Abstract
Lsr2 protein of Mycobacterium leprae was shown earlier to elicit B and T cell responses in leprosy patients (20, 28). Lymphoproliferation to M. leprae and Lsr2 antigens was observed in >70% of tuberculoid (T) patients and in 16 and 34% of lepromatous (L) patients, respectively. We focused on the M. leprae nonresponders in the lepromatous group using 22 synthetic Lsr2 peptides (end-to-end peptides A to F and overlapping peptides p1 to p16) in in vitro T cell responses. A total of 125 leprosy and 13 tuberculosis patients and 19 healthy controls from the area of endemicity (here, healthy controls, or HC) were investigated. The highest responses were observed (67 to 100%) in HC for all peptides except p1 to p3, and the lowest was observed in tuberculosis patients. Significant differences in lymphoproliferation were observed in T, L, and HC groups (analysis of variance [ANOVA], P = 0.000 to 0.015) for all end-to-end peptides except B and for p5 and p7 to p10. Hierarchical recognition between lepromatous and tuberculoid leprosy was noted for p8 (P < 0.05) and between the HC and L groups for p7 to p10, p15, and p16 (P < 0.005 to P < 0.02). Significant lymphoproliferation was observed to peptides A to F and p1 to p9, p11, p12, p15, p16 (P = 0.000 to 0.001) with 40% responding to peptides C and p16 in L patients. Lepromatous patients also showed significantly higher levels of a gamma interferon (IFN-γ) response to peptide C than to other peptides (P < 0.05). Major histocompatibility complex (MHC) class II bias for peptide recognition was not observed. These studies indicate that Lsr2 has multiple T cell epitopes that induce in vitro T cell responses in the highly infective lepromatous leprosy patients.
INTRODUCTION
Though the prevalence of leprosy has been reduced due to multidrug therapy regimens, the incidence continues to be a public health worry in some countries (40). Leprosy is caused by the noncultivable Mycobacterium leprae and is defined by distinct clinical-pathological types in humans (29), with the paucibacillary localized tuberculoid forms (borderline tuberculoid/tuberculoid [BT/TT]) having good in vitro and in vivo T cell functions and low levels of antibodies. In contrast, the multibacillary generalized lepromatous leprosy patients (borderline leprosy/lepromatous leprosy [BL/LL]) show abundant antibody responses and T cell unresponsiveness unique to the leprosy bacillus and not to other antigenically related mycobacteria such as Mycobacterium tuberculosis. Moreover, 10 to 15% of stable leprosy patients undergo inflammatory episodes of types 1 (reversal reactions [RR]) and 2 (erythema nodosum leprosum [ENL]) which are localized to the lesion or produce systemic signs of fever, joint pains, and skin nodules. The inverse relationship between cellular and humoral immunity as well as the dichotomy in the leprosy types has been intensely investigated (21, 30). The mechanisms underlying the antigen-specific anergy are not completely understood as it is long-lasting and not reversed by conventional therapy. Attempts to improve the host responses have been made with cross-reacting mycobacteria such as Mycobacterium bovis BCG, Mycobacterium W (32, 39), and Mycobacterium vaccae (38) as well as gamma interferon (IFN-γ) (15, 22).
Defining M. leprae antigens that would reverse the anergy in the highly infective patients would provide tools to break the infection cycle in the community as well as aid in early diagnosis of a disease that has a long incubation period. The annotation of the M. leprae genome has made it possible to study the role of several immunologically relevant proteins in leprosy (6, 7). Synthetic peptides and recombinant products are being increasingly used to design diagnostics for early detection of the disease. Both serological and whole-blood T cell assays using specific antigens, proteins, and peptides of the leprosy bacillus have been carried out (1, 18, 26, 31, 35, 36).
The Lsr2 gene coding for an immunodominant protein was identified in the 1990s by us (18) as well as others (31) using pooled lepromatous leprosy sera to screen the lambda gt11 (42) and a cosmid library. Whereas our clone expressed a 10-kDa Lsr2 protein, Sela et al. (31) cloned the full gene expressing the 15-kDa protein including the 5′ end missing in our clone. There was a paucity of information on the gene itself as M. leprae is not cultivable by conventional methods. Comparative genomics was not helpful in defining its functions until recently when several other organisms including the medically important M. tuberculosis, Mycobacterium avium-intracellulare (3, 6, 7), and Mycobacterium smegmatis (2) were shown to have Lsr2 homologues. In M. tuberculosis Lsr2 has been characterized to be a unique nucleoid-associated protein, akin to histone-like nucleoid structuring protein (H-NS) (3). It binds AT-rich regions including regions encoding virulence factors such as ESX secretion systems (8), lipid virulence factors, phthiocerol dimycocerosates (PDIM) (5), phenolic glycolipid (PGL) (14), and acidic asparagines or glycine-rich protein (PE/PPE) antigenic families (11). Lsr2 binding leads to transcription repression (11), influences cell wall synthesis (2), and may play an important role in drug resistance (5). Lsr2 is upregulated in the presence of iron (41). Lsr2 was shown to be recognized by T cells and antibodies of tuberculoid and lepromatous leprosy patients (18, 31, 34). Using overlapping synthetic peptides of Lsr2, we showed that ENL patients had sequence-specific antibodies during, as well as prior to, the inflammatory episodes (33). Others showed that T cell lines developed from Norwegian volunteers immunized with heat-killed leprosy bacilli recognized HLA-restricted epitopes of Lsr2 (26).
The present study investigates the specificities of T cell recognition of Lsr2 by peripheral blood mononuclear cells (PBMC) of clinically stable leprosy patients as well as of tuberculosis patients and healthy volunteers using 22 synthetic overlapping and end-to-end peptides spanning the Lsr2 sequence. Lymphoproliferation and gamma interferon release were used as in vitro correlates of T cell functions.
MATERIALS AND METHODS
Subjects.
A total of 125 freshly diagnosed untreated leprosy patients (86 males and 39 females aged between 18 to 54 years) were included in the study (Table 1). Of these, 118 were recruited from the Hansen's Disease Clinics of the Department of Dermatology, Safdarjung Hospital, New Delhi, India, and 7 were from the Voluntary Health Centre, Sakthinagar, Periyar district, Tamil Nadu, India. All patients were classified on the basis of Ridley-Jopling classification (29) and gave no history of prior treatment: care was taken to exclude patients with reactions both at the time of blood sampling as well as at subsequent follow-up for 12 to 18 months. Table 1 provides clinical details of the leprosy patients. Borderline and polar lepromatous patients and tuberculoid patients were grouped as L and T, respectively. Nineteen healthy subjects from the area of endemicity (here, healthy controls [HC]) exposed to leprosy for more than 10 years and 5 and 8 patients freshly diagnosed with skin (lupus vulgaris) and pulmonary tuberculosis, respectively, were included in the study as controls.
Table 1.
