Abstract
Sialylated glycoconjugates on the surfaces of mammalian cells play important roles in intercellular communication and self-recognition. The sialic acid preferentially expressed in human tissues is N-acetylneuraminic acid (Neu5Ac). In a process called molecular mimicry, many bacterial pathogens decorate their cell surface glycolipids with Neu5Ac. Incorporation of Neu5Ac into bacterial glycolipids promotes bacterial interactions with host cell receptors called Siglecs. These interactions affect bacterial adherence, resistance to serum killing and phagocytosis, and innate immune responses. Haemophilus ducreyi, the etiologic agent of chancroid, expresses lipooligosaccharides (LOS) that are highly sialylated. However, an H. ducreyi sialyltransferase (lst) mutant, whose LOS contain reduced levels of Neu5Ac, is fully virulent in human volunteers. Recently, a second sialyltransferase gene (Hd0053) was discovered in H. ducreyi, raising the possibility that Hd0053 compensated for the loss of lst during human infection. CMP-Neu5Ac is the obligate nucleotide sugar donor for all bacterial sialyltransferases; LOS derived from an H. ducreyi CMP-Neu5Ac synthetase (neuA) mutant has no detectable Neu5Ac. Here, we compared an H. ducreyi neuA mutant to its wild-type parent in several models of pathogenesis. In human inoculation experiments, the neuA mutant formed papules and pustules at rates that were no different than those of its parent. When grown in media with and without Neu5Ac supplementation, the neuA mutant and its parent had similar phenotypes in bactericidal, macrophage uptake, and dendritic cell activation assays. Although we cannot preclude a contribution of LOS sialylation to ulcerative disease, these data strongly suggest that sialylation of LOS is dispensable for H. ducreyi pathogenesis in humans.
INTRODUCTION
Sialic acids are 9-carbon sugars that decorate glycoconjugates found on the surfaces of eukaryotic cells. Sialylated glycoconjugates bind to receptors called sia-recognizing immunoglobulin superfamily lectins (Siglecs). Some Siglecs contain cytoplasmic signaling domains that modulate immune responses; others lack signaling domains and function in intercellular communication (20, 58). The sialic acids found in most mammalian tissues are N-acetylneuraminic acid (Neu5Ac) and its derivative N-glycolylneuraminic acid (Neu5Gc) (58, 60). Due to a mutation that occurred prior to the emergence of humans in a gene encoding an enzyme that catalyzes the conversion of Neu5Ac to Neu5Gc, Neu5Ac is expressed almost exclusively on human cells (58, 60). Not surprisingly, human Siglecs evolved to preferentially bind Neu5Ac over Neu5Gc (58).
Many bacterial pathogens incorporate Neu5Ac into their surface glycolipids, which can then bind to human Siglecs (58). These interactions either dampen immune responses and promote pathogen survival or activate immune responses and foster autoimmune responses (13, 20, 31). Incorporation of Neu5Ac into lipopolysaccharides, lipooligosaccharides (LOS), or capsular structures affects bacterial adherence to human cells, biofilm formation, resistance to complement-mediated killing, neutrophil function, and dendritic cell (DC) activation (13, 19, 20, 30, 49, 61). In animal models of infection, some pathogens require sialylation of LOS for virulence (11, 30).
Haemophilus ducreyi causes chancroid, a sexually transmitted genital ulcer disease endemic in resource-poor areas of Asia and Africa (51). H. ducreyi also causes a chronic lower-limb ulceration syndrome that is not sexually transmitted and is reported over a 4,800-mile range of the South Pacific from New Guinea to Samoa (38, 43, 56). In addition to causing its own morbidity, chancroid facilitates the sexual transmission and acquisition of HIV-1 (51). Clinical isolates of H. ducreyi are currently grouped into 2 classes, based on variations in their LOS structures and outer membrane proteins. Recent evidence suggests that these classes form distinct species that diverged from each other approximately 5 million years ago (46). While the LOS of class II strains lack sialic acid, the majority of clinical isolates belong to class I and express LOS that are highly sialylated (39, 45).
