | 0.125 | |
| 15 | 2 | 0.5 | 0.19 | 0.5 | 0.094 | 0.125 | |
| 16 | 2 | 0.25 | 0.19 | 0.5 | 0.19 | 0.125 | |
Gray shading corresponds to isolates with reduced azole susceptibility. cyp51A mutations were assessed in the 6 isolates with CLSI-confirmed reduced susceptibility.
Characteristics of the 131 CF subjects positive for A. fumigatus isolates are provided in Table 2. When comparing subjects harboring isolates with CLSI-confirmed reduced susceptibility to itraconazole (n = 6) to subjects with itraconazole-susceptible isolates (n = 125), no significant clinical or demographic difference was found between groups. However, previous exposure to itraconazole was found in 42/125 (33.6%) subjects with CLSI itraconazole MICs of <2 mg/liter versus 5/6 (83.3%) subjects with CLSI itraconazole MICs of ≥2 mg/liter (P = 0.02). One isolate from an itraconazole-nonexposed patient had the TR/L98H mutation. The other 5 resistant isolates were from itraconazole-exposed patients and involved G54E (n = 1), M220I (n = 1), M220R (n = 1), and TR/L98H (n = 1); no known cyp51A mutation was identified in 1 patient.
Table 2.
Characteristics of patients according to itraconazole MICs of A. fumigatus isolates
| Variablea | Results for: | P values | ||
|---|---|---|---|---|
| All subjects (n = 131) | Subjects with itraconazole-susceptible isolates (n = 125) | Subjects with itraconazole-resistant isolates (n = 6) | ||
| Age (yr [IQR]) | 27 [23; 35] | 27 [23; 34] | 23 [21; 36] | 0.37 |
| Age at CF diagnosis (yr [IQR]) | 1.0 [0.2; 6.0] | 1.5 [0.2; 6.0] | 0.6 [0.2; 1.0] | 0.53 |
| CFTR mutation (no. [%]) | ||||
| F508del/F508del | 56 (42.7) | 53 (42.4) | 3 (50.0) | 0.89 |
| F508del/other | 56 (42.7) | 54 (43.2) | 2 (33.3) | |
| Other/other | 19 (14.6) | 18 (14.4) | 1 (16.7) | |
| Male (no. [%]) | 77 (58.8) | 72 (57.6) | 5 (83.3) | 0.40 |
| Pancreatic insufficiency (no. [%]) | 112 (85.5) | 107 (85.6) | 5 (83.3) | 1.00 |
| FEV1 (% predicted [IQR]) | 54 [39; 74] | 55 [40; 73] | 44 [21; 78] | 0.36 |
| FVC (% predicted [IQR]) | 71 [55; 89] | 72 [55; 89] | 64 [34; 79] | 0.28 |
| Chronic P. aeruginosa (no. [%]) | 75 (57.3) | 70 (56.0) | 5 (83.3) | 1.00 |
| Chronic A. fumigatus (no. [%]) | 97 (74%) | 93 (74.4) | 4 (66.7) | 0.65 |
| ABPA (no. [%]) | 28 (21.4) | 25 (20.0) | 3 (50.0) | 0.11 |
| Previous itraconazole therapyb (no. [%]) | 47 (35.9) | 42 (33.6) | 5 (83.3) | 0.02 |
| Previous itraconazole therapyc (no. [%]) | ||||
| None | 84 (64.1) | 83 (66.4) | 1 (16.7) | 0.003 |
| Past | 22 (16.8) | 22 (17.6) | 0 (0) | |
| Recent | 25 (19.1) | 20 (16.0) | 5 (83.3) | |
| Previous voriconazole therapyb (no. [%]) | 7 (5.3) | 5 (4.0) | 2 (33.3) | 0.20 |
| Previous posaconazole therapyb (no. [%]) | 0 | 0 | 0 | |
| Sputum samples/patient (median [IQR]) | 3.0 [2.0; 4.0] | 3.0 [2.0; 4.0] | 3.5 [1.0; 5.0] | 0.81 |
| A. fumigatus-positive sputum samples/patient (median [IQR]) | 2.0 [1.0; 3.0] | 2.0 [1.0; 3.0] | 3.5 [1.0; 5.0] | 0.34 |
| Itraconazole MIC | ||||
| Median (IQR) by Etest | 0.75 [0.50; 1.00] | 0.75 [0.50; 1.00] | 32.00 [24.00; 32.00] | <0.001 |
| MIC of ≥2 mg/liter by CLSI (no. [%]) | 6 (4.6) | 0 (0) | 6 (100) | |
| Voriconazole MIC | ||||
| Median (IQR) by Etest | 0.125 [0.125; 0.190] | 0.125 [0.125; 0.190] | 0.750 [0.250; 1.500] | 0.005 |
| MIC of ≥2 mg/liter by CLSI (no. [%]) | 3 (2.3) | 0 (0) | 3 (50) | <0.0001 |
| Posaconazole MIC | ||||
| Median (IQR) by Etest | 0.094 [0.064; 0.125] | 0.094 [0.064; 0.125] | 1.75 [1.5; 2.0] | <0.0001 |
| MIC of ≥0.5 mg/liter by CLSI (no. [%]) | 6 (4.6) | 0 (0) | 6 (100) | <0.0001 |
FEV1, forced expiratory volume in 1 s. FVC, forced vital capacity.