Clinical details of 125 freshly diagnosed untreated leprosy patients from Delhi and Tamil Nadu clinics included in the study
| Leprosy type (abbreviation)a | Regionb | No. of patients | Age (yr) | Sexc |
Disease duration (mo.) | Range of BId | |
|---|---|---|---|---|---|---|---|
| M | F | ||||||
| Lepromatous (L) | All | 71 | 25–54 | 49 | 22 | 6–48 | 1.4–4.7 |
| Polar (LL) | Delhi | 44 | 25–54 | 28 | 16 | 10–48 | 2.4–4.7 |
| TN | 1 | 52 | 1 | 18 | 3.65 | ||
| Borderline (BL) | Delhi | 24 | 32–54 | 19 | 5 | 6–24 | 2.3–5.2 |
| TN | 2 | 25, 48 | 1 | 1 | 6,12 | 3.5–2.4 | |
| Tuberculoid (T) | All | 54 | 18–49 | 37 | 17 | 6–24 | 0 |
| Polar (TT) | Delhi | 4 | 21–35 | 3 | 1 | 9–11 | 0 |
| TN | 0 | ||||||
| Borderline (BT) | Delhi | 46 | 18–49 | 31 | 15 | 6–18 | 0 |
| TN | 4 | 34–48 | 3 | 1 | 12–24 | 0 | |
| Total | 125 | 18–54 | 86 | 39 | 6–48 | 0–5.2 | |
Patients were classified on the basis of Ridley-Jopling classification (see Materials and Methods).
TN, Tamil Nadu.
M, male; F, female.
BI, mean bacillary index of six skin sites.
The study was approved by Institutional Ethics Committee of the All India Institute of Medical Sciences, Safdarjung, and VHS hospitals, and informed consent was taken from patients prior to collection of samples. For the M. leprae antigen, integral heat-killed armadillo-derived M. leprae was kindly provided by P. J. Brennan (Colorado State University, Fort Collins, CO). The 10-kDa recombinant Lsr2 antigen was prepared in our laboratory as described earlier (34). Essentially, lysates of Escherichia coli with and without the insert were centrifuged at 12,000 × g, and the insoluble pellet containing the Lsr2 protein was resuspended in endotoxin-free phosphate-buffered saline (PBS), pH 7.4. The 15-kDa Lsr2 clone (A15) was kindly provided by J. E. Clark Curtiss, Washington University, St. Louis, MO. In brief, the Lsr2 gene was amplified by PCR from the plasmid pYA1164 (31) and subcloned into a pMAL-c (New England BioLabs) to yield plasmid pYA1287. This plasmid was then digested with BamHI and PstI to yield a fragment of 460 bp that contained the coding sequences for A15 protein plus the amino acids that comprise the factor Xa recognition site from pMAL-c. This fragment was cloned into pQE9 (Qiagen, Inc., Chatsworth, CA) to form an in-frame fusion of A15 to six histidines of pQE9, put in the E. coli M15 strain, and grown in the presence of kanamycin and ampicillin at a final concentration of each of 25 μg/ml (J. E. Clark Curtiss, personal communication). The A15 protein was purified from lysates by mixing with a slurry of Ni-iminodiacetic acid (IDA) resin and washed with buffer containing various concentrations of imidazole as per the manufacturer's instructions (Qiagen, Inc., CA). The purification trail of Lsr2 as assessed in 12% SDS-PAGE gels is shown in Fig. S1a in the supplemental material. The pooled, dialyzed fractions were suspended in endotoxin-free PBS and checked for purity by 12% SDS-PAGE gel electrophoresis. Figure S1b depicts a Western blot of purified Lsr2 protein blotted on a polyvinylidene difluoride (PVDF) membrane treated with horseradish peroxidase (HRP)-conjugated mouse His antibody as per the manufacturer's instructions (Qiagen Inc.). Endotoxin contamination was further assessed using a chromogenic microplate Limulus amebocyte lysate assay (QCL-1000) detectable within a range of 0.1 to 1 endotoxin units (EU)/ml as per the manufacturer's instructions (Lonza, Walksville, MD). The two batches of Lsr2 used in the study showed 0.3 and 0.4 EU/ml which is in conformity with FDA regulations.
Synthetic peptides.
Using algorithms for T cell epitopes, M. E. Pattaroyo and F. Guzman (Institute de Immunologia, Bogota, Colombia) designed and kindly synthesized six end-to-end native peptides (A to F) spanning the contiguous sequences from amino acids 1 to 112 of Lsr2 (Fig. 1). Overlapping peptides (p1 to p16) were prepared as described earlier (34) in the laboratory of the late M. J. Colston at the National Institute of Medical Research, Mill Hill, United Kingdom (Fig. 1). In brief, both types of peptides were synthesized by solid phase using the N-tert-butyloxycarbonyl (t-boc) method (13) in propylene bags (74-μm pore size) with 150 mg of p-methyl benzhydrylamine HCl resin (Bachem, Inc., Torrance, CA).
Fig 1.
The amino acid sequences of the 15-kDa Lsr2, end-to-end (aa 1 to 112; peptides A to F), and overlapping (p1 to p16) peptides, and their locations in the protein are provided along with the percentages of responders showing an SI of >2 in lymphoproliferative responses of PBMC. The end-to-end peptides were investigated in 9 HC, 34 borderline/polar tuberculoid (T) patients, and 38 borderline/polar lepromatous patients (L). The overlapping peptides p1 to p16 were investigated in an additional 5 HC and 14 T and 10 L patients. GenBank accession numbers are X53487 for the 10-kDa Lsr2 and M67510 for the 15-kDa Lsr2.
Ex vivo PBMC cultures with antigens and mitogen. (i) Lymphoproliferation assay.
Heparinized peripheral blood obtained by venipuncture from the field areas was transported by air in ice packs and processed within 10 to 12 h of bleeding, whereas samples from the Delhi clinic were processed within 1 h. PBMC were separated using Histopaque (Sigma-Aldrich, St. Louis, MO) and density gradient centrifugation. PBMC were washed in Hank's balanced salt solution (HBSS) and resuspended in RPMI 1640 medium (Gibco-BRL, MD) containing 10% AB serum, 2 mM l-glutamine, 100 units of penicillin (Alembic chemicals, India) and 100 μg of streptomycin (Sarabhai Chemicals, India). Staining with 0.2% trypan blue dye exclusion showed 95 to 98% cell viability. PBMC (100 μl) were dispensed in triplicate at a concentration of 1 × 105 cells/well into 96-well sterile flat bottom culture plates (Nunc, Intermed, and Kampstrup, Denmark). The optimal concentrations of antigens, peptides, and mitogen phytohemagglutinin ([PHA] Difco Laboratories, Mosley, United Kingdom) were determined following preliminary testing with three concentrations of each antigen/peptide on five healthy controls and 10 each of tuberculoid and lepromatous leprosy patients. The cells were exposed to 25 μl of each of the following antigens: (i) integral heat-killed armadillo-derived M. leprae at a concentration of 5 × 106 bacilli/ml; (ii) recombinant Lsr2 (15 kDa) antigen at 1 μg/ml; (iii) synthetic peptides at a concentration of 50 μg/ml; (iv) purified protein derivative (PPD) at 50 μg/ml (Ministry of Agriculture and Fisheries, Weybridge, Surrey, United Kingdom); (v) PHA at 1 μg/ml; and (vi) no antigen. All antigen- and mitogen-stimulated cultures were incubated for 5 and 3 days, respectively, at 37°C in humidified 5% CO2 plus air. The cultures were pulsed with [3H]thymidine (2 Ci/mM; Amersham International, Buckinghamshire, United Kingdom) 16 to 18 h prior to being harvested on glass fiber discs (GF/C; Whatman, Maidstone, England) by a semiautomatic harvester (PHD Cell Harvester, Cambridge, MA). The mitogen-stimulated cultures were similarly treated and harvested at 72 h. The incorporated radioactivity was assessed by liquid scintillation counting on an LKB RackBeta 1209 counter (Piscataway, NJ) using toluene-based scintillation counting fluid. The counts per minute (cpm) were obtained, and the results were expressed as the mean change in cpm (Δcpm) ± standard deviation ([SD] mean cpm of stimulated cultures − mean cpm of unstimulated cultures) and the stimulation index (SI; mean cpm of stimulated cultures/mean cpm of unstimulated cultures). An SI value of >2 was considered a positive response to the antigens and peptides as the receiver operating characteristic (ROC) analysis showed the statistically significant area under the curve to be a P value of <0.001.