The major glycoform of the LOS of class I strains is Galβ1-4GlcNAcβ1-3Galβ1-4Hepα1-6Glcβ1-4Hepα1-KDO, which resembles paragloboside, a precursor of major human blood group antigens (45, 64). As is the case with paragloboside, Neu5Ac is added to the terminal galactose of this structure by a α2,3-sialyltransferase encoded by lst (12). H. ducreyi does not synthesize Neu5Ac and uses an ABC transporter system to scavenge environmental Neu5Ac (44, 47). When H. ducreyi is grown on chocolate agar, which contains approximately 0.5 μM Neu5Ac, up to 15% of the major LOS glycoform is sialylated (18, 47). When cultured on agar supplemented with 1 mM Neu5Ac, up to 62% of the major LOS glycoform is sialylated; supplementation with higher concentrations of Neu5Ac does not result in additional sialic acid incorporation (18, 47). Addition of Neu5Ac to cocultures of H. ducreyi and human foreskin fibroblasts (HFF) significantly increases the adherence of H. ducreyi to HFF (2). However, an H. ducreyi lst mutant, whose LOS contain only 3% of the wild-type level of Neu5Ac, is fully virulent in experimentally infected human volunteers, suggesting that sialylation of LOS is dispensable for pathogenesis (12, 64).
Recently, a gene (Hd0053) that encodes a second α2,3-sialyltransferase was discovered in H. ducreyi (35), raising the possibility that Hd0053 may have compensated for the loss of lst during human infection. The obligate nucleotide sugar donor for all bacterial sialyltransferases is CMP-Neu5Ac. In contrast to the lst mutant, LOS derived from an H. ducreyi CMP-Neu5Ac synthetase (neuA) mutant contain no detectable Neu5Ac (12, 40, 60).
Since sialic acid plays many key roles in bacterial pathogenesis, here we reexamined whether the ability of a class I strain of H. ducreyi to sialylate its LOS contributes to virulence. We compared the neuA mutant to its parent in human inoculation experiments. Given that DC responses and resistance to serum killing and phagocytosis are important features of H. ducreyi pathogenesis (8, 10, 24), we also compared the strains grown with and without Neu5Ac supplementation in DC activation, bactericidal, and macrophage uptake assays.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The bacterial strains used in this study are included in Table 1. H. ducreyi strains were grown on chocolate agar plates supplemented with 1% IsoVitaleX in the presence of 5% CO2. In some experiments, the chocolate agar plates were supplemented with 1 mM Neu5Ac (Calbiochem, La Jolla, CA) (44). For the human inoculation experiments, H. ducreyi was grown in a proteose peptone broth-based medium with 5% heat-inactivated fetal calf serum (27). All cultures were grown at 33°C. Where appropriate, the media were supplemented with chloramphenicol or kanamycin at 0.3 μg/ml or 20 μg/ml, respectively.
Table 1.
Bacterial strains used in this study
Analysis of LOS.
35000HP and the neuA mutant were grown in the broth described above with and without 50 μg/ml CMP-Neu5Ac (Sigma-Aldrich) (48). Bacteria were harvested at mid-log phase, and LOS was prepared by proteinase K digestion followed by phenol extraction and analyzed on 14% polyacrylamide gels as described previously (4).
Human inoculation experiments.
Dedicated stocks of 35000HP and 35000HP-RSM208 were prepared according to FDA guidelines under BB-IND 13064. Five healthy adult volunteers (four females and one male; 4 white and 1 black; mean age ± standard deviation [SD], 34.2 ± 13.7 years) over 21 years of age participated in the study. Subjects gave informed consent for participation and for HIV serology, in accordance with the human experimentation guidelines of the Institutional Review Board of Indiana University-Purdue University of Indianapolis and the U.S. Department of Health and Human Services. The preparation and inoculation of the bacteria, calculation of the estimated delivered dose (EDD), clinical observations, surface cultures, definitions of clinical endpoints, biopsies, and antibiotic treatment of the volunteers were carried out exactly as described previously (28). To confirm that the bacteria isolated from the inocula, surface cultures, or biopsy specimens had the intended phenotype, colonies were screened for susceptibility to Kan (28).