At any time point.
Recent exposure, within the previous 3 years; past exposure, before the previous 3 years.
We next examined the prevalence of azole-resistant A. fumigatus in CF subjects, taking into account previous exposure to itraconazole. Forty-seven (35.9%) subjects had previously received itraconazole therapy, 24 (51.0%) subjects for ABPA and 23 (49.0%) subjects for Aspergillus bronchitis. The median (IQR) duration of itraconazole therapy was 25.0 (11.0; 44.3) months, and most subjects received 400 mg/day itraconazole. Because we hypothesized that recent itraconazole therapy was associated with the increased prevalence of azole-resistant isolates, we compared subjects with recent (within the previous 3 years) itraconazole treatment to subjects with past itraconazole treatment (but no treatment within the previous 3 years) and to subjects who had never been treated with itraconazole (Table 3). Isolates with reduced susceptibilities to itraconazole (CLSI MICs of ≥2 mg/liter) were found in 1/84 (1.2%) and 0/22 (0%) subjects without itraconazole treatment or with past itraconazole treatment, respectively. However, isolates with reduced susceptibilities to itraconazole and to posaconazole were found in 5/25 (20%) of subjects with recent itraconazole treatment (P = 0.003) (Table 3). No significant association was found between the number of months on itraconazole or daily dosage of itraconazole and reduced susceptibilities to itraconazole and posaconazole (not shown).
Table 3.
Characteristics of patients according to itraconazole exposure
| Variablea | Result according to itraconazole exposure |
P values | ||
|---|---|---|---|---|
| No exposure (n = 84) | Past exposure (n = 22) | Recent exposure (n = 25) | ||
| Age (yr [IQR]) | 27 [23; 36] | 30 [27; 34] | 24 [21; 37] | 0.01 |
| Age at CF diagnosis (yr [IQR]) | 1.5 [0.2; 9.0] | 1.4 [0.1; 7.0] | 1.0 [0.2; 3.0] | 0.39 |
| Male (no. [%]) | 50 (59.5) | 14 (63.6) | 13 (52.0) | 0.73 |
| Pancreatic insufficiency (no. [%]) | 71 (84.5) | 18 (81.8) | 23 (92.0) | 0.55 |
| FEV1 (% predicted [IQR]) | 58 [42; 74] | 57 [34; 66] | 48 [36; 78] | 0.29 |
| FVC (% predicted [IQR]) | 75 [63; 92] | 67 [54; 81] | 62 [54; 83] | 0.04 |
| Chronic P. aeruginosa (no. [%]) | 49 (58.3) | 11 (50.0) | 15 (60.0) | 0.76 |
| ABPA (no. [%]) | 4 (4.8) | 11 (50.0) | 13 (52.0) | <0.001 |
| Voriconazole therapyb (no. [%]) | 1 (1.2) | 3 (13.6) | 3 (12) | 0.01 |
| Posaconazole therapyb (no. [%]) | 0 | 0 | 0 | |
| Sputum samples/patient (median [IQR]) | 3.0 [2.0; 4.0] | 3.0 [2.0; 4.0] | 4.0 [3.0; 5.0] | 0.008 |
| A. fumigatus-positive sputum samples/patient (median [IQR] | 2.0 [1.0; 3.0] | 2.5 [1.0; 4.0] | 3.0 [2.0; 4.0] | 0.24 |
| Itraconazole MIC | ||||
| Median (IQR) by Etest | 0.750 [0.440; 1.000] | 0.750 [0.500; 1.000] | 1.000 [0.500; 2.000] | 0.03 |
| MIC of ≥2 mg/liter by CLSI (no. [%]) | 1 (1.2) | 0 | 5 (20.0) | 0.003 |
| Voriconazole MIC | ||||
| Median (IQR) by Etest | 0.125 [0.125; 0.190] | 0.158 [0.125; 0.190] | 0.190 [0.125; 0.250] | 0.50 |
| MIC of ≥2 mg/liter by CLSI (no. [%]) | 1 (1.2) | 0 | 2 (8.0) | 0.17 |
| Posaconazole MIC | ||||
| Median (IQR) by Etest | 0.094 [0.064; 0.125] | 0.094 [0.064; 0.125] | 0.125 [0.064; 0,19] | 0.001 |
| MIC of ≥0.5 mg/liter by CLSI (no. [%]) | 1 (1.2) | 0 | 5 (20.0) | 0.003 |
FEV1, forced expiratory volume in 1 s. FVC, forced vital capacity.