(ii) IFN-γ.
PBMC were cultured with Lsr2, end-to end peptides, and PHA as described above for 48 h. The supernatants were removed and stored at −20°C until further use. IFN-γ was estimated in duplicate as per the manufacturer's instructions using an eBioscience Ready-Set-Go enzyme-linked immunosorbent assay (ELISA) kit (Biogene, New Delhi, India) with a sensitivity of 4 pg/ml.
HLA-DRB1, -DQA1, and -DQB1 polymorphism.
HLA-DRB1 alleles were checked in 19 lepromatous and 4 tuberculoid patients and two family contacts. Alleles of the HLA-DRB1 locus were determined by amplifying the second exon of the DRB1 gene using PCR and hybridization with sequence-specific oligonucleotide probes (SSOP) as described earlier (9, 27). Briefly, 500 ng of genomic DNA was amplified by using primers for generic amplification of the DRB1 second exon using standard conditions and run on a 1% agarose gel to check for amplification. The amplicons were subjected to dot blots on Zeta Probe membranes (Bio-Rad, CA), UV cross-linked (Syngene, San Carlos, CA), and hybridized with 20 32P-labeled probes for generic DRB1. This was followed by group-specific amplifications and hybridizations for DR2-, DR4-, and DR52-associated alleles since each one of these groups has several alleles. Similarly, the second exons of the DQB1 and DQA1 alleles were amplified; dot blots were made and hybridized with sequence-specific oligonucleotide probes. Interpretation of the alleles was based on the hybridization patterns of the sequence-specific oligonucleotide probes as described earlier (27, 28).
Evidence-based computer algorithms used for major histocompatibility complex (MHC) class II binding and transporter-associated protein (TAP) were HLAPred (http://www.imtech.res.in/raghava/hlapred/) and TAPPred (http://www.imtech.res.in/raghava/tappred/).
Statistical analysis.
Nonparametric statistics used to evaluate significance were one-way analysis of variance (ANOVA), Tukey, Wilcoxon ranked sign test, two-tailed Mann-Whitney, and ROC analysis; multiple-discriminant analysis (MDA) was performed to evaluate the relative importance of peptides, and results were interpreted in terms of Wilk's lambda statistics along with P value and canonical correlation territorial maps, using GraphPad Prism, version 5, and SPSS, version 19. A two-sided P value of <0.05 was considered to be statistically significant.
RESULTS
A total of 125 freshly diagnosed clinically stable leprosy patients (71 lepromatous [L]) and (54 tuberculoid [T]) were investigated prior to receiving treatment (Table 1). Care was taken to exclude patients with types 1 (RR) and 2 (ENL) leprosy reactions by extended clinical follow-up after the initial diagnosis. Borderline and polar lepromatous were grouped together as L, whereas polar (TT) and borderline tuberculoid leprosy patients (BT) were grouped as T as they showed nonsignificant differences in T cell responses (P < 0.7, ANOVA). Thirteen freshly diagnosed tuberculosis patients and 19 healthy contacts from the area of endemicity (HC) were included as controls.
Heat-killed integral armadillo-derived M. leprae, recombinant Lsr2, and end-to-end as well as overlapping peptides of Lsr2 were used as antigens. The amino acid sequences of Lsr2 and the peptides are given in Fig. 1. Subjects showing a stimulation index (SI) of <2 in the lymphoproliferation assay were considered nonresponders based on ROC analysis.
Lymphoproliferative responses of PBMC: whole antigens/mitogen.
The results of lymphoproliferative responses (mean Δcpm ± SD and mean SI ± SD) obtained with whole antigens, M. leprae, Lsr2, PPD, and a T cell mitogen PHA in healthy contacts and lepromatous and tuberculoid patients are provided in Table 2. Preliminary studies on 10 each of T and L patients did not show significant differences between concurrent PBMC cultures stimulated with the 10-kDa (18) or 15-kDa Lsr2 protein in either individual subjects or clinical groups (data not shown) (P < 0.666, Mann-Whitney), and the 15-kDa Lsr2 protein was used for subsequent studies. As expected HC and tuberculoid patients showed higher in vitro responses to antigens and PHA than lepromatous patients (Table 2) (P < 0.001, ANOVA) with 79% and 54.1% of tuberculoid leprosy subjects responding to M. leprae and Lsr2, respectively, compared to 23% and 35.5% of lepromatous leprosy patients showing lymphoproliferation to M. leprae and Lsr2, respectively (SI >2 to >3) (Table 2). The 10 lepromatous subjects who responded to both M. leprae and Lsr2 also recognized 3 to 6 end-to-end and 11 to 13 overlapping peptides (SI, 2.5 to 3.6) (data not shown). Of interest was the observation that anergic lepromatous patients who did not show in vitro responses to M. leprae antigens nevertheless responded to Lsr2 protein (P < 0.001, Wilcoxon signed-rank test). Lepromatous patients who did not respond to M. leprae antigens were further explored along with clinical groups of HC, T, and tuberculosis patients for T cell responses (see figures).
Table 2.
Mean Δcpm and mean SI of lymphoproliferative responses of PBMC to whole heat-killed M. leprae, recombinant 15-kDa Lsr2, and mitogen PHA in 14 healthy controls and 48 tuberculoid and 62 lepromatous patientsa
| Antigen/mitogen | Mean Δcpm ± SD |
Mean SI ± SD |
||||
|---|---|---|---|---|---|---|
| HC | T | L | HC | T | L | |
| Lsr2 | 1,782.4 ± 525.3 | 3,360.1 ± 1,020.2 | 2,345 ± 1,271.0* | 2.29 ± 1.86 | 2.74 ± 1.12 | 0.89 ± 0.53** |
| M. leprae | 2,390.2 ± 711.1 | 2,610.1 ± 1,019.4 | 1,019 ± 929.2* | 3.29 ± 2.73 | 2.54 ± 0.72 | 0.82 ± 1.25* |
| PPD | 9,090.3 ± 5,501.3 | 5,793.8 ± 1,501.4 | 6,102.4 ± 4,120.5 | 10.73 ± 4.98 | 5.62 ± 2.15 | 5.32 ± 2.81 |
| PHA | 62,105.4 ± 12,542.0 | 53,195.3 ± 14,206.2 | 34,559.2 ± 10,914.4 | 72.62 ± 15.7 | 52.58 ± 12.3 | 35.30 ± 8.46* |
Δcpm, cpm of stimulated cultures − cpm of unstimulated cultures; SI, cpm of stimulated cultures/cpm of unstimulated cultures. Asterisks indicate significant differences between results for tuberculoid (T) versus lepromatous (L) leprosy (*, P < 0.001; **, P < 0.0001 by ANOVA). The median (Q1,Q3) values of concurrent unstimulated cultures for PHA were 933.5 (873.5, 1,187.5), 845 (750, 926), and 821 (762.8, 901) for HC, T, and L, respectively. Six-day unstimulated cultures for three antigens were the same, with median (Q1, Q3) values being 872 (628, 1,256), 827 (664.5, 1,009), and 859 (670.5, 1055.8) for HC, T, and L respectively. Statistical differences between the clinical groups were not observed (ANOVA). Percent responders (SI > 2) were the following: for M .leprae, the values were 93% for HC, 79% for T, and 23% for L; for Lsr2, the values were 71% for HC, 54.1% for T, and 35.5% for L. All subjects responded to PPD and PHA.