In the human model, site outcomes within a subject are not independent. To account for the correlation among site outcomes within an individual, comparison of papule and pustule formation rates for the two strains was performed using a logistic regression model with generalized estimating equations (GEE) (54). The GEE sandwich estimate for the standard errors was used to calculate 95% confidence intervals (95% CI) for these rates.
Bactericidal assays.
Bactericidal assays were done using normal human serum (NHS) obtained from a single healthy donor (1, 6, 53). In the first set of experiments, we compared the survival rates in 50% NHS of 35000HP and its isogenic neuA, lst, and dsrA mutants, using bacteria grown on chocolate agar for 16 h. In the second set of experiments, we compared the survival rates of 35000HP, the neuA mutant, and the dsrA mutant grown on chocolate agar supplemented with Neu5Ac with that of the dsrA mutant grown without supplementation. Data were reported as percent survival in active NHS compared to that in heat-inactivated serum [(geometric mean CFU in active NHS/geometric mean CFU in heat-inactivated NHS) × 100]. Each experiment was repeated five times, and the arithmetic mean and standard deviation (SD) of the percent survival were calculated. Comparison of the strains was performed using paired Student's t tests. With the Bonferroni adjustment for multiple comparisons, a P value of <0.0083 was considered significant for these assays.
Macrophage assays.
Peripheral blood mononuclear cells (PBMC) were isolated from leukopacks obtained from 8 anonymous donors from the Central Indiana Regional Blood Center or from blood obtained from 4 healthy uninfected adult volunteers by Ficoll-Paque Plus gradient centrifugation. Informed consent was obtained from the volunteers according to institutional and federal guidelines. CD14+ monocytes were purified from PBMC by positive selection using magnetic CD14 microbeads (Miltenyi Biotech) according to the manufacturer's instructions. CD14+ cells were differentiated into monocyte-derived macrophages (MDM) in X-vivo 15 medium (Lonza) supplemented with 1% heat-inactivated human AB serum (Invitrogen) for 5 days. MDM were harvested and seeded onto glass coverslips in 24-well tissue culture plates at a density of 2.5 × 105 cells/well and grown for 24 h. 35000HP and the neuA mutant were grown on chocolate agar plates with and without Neu5Ac supplementation for 18 h, harvested, and suspended in Hanks buffered salt solution (HBSS). The bacterial suspension was vortexed for 30 s, and clumps were allowed to settle for 2 min. The bacteria were either not opsonized or opsonized with 100% complement-replete autologous serum for 20 min at room temperature. Approximately 2.5 × 106 CFU was added to wells containing MDM, centrifuged at 180 × g for 10 min, and incubated for 30 min at 35°C in 5% CO2. Nonadherent bacteria were removed by washing three times with HBSS. To determine the number of MDM-associated bacteria, wells were incubated with HBSS containing 0.2% saponin for 15 min at room temperature and quantitatively cultured. To measure the number of internalized bacteria, other wells were incubated in HBSS containing gentamicin (50 μg/ml) for 30 min, washed three times with HBSS, treated with HBSS containing 0.2% saponin, and quantitatively cultured. The percentages of associated and internalized bacteria were calculated as the ratios of MDM-bound CFU and gentamicin-protected CFU, respectively, to the initial CFU added per well.
DC activation assays.