At any time point.
DISCUSSION
In the present study, we prospectively examined azole susceptibilities of A. fumigatus isolates obtained from the sputum of adult CF subjects. In this single-center cohort, we found that 4.6% of subjects harboring A. fumigatus had isolates with reduced susceptibilities to itraconazole. All of the isolates with reduced susceptibilities to itraconazole also had reduced susceptibilities to posaconazole, and half of these isolates also were resistant to voriconazole. A strong association was found between itraconazole treatment within the previous 3 years and reduced susceptibilities to both itraconazole and posaconazole.
To the best of our knowledge, only three studies reported systematic screening for A. fumigatus azole susceptibilities in CF subjects. Mortensen et al., who studied azole susceptibilities using an itraconazole screening agar and the EUCAST method, found itraconazole MICs of ≥2 mg/liter in 6/133 (4.5%) CF subjects with A. fumigatus isolates (18). A recent Dutch study reported a prevalence of 4.2% azole resistance among 442 isolates after screening with a real-time PCR (25). Amorim et al. studied 159 A. fumigatus isolates collected from the sputa of 11 adult CF patients using the CLSI broth dilution method and reported no resistance to itraconazole or posaconazole (5). In the latter study, 4/11 patients had isolates with itraconazole MICs of 2 mg/liter, indicating intermediate susceptibility to itraconazole, and A. fumigatus also had intermediate susceptibility to posaconazole in one of these patients (5). Our findings confirmed that A. fumigatus isolates with reduced susceptibility to itraconazole were present among CF subjects and extended previous data by showing that cross-resistance to posaconazole and to voriconazole also was prevalent.
Subjects with reduced itraconazole susceptibility were more likely to have received itraconazole therapy within the previous 3 years. This finding is in line with the report by Mortensen et al., who found that the 6 subjects with reduced A. fumigatus susceptibility to itraconazole were previously exposed to itraconazole (18). However, no data were provided on the rates of azole resistance in subjects exposed to azole therapies (18). Here, we show that reduced itraconazole and posaconazole susceptibilities were present in 20% of subjects with recent itraconazole treatment. These data suggest a major role for itraconazole therapy in the selection of azole-resistant isolates.
Subjects with past treatment with itraconazole (but without treatment within the previous 3 years) had no evidence of decreased azole susceptibility. In a recent study, de Valk et al. performed the molecular typing of A. fumigatus isolates obtained during longitudinal follow-up in 36 adult CF subjects (9). The authors found that only 17% of the patients were chronically colonized with a single genotype, suggesting that in many subjects A. fumigatus isolates were cleared but that recolonization with other genotypes of A. fumigatus occurred (9). We speculate that the clearance of A. fumigatus isolates, followed by the acquisition of novel isolates, explains the low rates of reduced susceptibility to itraconazole in CF subjects with past (but without recent) itraconazole treatment. It also is possible that azole resistance in A. fumigatus isolates results in the alteration of growth and virulence (loss of fitness), favoring their disappearance from CF airways (6). Further studies will be required to confirm these hypotheses.
The present study has several strengths. It was performed in a prospective design in consecutive adult CF subjects with various degrees of respiratory impairment. The size of the cohort allowed the assessment of the impact of itraconazole exposure on azole-resistant A. fumigatus. We also recognize limitations. Susceptibility to azoles was initially tested using Etest, which is not the reference method (8, 21). The confirmation of azole resistance was obtained using the reference CLSI broth dilution technique. Although isolates that had CLSI-confirmed azole resistance had the highest Etest MICs (Table 1), our data suggest that breakpoints or epidemiological cutoff values (ECVs) are method specific. The use of Etest had the advantage of being commercially available, and it can be routinely performed in most laboratories (14). However, the confirmation of resistance using a broth dilution technique and/or genetic analysis appears necessary until Etest-specific breakpoints are established. Only 13/47 (27.6%) subjects with itraconazole treatment had therapeutic drug monitoring, and itraconazole concentrations were lower than recommended in 4 subjects (data not shown). Thus, we were unable to examine the effect of long-term underdosing on azole susceptibility to A. fumigatus. Because azole plasma concentrations may be widely variable in CF subjects receiving similar doses of azole therapies (7), the role of pharmacokinetic variability in the reduced azole susceptibility of A. fumigatus isolates will require further studies.