End-to-end peptides of Lsr2. (i) Lymphoproliferative responses of PBMC.
The sequence of the Lsr2 protein and its peptides and the percentages of responders in the clinical groups are given in Fig. 1. The peptides were essentially 18 to 20 residues except for the longer peptide A (amino acids [aa] 1 to 23), which covered the additional sequence seen in the 15-kDa protein (31), and the shorter peptide F consisting of the 12 terminal residues.
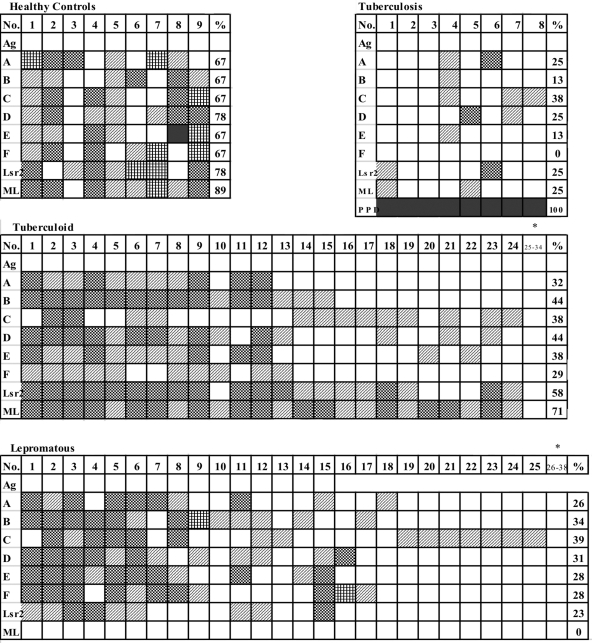
Individual lymphoproliferative responses (SI of >2) to the end-to-end peptides (Fig. 2) showed both responders and nonresponders to Lsr2 protein and its peptides among the clinical groups examined. The T cell recognition pattern of peptides showed variation between and within the clinical groups (Fig. 2). As may be seen from Fig. 1 and 2, all end-to-end peptides were stimulatory in the clinical groups. Moreover, some subjects responded to multiple peptides, some to a restricted number, and others to a single peptide. The best responses were noted in healthy controls, where all except one recognized multiple peptides at SIs ranging from 2 to >4. However, the percentage of responders varied, with HC showing the highest rate of responders (67 to 78%) followed by the tuberculoid (32 to 44%) and lepromatous leprosy patients (26 to 39%). Interestingly, 24/34 tuberculoid patients (70.6%) and 25/38 lepromatous patients (65.8%) showed responses to at least one of the Lsr2 peptides tested (Fig. 2). Moreover, in conformity with results obtained with the whole Lsr2 protein, the anergic lepromatous patients showed significantly higher responses to peptides A to F (<0.000 to <0.001; Wilcoxon signed-rank test) than M. leprae or the Lsr2 protein.
Fig 2.
Individual lymphoproliferative responses of PBMC to end-to-end peptides (A to F) in healthy controls and tuberculoid, lepromatous leprosy, and pulmonary tuberculosis patients expressed as SIs are indicated by each box. The data on additional tuberculoid (subjects 25 to 34) and lepromatous (subjects 26 to 38) subjects who showed SIs of <2 to all of the antigens are represented as an end block marked with an asterisk. The percentage of responders (SI of >2) for each peptide is provided in the right-hand panel for each antigen. ML, heat-killed M. leprae; PPD, purified protein antigen of M. tuberculosis. The SI for each subject and peptide/antigen is graded as follows: white box, <2; diagonally striped box, >2; black and white checked box, >3; vertically striped box, >4; black box, >10.
Hierarchical peptide recognition was evident in the clinical groups (Fig. 1 and 2). Significant differences in response to the peptides were observed between the groups for all peptides except B (P < 0.015 to 0.000, ANOVA). Maximally, 78% of HC recognized peptide D, and 44% of tuberculoid leprosy patients recognized both peptides B and D, whereas 39% of lepromatous leprosy patients recognized peptide C. For lepromatous leprosy patients and tuberculoid patients, 13/38 and 10/34, respectively, did not show lymphoproliferation to any peptide of Lsr2, as indicated by SIs of <2 and mean Δcpms of <800.
Though the eight pulmonary tuberculosis patients showed high lymphoproliferative responses to PPD, with SIs ranging from 12 to 35, responsiveness to Lsr2 and its peptides A to E was poor (Fig. 2).
(ii) IFN-γ responses of PBMC.
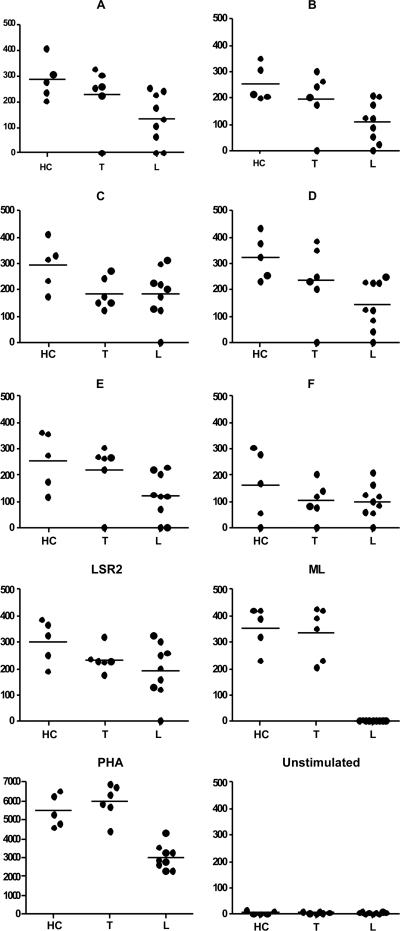
The results of IFN-γ responses to the peptides by 48-h cultured PBMC from an additional five HC, six tuberculoid, and nine lepromatous subjects are shown in Fig. 3. In conformity with the lymphoproliferation data, in general the HC and tuberculoid patients showed higher levels of IFN-γ than the lepromatous patients to Lsr2, M. leprae, end-to-end peptides, and PHA. Except for one patient, none of the lepromatous patients showed detectable levels of IFN-γ in M. leprae-stimulated PBMC cultures. All the end-to-end peptides stimulated IFN-γ, with peptide F showing the lowest levels in both leprosy types. There was individual variability in levels of IFN-γ to the same peptide, with HC showing significantly higher levels than lepromatous leprosy patients, with P values of <0.02, <0.007, <0.04, and <0.002 for peptides A, B, C, and D, respectively (Wilcoxon rank test). Peptide E showed significance between tuberculoid and lepromatous leprosy (P < 0.04). The tuberculoid group showed significant differences between peptide A and peptides C, D, and F (P < 0.05, Mann-Whitney), whereas the lepromatous group showed significance with only peptide C (P < 0.05), which is in conformity with the lymphoproliferation data, where this peptide was maximally recognized by 39% of the patients.
Fig 3.
Mean IFN-γ levels estimated by ELISA in duplicate samples of supernatants from PBMC cultures from 5 HC, 6 T, and 9 L subjects which were left unstimulated or stimulated with peptides A to F, Lsr2, M. leprae, and mitogen PHA for 48 h. Median values are represented by horizontal lines. Each dot represents an individual subject.
Overlapping peptides of Lsr2.