The molecular characterization of the azole-resistant isolates identified mutations in the cyp51A gene (26), including TR/L98H, and mutations at positions M220 and G54 (14). A variety of mutations at M220 and G54 have been previously implicated as causing azole resistance (including G54R, G54E, G54W, G54V, G54K, M220V, M220K, M220T, M220R, and M220I) and were associated with azole exposure. In contrast, the TR/L98H mutation also is described in azole-naïve patients and was present in one patient without previous therapeutic exposure to itraconazole, suggesting that it resulted from environmental exposure. One isolate, with cross-resistance to itraconazole, voriconazole, and posaconazole, had no mutation at any of the positions covered by the real-time PCR-based screening assay (also including G138) (17). Further studies will be necessary to determine the molecular basis of azole resistance in this isolate.
Itraconazole therapy has been advocated in subjects with Aspergillus bronchitis (22) and as an adjunctive treatment to oral steroids in subjects with ABPA (24). These attitudes are based on relatively short-term studies. However, many patients in our study were treated for long periods, and the median time of itraconazole therapy was 25 months. Because exposure to itraconazole is associated with a marked increase in A. fumigatus MICs to both itraconazole and posaconazole, we suggest that the benefits of long-term treatment with itraconazole are uncertain. For example, the in vitro resistance of A. fumigatus to oral azole therapies had been associated with treatment failure in subjects with aspergillosis (15). These findings may be particularly relevant for subjects with severe CF in whom lung transplantation is associated with a higher risk of invasive aspergillosis (16).
In conclusion, we found a prevalence of 4.6% azole-resistant A. fumigatus isolates in CF adults, which was similar to studies in Denmark and the Netherlands. Reduced susceptibilities to itraconazole and posaconazole were clearly associated with recent exposure to itraconazole. We suggest that the in vitro testing of A. fumigatus azole susceptibility should be performed systematically when considering azole therapy in CF subjects. Although further studies are clearly required, we speculate that a combination of limiting azole therapy to short periods of time and the monitoring of therapeutic drug concentrations will reduce the emergence of azole-resistant Aspergillus isolates.
ACKNOWLEDGMENTS
We thank Marie-Elisabeth Bougnoux (Hôpital Necker, Université Paris Descartes, Paris, France) for help with mass spectrometry and Vincent Julien (Hôpital Saint Vincent de Paul, Paris, France) for help with therapeutic drug monitoring.
J.F.M. was partly supported by the Dutch Cystic Fibrosis Foundation.
Footnotes
Published ahead of print 28 November 2011
REFERENCES
- 1. Alanio A, et al. 2011. Matrix-assisted laser desorption ionization time-of-flight mass spectrometry for fast and accurate identification of clinically relevant Aspergillus species. Clin. Microbiol. Infect. 17:750–755 [DOI] [PubMed] [Google Scholar]
- 2. Alanio A, et al. 2011. Low prevalence of resistance to azoles in Aspergillus fumigatus in a French cohort of patients treated for haematological malignancies. J. Antimicrob. Chemother. 66:371–374 [DOI] [PubMed] [Google Scholar]
- 3. Alcazar-Fuoli L, Mellado E, Alastruey-Izquierdo A, Cuenca-Estrella M, Rodriguez-Tudela JL. 2008. Aspergillus section Fumigati: antifungal susceptibility patterns and sequence-based identification. Antimicrob. Agents Chemother. 52:1244–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Amin R, Dupuis A, Aaron SD, Ratjen F. 2010. The effect of chronic infection with Aspergillus fumigatus on lung function and hospitalization in patients with cystic fibrosis. Chest 137:171–176 [DOI] [PubMed] [Google Scholar]
- 5. Amorim A, Guedes-Vaz L, Araujo R. 2010. Susceptibility to five antifungals of Aspergillus fumigatus strains isolated from chronically colonised cystic fibrosis patients receiving azole therapy. Int. J. Antimicrob. Agents 35:396–399 [DOI] [PubMed] [Google Scholar]