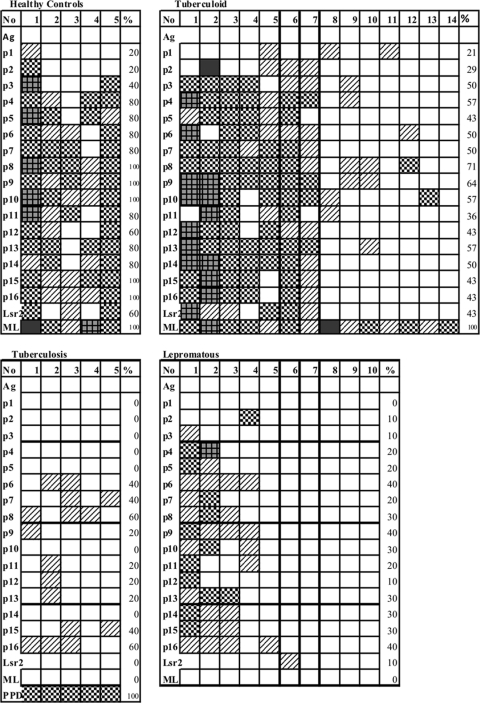
With a view to define the specificities of T cell recognition, 14- to 18-mer peptides of only the 10-kDa Lsr2 (p1 to p16, aa 24 to 112) offset by 5-mer at the amino terminal were used in the lymphoproliferation assays (Fig. 1 and 4). All peptides except p1, p2, and p3 showed higher percentages of responders than with the corresponding end-to-end peptides. In conformity with end-to-end peptides, there was multipeptide (more than four peptides) recognition in some subjects followed by response to two to four peptides, with an occasional patient responding to a single peptide. For p4 to p16 the range of percent responders was highest in HC (60 to 100%), followed by tuberculoid (43 to 71%) and lepromatous (20 to 40%) subjects. Significant differences between groups were noted for p5 (P < 0.05), p7 (<0.04), p8 (<0.005), and p10 (<0.50) by ANOVA. All HC recognized p8 to p10, which overlap with peptide D (Fig. 1), which was best recognized by this group. In addition, p15 and p16 were also seen to be stimulatory in all HC. As expected, the tuberculoid group responded better than the lepromatous group to all peptides. In conformity with data obtained on peptide D (aa 61 to 80), the overlapping p8 (aa 59 to 73) and p9 (aa 64 to 81) were recognized by 71% and 64% of tuberculoid subjects, respectively. In contrast, maximum recognition (40%) in lepromatous subjects was observed for p6 (aa 49 to 64) which shared 11 residues (RQWVSAGRRVG) with the best recognized peptide C (Fig. 1 and 2). p16 (aa 99 to 112) at the C′ terminal of the protein was also recognized by 40% of lepromatous subjects and induced a better response than peptide F, which lacked two residues, arginine and isoleucine (P = 0.8989, Mann-Whitney).
Fig 4.
Individual lymphoproliferative responses of PBMC to overlapping peptides (p1 to p16) in 6 healthy controls, 14 tuberculoid subjects, 10 lepromatous leprosy subjects, and 5 dermal tuberculosis patients expressed as SIs are indicated by each box. Percentages of responders, abbreviations, and key for grading are as described in the legend of Fig. 2.
Intergroup comparisons (Mann-Whitney two-tailed analysis) between HC and tuberculoid leprosy groups did not show statistical significance to any of the peptides (P > 1.0 to 0.3). However, several peptides, p7 (P < 0.04), p8 (<0.005), p9 (<0.02), p10 (<0.01), p15 (0.01) and p16 (0.02), showed differential recognition when HC were compared to lepromatous leprosy. p8 showed recognition by 71% and 30% of tuberculoid and lepromatous leprosy subjects, respectively (P < 0.05). Whereas 40% of the lepromatous subjects showed maximal responses to p6 and p16, a statistically significant difference was not seen compared to tuberculoid leprosy subjects (P > 0.6)
The number of nonresponders to all peptides was 1/14 in tuberculoid patients and increased to 4/10 in the lepromatous subjects. The five dermal tuberculosis patients showed low responses (SI > 2) to p6 to p9, p11 to p13, and p16 peptides (Fig. 4).
RGR motif in Lsr2, known to bind to the DNA minor groove in mycobacteria and play a regulatory role in the pathogen (10), was present in peptides D, E, p7 to p10, and p13 to p15. Peptide 8 with two repeats of RGR was recognized by all HC and by >70% of tuberculoid patients. RGD, a fibronectin binding motif earlier described by us to be important for seroreactivity in patients with ENL reactions (31), also elicited moderately higher responses when present in a central location in p4 (80% and 57%, respectively) in HC and tuberculoid patients.
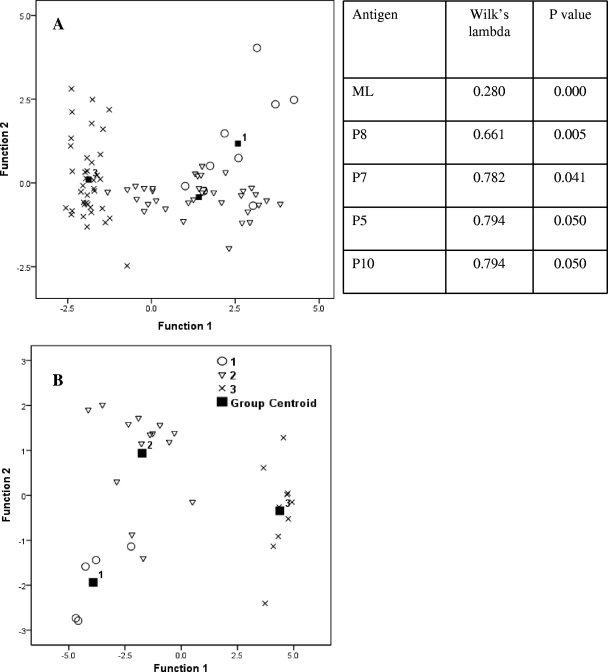
Multiple discriminant analysis (MDA) was performed to evaluate the relative importance of peptides in discriminating the clinical groups using Wilk's lambda statistics and canonical correlation territorial maps (Fig. 5). It may be observed that the lepromatous group is distinct from the HC and tuberculoid groups, and the accompanying table (Fig. 5) gives the decreasing order of statistical significance for the discriminating peptides p8, p7, p5, and p10 (P < 0.005 to P < 0.050).
Fig 5.
Canonical correlation territorial maps. Multidiscriminant analysis on lymphoproliferation responses showing separation of lepromatous (group 3) from tuberculoid (group 2) patients and healthy contacts (group 1), with the centroid giving the number of each group. (A) End-to-end peptides. (B) Overlapping peptides. The x and y axes represent two discriminant functions. The table shows only the statistically significant peptides discriminating the clinical groups by Wilk's lambda statistics.
Protein-protein search for homology within M. tuberculosis and other bacteria for the individual peptides was undertaken using BLASTP (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Peptide D, which showed responses in all clinical groups, had strong homology with M. avium-intracellulare, Mycobacterium kansasii, and M. tuberculosis, with a total score of 59.6 at 100% coverage and an e value of 9e−11. Peptide E had homology, with a total score of 67.7 and e value of 2e−10, for M. avium-intracellulare and M. kansasii and a score of 84 and 3e−13 for M. tuberculosis, whereas the corresponding p15 and p16 showed total scores of 46 and 44 and e values of 1e−06 and 4e−06, respectively. Other mycobacteria such as M. smegmatis, M. bovis, and Mycobacterium ulcerans also showed sequence homology for many of the peptides used in the study (p1 to p8, p12, p13, p14, A C, D, and E). Other species that showed homology included Rhodococcus and Nocardia for peptides A, B, p1, p4, p12, and p13 in addition to a few rare medically nonrelevant organisms.
Lsr2 peptide association with MHC class II and TAP binding.
With a view to investigate whether the selective recognition or nonrecognition of peptides had MHC class II bias, we undertook typing for HLA-DRB1 and -DQA1 and -DQB1 in 19 lepromatous and 4 tuberculoid patients and 2 family contacts. The lepromatous and tuberculoid patients and both family contacts had HLA-DRB1*1501 and -DRB1*1502, the two alleles of HLA-DR2. Fourteen lepromatous patients, of which 11 were homozygous, as well as the family contacts had DQB1* 0601. DQA1 showed greater diversity with eight lepromatous patients showing DQA1*0101 in heterozygosity with DQA1*0103, DQA1*0501, DQA1*0301, and DQA1*0201. HLA bias was not observable in peptide recognition or in subjects showing multiple or restricted peptide recognition or an absence peptide recognition. Peptides showing considerable immune responses were checked for their binding affinities using HLAPred (http://www.imtech.res.in/raghava/hlapred/) programs. Table 3 shows the predicted binding to the commonly observed alleles of 19 lepromatous patients. HLA-DRB1*1501 and HLA-DRB1*1502 showed binding of peptides C, p5, and more peptide E, p14, and p15 recognized by lepromatous subjects. The default thresholds of binding were 6 and 7 for HLA-DRB1*1501 and -DRB1*1502 (Table 3). RGD, a fibronectin binding motif, formed part of the binding sites when both the peptides and the total protein were scanned.
Table 3.
Comparison of predicted binding sites of Lsr2 peptides for HLA-DRB1*1501 and HLA-DRB1*1502 commonly observed in 19 lepromatous subjects investigated in lymphoproliferative assays
| HLA epitope and peptide | Peptide sequencea | Binding threshold (score)b |
|---|---|---|
| HLA DRB1*1501 | ||
| p4 | KNAAKLRGDLRQWVSAG | 4 (3.0) |
| C | KLRGDLRQWVSAGRRVGG | 4 (3.0) |
| C | KLRGDLRQWVSAGRRVGG | 7 (2.2) |
| p5 | LRGDLRQWVSAGRRVG | 4 (3.0) |
| p5 | LRGDLRQWVSAGRRVG | 7 (2.2) |
| p7 | AGRRVGGRRRGRSNSG | 9 (1.6) |
| p14 | RNGHNVSTRGRIPAD | 6 (2.4) |
| p15 | VSTRGRIPADVIDA | 6 (2.4) |
| HLA DRB1*1502 | ||
| p4 | KNAAKLRGDLRQWVSAG | 7 (2.0) |
| C | KLRGDLRQWVSAGRRVGG | 7 (2.0) |
| C | KLRGDLRQWVSAGRRVGG | 7 (2.0) |
| p5 | LRGDLRQWVSAGRRVG | 7 (2.0) |
| p5 | LRGDLRQWVSAGRRVG | 7 (2.0) |
| p6 | RQWVSAGRRVGGRRRG | 7 (2.0) |
| p12 | QSAAIREWARRNGHNV | 6 (2.3) |
| E | AAIREWARRNGHNVSTRGRI | 6 (2.3) |
| p13 | REWARRNGHNVSTRGRI | 6 (2.3) |
Predicted binders for the given peptides are highlighted in bold.
Values were determined using HLAPred software (http://www.imtech.res.in/raghava/hlapred/).
TAP (transporter-associated protein) binding (TAPPred [http://www.imtech.res.in/raghava/tappred/]) scores were highest (8.648) within peptide 16 (aa 103 to 111; DVIDAFHAAT), which was found to be antigenic in the lepromatous subjects. Consistent with this, REQSAAIRE (aa 78 to 85) within p9, p10, and p11 preferentially recognized by lepromatous leprosy (90%) subjects (Fig. 1) also gave a high score of 8.573. The TAP binding score was also high in peptide B and C, which were recognized universally by all clinical subjects or only the tuberculoid and healthy subjects, respectively. Peptide D, recognized best by HC, had the sequence AAIREW, which also gave a high TAP binding score of 8.602. That the TAP binding scores may not be sufficient to predict T cell binding was indicated by peptides A and C, which were not strong stimulators but had sequences with high TAP scores of 8.3 and 8.218, respectively.
In summary, it would appear that Lsr2 protein has multiple epitopes recognized by peripheral T cells of individuals exposed to the leprosy bacillus. The lymphoproliferation elicited by Lsr2 peptides in lepromatous patients who were unresponsive to M. leprae demonstrates that the antigen-specific T cell anergy associated with this disease is not total.
DISCUSSION
The leprosy spectrum with its different clinical forms has been a challenge for the understanding of host responses involved in infections caused by the same intracellular pathogen. This challenge was further complicated by the noncultivable nature of the leprosy bacillus and the lack of suitable animal models. The discovery of phenolic glycolipid (PGL) was a hallmark in identifying a unique antigen of M. leprae (14) and paved the way for understanding antibody responses and designing diagnostic markers of early disease and lepromatous leprosy (4). IgG antibodies to Lsr2 had also been shown in lepromatous leprosy subjects (34). However, the search for an equivalent antigen to define T cell immune responses continues to be elusive, and strategies to reverse the M. leprae-specific T cell unresponsiveness/anergy have been unsuccessful to date. Using the genomic approach several antigens of immunological relevance are being actively pursued for diagnosis of early leprosy or as candidate vaccines (1, 35, 36). Over a decade after the identification of Lsr2 in M. leprae, its presence in several commonly pathogenic and nonpathogenic mycobacteria as well as other organisms is being continually reported. A BLASTP search in March 2011 showed significant homologies in medically important organisms such as M. tuberculosis, M. ulcerans, the M. avium-intracellulare group, and M. kansasii and a putative Lsr2 in Nocardia farcinica. Our present investigation using Lsr2 peptides was aimed at studying the variation in T cell responses in the different leprosy types. Lymphoproliferation and IFN-γ levels in antigen-stimulated PBMC cultures were used as an in vitro correlate of T cell functions. Our earlier reports had shown that gamma interferon was expressed/released along with interleukin-4 (IL-4)/IL-10 in some lepromatous leprosy patients (20, 37). Both responders and nonresponders were noted in all the clinical groups for all the antigens, including the whole Lsr2 protein, native integral bacilli, and the peptides. The number of responders and number of peptides recognized were highest in healthy contacts, followed by tuberculoid leprosy patients. Though the percentage of responders decreased further in the lepromatous group, it is important to note that Lsr2 and some of its peptides were nevertheless recognized by many subjects who were unresponsive to the native bacillus (P < 0.05, ANOVA). Earlier data from our laboratory had demonstrated transient emergence of antigen-specific T cell functions to M. leprae antigen during natural leprosy reactions in lepromatous leprosy patients (17, 37). Further evidence of antigen-reactive T cells in circulation in lepromatous patients was provided by improved lymphoproliferation on the addition of interleukin-2 (12, 23) and reconstitution of T cells from anergic patients with HLA-identical accessory cells from tuberculoid leprosy siblings in in vitro cultures (24). The present study not only reinforces the presence of antigen-reactive T cells in some of the apparently anergic lepromatous leprosy patients but also draws attention to a feasible strategy to reverse T cell unresponsiveness in these patients.