- 6. Arendrup MC, et al. 2010. Development of azole resistance in Aspergillus fumigatus during azole therapy associated with change in virulence. PLoS One 5:e10080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Billaud EM, et al. 2010. Pharmacological considerations for azole antifungal drug management in cystic fibrosis lung transplant patients. Med. Mycol. 48(Suppl. 1):S52–S59 [DOI] [PubMed] [Google Scholar]
- 8. Clinical and Laboratory Standards Institute 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi-second edition: approved standard M38-A2. CLSI, Wayne, PA [Google Scholar]
- 9. de Valk HA, et al. 2009. Molecular typing and colonization patterns of Aspergillus fumigatus in patients with cystic fibrosis. J. Cyst. Fibros. 8:110–114 [DOI] [PubMed] [Google Scholar]
- 10. de Vrankrijker AM, et al. 2011. Aspergillus fumigatus colonization in cystic fibrosis: implications for lung function? Clin. Microbiol. Infect. 17:1381–1386 [DOI] [PubMed] [Google Scholar]
- 11. Farrell PM, et al. 2008. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J. Pediatr. 153:S4–S14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hilliard T, et al. 2005. Voriconazole therapy in children with cystic fibrosis. J. Cyst. Fibros. 4:215–220 [DOI] [PubMed] [Google Scholar]
- 13. Horré R, Symoens F, Delhaes L, Bouchara JP. 2010. Fungal respiratory infections in cystic fibrosis: a growing problem. Med. Mycol. 48(Suppl. 1):S1–S3 [DOI] [PubMed] [Google Scholar]
- 14. Howard SJ, Arendrup MC. 2011. Acquired antifungal drug resistance in Aspergillus fumigatus: epidemiology and detection. Med. Mycol. 49(Suppl. 1):S90–S95 [DOI] [PubMed] [Google Scholar]
- 15. Howard SJ, et al. 2009. Frequency and evolution of azole resistance in Aspergillus fumigatus associated with treatment failure. Emerg. Infect. Dis. 15:1068–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Iversen M, et al. 2007. Aspergillus infection in lung transplant patients: incidence and prognosis. Eur. J. Clin. Microbiol. Infect. Dis. 26:879–886 [DOI] [PubMed] [Google Scholar]
- 17. Klaassen CH, De Valk HA, Curfs-Breuker IM, Meis JF. 2010. Novel mixed-format real-time PCR assay to detect mutations conferring resistance triazoles in Aspergillus fumigatus and prevalence of multi-triazole resistance among clinical isolates in the Netherlands. J. Antimicrob. Chemother. 65:901–905 [DOI] [PubMed] [Google Scholar]
- 18. Mortensen KL, et al. 2011. Aspergillus species and other moulds in respiratory samples from cystic fibrosis patients: a laboratory-based study with focus on azole resistance in Aspergillus fumigatus. J. Clin. Microbiol. 49:2243–2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Paugam A, et al. 2010. Characteristics and consequences of airway colonization by filamentous fungi in 201 adult patients with cystic fibrosis in France. Med. Mycol. 48(Suppl. 1):S32–S36 [DOI] [PubMed] [Google Scholar]
- 20. Rodriguez-Tudela JL, et al. 2008. Epidemiological cutoffs and cross-resistance to azole drugs in Aspergillus fumigatus. Antimicrob. Agents Chemother. 52:2468–2472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rodriguez-Tudela JL, et al. 2008. EUCAST definitive document EDef 7.1: method for the determination of broth dilution MICs of antifungal agents for fermentative yeasts. Clin. Microbiol. Infect. 14:398–405 [DOI] [PubMed] [Google Scholar]
- 22. Shoseyov D, Brownlee KG, Conway SP, Kerem E. 2006. Aspergillus bronchitis in cystic fibrosis. Chest 130:222–226 [DOI] [PubMed] [Google Scholar]
- 23. Snelders E, et al. 2008. Emergence of azole resistance in Aspergillus fumigatus and spread of a single resistance mechanism. PLoS Med. 5:e219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stevens DA, et al. 2003. Allergic bronchopulmonary aspergillosis in cystic fibrosis–state of the art: Cystic Fibrosis Foundation Consensus Conference. Clin. Infect. Dis. 37(Suppl. 3):S225–S264 [DOI] [PubMed] [Google Scholar]
- 25. Terpstra PD, et al. 2011. Filamentous fungi in the Netherlands among CF patients. Mycoses 54(Suppl. 2):152 [Google Scholar]
- 26. Verweij PE, Howard SJ, Melchers WJ, Denning DW. 2009. Azole-resistance in Aspergillus: proposed nomenclature and breakpoints. Drug Resist. Updat. 12:141–147 [DOI] [PubMed] [Google Scholar]
- 27. Verweij PE, Snelders E, Kema GH, Mellado E, Melchers WJ. 2009. Azole resistance in Aspergillus fumigatus: a side-effect of environmental fungicide use? Lancet Infect. Dis. 9:789–795 [DOI] [PubMed] [Google Scholar]