It is intriguing that lepromatous patients unresponsive to the native bacillus respond to its proteins and its peptides when presented individually. It is possible that in the natural disease many factors come into play before T cell proliferation or function is manifested. Antigen processing in the natural state may generate many peptides, including inhibitory ones, which have a hierarchy for binding to MHC and the T cell receptor. This hierarchy may be overcome when peptides are presented individually. Moreover, the dynamics in the loading of peptides to MHC and critical epitope conformation in the synthetic peptide would also influence T cell recognition. The flanking residues of the peptide in the MHC groove may also influence T cell receptor (TCR) signaling. Recent studies indicate that disruption of HLA-DR lipid raft in lepromatous leprosy may result in defective antigen presentation and dephosphorylation of imperative T cell signaling molecules and thereby induce unresponsiveness (16).
In lymphoproliferation assays peptide B, which differs from p1 by one extra alanine, and p16, with two extra residues compared to peptide F, showed a significant reduction in the percentage of responders (P < 0.05, ANOVA) in all clinical groups (Fig. 1 and 2), indicating the influence of flanking residues in T cell recognition. Peptides such as A and F with sequences at the amino and carboxy terminus of the Lsr2 protein were poorly recognized by the leprosy patients compared to HC (P < 0.015 and 0.002, respectively, ANOVA). Peptide B (aa 24 to 42), in contrast, showed similar recognition patterns in both HC and leprosy patients, indicative of exposure to M. leprae infection, (P < 0.65, ANOVA). Peptide p8 (P < 0.05) showed differential recognition in tuberculoid and lepromatous patients. The latter recognized p6 and p16 maximally and showed significant differences compared to HC (P < 0.05 and 0.02, respectively) but not with the tuberculoid group (P = 0.5955 and 0.6172, respectively). Multideterminant discrimination analysis showed the lepromatous group to be a distinct from the other two clinical groups, with some discriminating Lsr2 peptides. It is of interest that the RGR motif, recently shown to be important for binding to the DNA minor groove in M. tuberculosis-derived Lsr2 (10), was present in many stimulatory M. leprae-derived Lsr2 peptides in leprosy but not in tuberculosis patients.
Surprisingly, p2 and p3, recognized by ENL sera in our earlier study, were poor stimulators of T cells in the present context, indicating that T cell help for IgG class differentiation may have been provided by other Lsr2 epitopes. The peptides of Lsr2 appear to be promiscuous and may be recognized by diverse MHC class II molecules. However, HLA-DRB1*1501, -DRB1*1502, and -DQB1*0601 were commonly observed in this group, and at least 10 of the peptides tested showed binding to HLA-DRB1*1501 and -DRB1*1502 (Table 3) in the bioinformatics analysis. These had been reported earlier to be susceptibility factors in Indian leprosy patients (27, 28). Recognition of Lsr2 peptides by T cell clones from Norwegian volunteers showed different HLA-DR restriction epitopes which further confirms promiscuous recognition of this protein (25). In conformity with the role of TAP in peptide loading of MHC (19), several stimulatory Lsr2 peptides showed high TAP binding scores.
The peptides used in the study were designed to cover the M. leprae Lsr2 at a time when databases had not shown homologues or orthologues in other species. To check, cross-reactivity of the peptides, we also investigated 13 tuberculosis patients who showed SIs ranging from 12 to 35 to PPD antigen in lymphoproliferative responses. These also included five patients with dermal tuberculosis as leprosy is primarily manifested in the skin. Though the Lsr2 gene has been detected in the causative organism, it is intriguing that M. leprae-derived Lsr2 and/or its peptides showed poor T cell recognition by tuberculosis patients. Our earlier study had shown 63 to 89% seroreactivity to p5, p6, p11, p12, and p15 (34), and in the present study p6 and p11 to p13 were also stimulatory in patients. Larger studies would be required not only to confirm these findings but also to address whether immunodominant proteins other than Lsr2 drive the human immune responses in diseases caused by different mycobacteria. As far as we are aware no published data have been reported on Lsr2-driven immune responses in tuberculosis or other mycobacterial infections in humans. Moreover, no reports are available to indicate whether Lsr2 obtained from different organisms would elicit different responses in diseased states
The stimulation of gamma interferon in leprosy patients by Lsr2 peptides A to F further confirms the findings of lymphoproliferation. In general, all Lsr2 peptides stimulated this cytokine, with HC and tuberculoid patients producing higher levels than the anergic patients (P < 0.02 to 0.04)
It is of interest that peptide C, which stimulated the anergic patients maximally, also induced significant levels of in vitro IFN-γ (mean ± SD, 187 + 96 pg/ml; P < 0.05, Mann-Whitney) compared to other end-to-end peptides.
In conclusion, Lsr2 protein which was first discovered using human sera from LL patients contains many T cell epitopes that are recognized by subjects exposed to M. leprae infection. However, its immunodominance for T cells is predominant in tuberculoid leprosy compared to lepromatous patients. Variation in epitope dominance was observed in clinical leprosy types. More importantly, certain sequences were recognized preferentially by lepromatous patients, indicating that the anergy associated with this disease is amenable to reversal by selective peptides of M. leprae proteins such as the Lsr2. The stimulatory peptides in our study appear to be promiscuous, which may facilitate therapeutic or prophylactic strategies against the more clinically recalcitrant lepromatous leprosy.
Supplementary Material
ACKNOWLEDGMENTS
The work was supported by the Indian Council of Medical Research and the Department of Science and Technology.
We are grateful to the late M. J. Colston, NIMR, Mill Hill, London, United Kingdom, and M. E. Pattarroyo, Institute de Immunologia, Bogota, Colombia, for technical advice and providing the peptides. Aswhini Kumar Mishra assisted in statistical analysis, and P. Manickam and Chaman Saini provided technical assistance beyond the call of duty.
Footnotes
Published ahead of print 5 December 2011
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1. Brahmbhatt S, et al. 2002. Human T cell responses to peptides of the Mycobacterium leprae 45-kD serine-rich antigen. Clin. Exp. Immunol. 128:140–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen JM, et al. 2006. Roles of Lsr2 in colony morphology and biofilm formation of Mycobacterium smegmatis. J. Bacteriol. 188:633–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen JM, et al. 2008. Lsr2 of Mycobacterium tuberculosis is a DNA-bridging protein. Nucleic Acids Res. 36:2123–2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cho SN, Yanagihara DL, Hunter SW, Gelber RH, Brennan PJ. 1983. Serological specificity of phenolic glycolipid I from Mycobacterium leprae and use in serodiagnosis of leprosy. Infect. Immun. 41:1077–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Colangeli R, et al. 2007. Transcriptional regulation of multi-drug tolerance and antibiotic-induced responses by the histone-like protein Lsr2 in M. tuberculosis. PLoS Pathog. 3:e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cole ST, et al. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537–544 [DOI] [PubMed] [Google Scholar]
- 7. Cole ST, et al. 2001. Massive gene decay in the leprosy bacillus. Nature 409:1007–1011 [DOI] [PubMed] [Google Scholar]
- 8. Coros A, Callahan B, Battaglioli E, Derbyshire KM. 2008. The specialized secretory apparatus ESX-1 is essential for DNA transfer in Mycobacterium smegmatis. Mol. Microbiol. 69:794–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fernandez-Vina MA, et al. 1991. Alleles at four HLA class II loci determined by oligonucleotide hybridization and their associations in five ethnic groups. Immunogenetics. 34:299–312 [DOI] [PubMed] [Google Scholar]
- 10. Gordon BR, et al. 2011. Structural basis for recognition of AT-rich DNA by unrelated xenogeneic silencing proteins. Proc. Natl. Acad. Sci. U. S. A. 108:10690–10695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gordon BR, et al. 2010. Lsr2 is a nucleoid-associated protein that targets AT-rich sequences and virulence genes in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 107:5154–5159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Haregewoin A, Godal T, Mustafa AS, Belehu A, Yemaneberhan T. 1983. T-cell conditioned media reverse T-cell unresponsiveness in lepromatous leprosy. Nature 303:342–344 [DOI] [PubMed] [Google Scholar]
- 13. Houghten RA. 1985. General method for the rapid solid-phase synthesis of large numbers of peptides: specificity of antigen-antibody interaction at the level of individual amino acids. Proc. Natl. Acad. Sci. U. S. A. 82:5131–5135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hunter SW, Brennan PJ. 1981. A novel phenolic glycolipid from Mycobacterium leprae possibly involved in immunogenicity and pathogenicity. J. Bacteriol. 147:728–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kaplan G, Mathur NK, Job CK, Nath I, Cohn ZA. 1989. Effect of multiple interferon gamma injections on the disposal of Mycobacterium leprae. Proc. Natl. Acad. Sci. U. S. A. 86:8073–8077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kumar S, Naqvi RA, Khanna N, Rao DN. 2011. Disruption of HLA-DR raft, deregulations of Lck-ZAP-70-Cbl-b cross-talk and miR181a towards T cell hyporesponsiveness in leprosy. Mol. Immunol. 48:1178–1190 [DOI] [PubMed] [Google Scholar]
- 17. Laal S, Bhutani LK, Nath I. 1985. Natural emergence of antigen-reactive T cells in lepromatous leprosy patients during erythema nodosum leprosum. Infect. Immun. 50:887–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Laal S, et al. 1991. Recombinant fusion protein identified by lepromatous sera mimics native Mycobacterium leprae in T-cell responses across the leprosy spectrum. Proc. Natl. Acad. Sci. U. S. A. 88:1054–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li S, Sjogren HO, Hellman U, Pettersson RF, Wang P. 1997. Cloning and functional characterization of a subunit of the transporter associated with antigen processing. Proc. Natl. Acad. Sci. U. S. A. 94:8708–8713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Misra N, et al. 1995. Cytokine profile of circulating T cells of leprosy patients reflects both indiscriminate and polarized T-helper subsets: T-helper phenotype is stable and uninfluenced by related antigens of Mycobacterium leprae. Immunology 86:97–103 [PMC free article] [PubMed] [Google Scholar]
- 21. Nath I, Mehervani C. 2010. Immunological aspects. Jaypee Brothers Medical Publishers (P), Ltd., New Delhi, India [Google Scholar]
- 22. Nath I, et al. 1990. Human immune response to recombinant interferon gamma and protein antigen LSR2. Trop. Med. Parasitol. 41:324–325 [PubMed] [Google Scholar]
- 23. Nath I, Sathish M, Jayaraman T, Bhutani LK, Sharma AK. 1984. Evidence for the presence of M. leprae reactive T lymphocytes in patients with lepromatous leprosy. Clin. Exp. Immunol. 58:522–530 [PMC free article] [PubMed] [Google Scholar]
- 24. Nath I, Van Rood JJ, Mehra NK, Vaidya MC. 1980. Natural suppressor cells in human leprosy: the role of HLA-D-identical peripheral lymphocytes and macrophages in the in vitro modulation of lymphoproliferative responses. Clin. Exp. Immunol. 42:203–210 [PMC free article] [PubMed] [Google Scholar]
- 25. Oftung F, Lundin KE, Meloen R, Mustafa AS. 1999. Human T cell recognition of the Mycobacterium leprae LSR antigen: epitopes and HLA restriction. FEMS Immunol. Med. Microbiol. 24:151–159 [DOI] [PubMed] [Google Scholar]
- 26. Ottenhoff TH, et al. 1986. Mycobacterium leprae-specific protein antigens defined by cloned human helper T cells. Nature 319:66–68 [DOI] [PubMed] [Google Scholar]
- 27. Rani R, Fernandez-Vina MA, Zaheer SA, Beena KR, Stastny P. 1993. Study of HLA class II alleles by PCR oligotyping in leprosy patients from north India. Tissue Antigens 42:133–137 [DOI] [PubMed] [Google Scholar]
- 28. Rani R, Mukherjee R, Stastny P. 1998. Diversity of HLA-DR2 in North Indians: the changed scenario after the discovery of DRB1*1506. Tissue Antigens 52:147–152 [DOI] [PubMed] [Google Scholar]
- 29. Ridley DS, Jopling WH. 1966. Classification of leprosy according to immunity. A five-group system. Int. J. Lepr. Other Mycobact. Dis. 34:255–273 [PubMed] [Google Scholar]
- 30. Scollard DM, et al. 2006. The continuing challenges of leprosy. Clin. Microbiol. Rev. 19:338–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sela S, Thole JE, Ottenhoff TH, Clark-Curtiss JE. 1991. Identification of Mycobacterium leprae antigens from a cosmid library: characterization of a 15-kilodalton antigen that is recognized by both the humoral and cellular immune systems in leprosy patients. Infect. Immun. 59:4117–4124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sharma P, et al. 2000. Mycobacterium w vaccine, a useful adjuvant to multidrug therapy in multibacillary leprosy: a report on hospital based immunotherapeutic clinical trials with a follow-up of 1–7 years after treatment. Lepr. Rev. 71:179–192 [DOI] [PubMed] [Google Scholar]
- 33. Singh S, et al. 1994. Critical residues of the Mycobacterium leprae LSR recombinant protein discriminate clinical activity in erythema nodosum leprosum reactions. Infect. Immun. 62:5702–5705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Singh S, et al. 1994. Sera of leprosy patients with type 2 reactions recognize selective sequences in Mycobacterium leprae recombinant LSR protein. Infect. Immun. 62:86–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Spencer JS, et al. 2005. Identification of specific proteins and peptides in Mycobacterium leprae suitable for the selective diagnosis of leprosy. J. Immunol. 175:7930–7938 [DOI] [PubMed] [Google Scholar]
- 36. Spencer JS, et al. 2004. Comparative analysis of B- and T-cell epitopes of Mycobacterium leprae and Mycobacterium tuberculosis culture filtrate protein 10. Infect. Immun. 72:3161–3170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sreenivasan P, Misra RS, Wilfred D, Nath I. 1998. Lepromatous leprosy patients show T helper 1-like cytokine profile with differential expression of interleukin-10 during type 1 and 2 reactions. Immunology 95:529–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stanford JL, et al. 1989. Vaccination and skin test studies on the children of leprosy patients. Int. J. Lepr. Other Mycobact. Dis. 57:38–44 [PubMed] [Google Scholar]
- 39. Talwar GP, et al. 1990. Immunotherapeutic effects of a vaccine based on a saprophytic cultivable mycobacterium, Mycobacterium w in multibacillary leprosy patients. Vaccine 8:121–129 [DOI] [PubMed] [Google Scholar]
- 40. WHO 2010. Global leprosy situation, 2010. Wkly. Epidemiol. Rec. 85:337–348 [PubMed] [Google Scholar]
- 41. Wong DK, Lee BY, Horwitz MA, Gibson BW. 1999. Identification of fur, aconitase, and other proteins expressed by Mycobacterium tuberculosis under conditions of low and high concentrations of iron by combined two-dimensional gel electrophoresis and mass spectrometry. Infect. Immun. 67:327–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Young RA, et al. 1985. Genes for the major protein antigens of the leprosy parasite Mycobacterium leprae. Nature 316:450–452 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.