DC were differentiated from CD14+ cells in RPMI 1640 medium supplemented with 2 mM l-glutamine, 50 μM beta-mercaptoethanol, 10% heat-inactivated fetal bovine serum, and 5 ng/ml of recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4) (R&D Systems) for 7 days (7). DC were collected, washed once, and suspended in the DC growth medium without GM-CSF and IL-4 in 48-well tissue culture plates. 35000HP and the neuA mutant grown on chocolate agar plates with and without Neu5Ac for 18 h were centrifuged onto wells containing 4 × 105 DC at a multiplicity of infection (MOI) of 10:1; control wells were not infected. After 90 min of incubation at 35°C, gentamicin was added at a final concentration of 50 μg/ml, and the DC were incubated at 37°C for 22.5 h.
Uninfected and H. ducreyi-infected DC were stained with allophycocyanin-conjugated antibodies to CD40 or CD86, phycoerythrin-conjugated anti-CD80 or anti-CD83 antibodies, and isothiocyanate-conjugated anti-HLA-DR antibodies (BD Biosciences). Samples were analyzed using a FACSCalibur flow cytometer and BD CellQuest Pro version 4.0.1 software (BD Biosciences). DC culture supernatants were collected and frozen at −80°C. The levels of IL-6, IL-10, IL-12, and tumor necrosis factor alpha (TNF-α) were measured by cytokine enzyme-linked immunosorbent assay (ELISA) kits from BD Biosciences according to the manufacturer's instructions. All comparisons between groups in the macrophage and DC assays were made using paired Student's t tests.
RESULTS
An H. ducreyi neuA mutant is not impaired in its ability to infect human volunteers.
We had shown previously that an H. ducreyi lst mutant is fully virulent in experimentally infected human volunteers (64). A gene (Hd0053) that encodes a second α2,3-sialyltransferase was subsequently discovered in H. ducreyi (35), raising the possibility that Hd0053 may have compensated for the loss of lst in vivo. As CMP-Neu5Ac is the obligate nucleotide sugar donor for all sialyltransferases, LOS derived from an H. ducreyi neuA mutant contain no detectable Neu5Ac (12). The LOS of the neuA mutant lack the Neu5Ac a-branch and contain a lactosamine b-branch on the major glycoform; the parental LOS profile is restored by complementation (12). The outer membrane protein profiles and growth rates of 35000HP and the neuA mutant in broth are identical (12).
To test whether sialylated LOS are required for H. ducreyi infection in humans, we inoculated 2 groups of volunteers. The first group, with 3 participants, was inoculated with an EDD of 32 CFU of 35000HP at 3 sites on one arm and with EDDs of 24, 48, and 97 CFU of the neuA mutant at 3 sites on the other arm. The second group, with 2 participants, was inoculated with an EDD of 63 CFU of 35000HP at 3 sites on one arm and with EDDs of 23, 46, and 91 CFU of the neuA mutant at 3 sites on the other arm. Overall, papules formed at 86.7% (95% CI, 63.3 to 99.9%) of parent-inoculated sites and at 86.7% (95% CI, 72.4 to 99.9%) of mutant-inoculated sites (P = 1.0) (Table 2). After 24 h of infection, the mean area of the papules was 18.5 ± 10.7 mm2 at parent sites and 16.9 ± 17.6 mm2 at mutant sites (P = 0.69). Pustules formed at 46.7% (95% CI, 11.6 to 81.7%) of parent sites and 40% (95% CI, 5.9 to 74.1%) of mutant sites (P = 0.39).
Table 2.
Response to inoculation with live H. ducreyi
| Volunteera (gender) | Observation period (days) | Strainb | Dose (CFU)c | No. of initial papules | Final outcome of papule |
|
|---|---|---|---|---|---|---|
| No. resolved | No. of pustules | |||||
| 395 (F) | 7 | P | 32 | 3 | 1 | 2 |
| M | 24–97 | 3 | 3 | |||
| 396 (F) | 13 | P | 32 | 1 | 1 | |
| M | 24–97 | 2 | 2 | |||
| 397 (F) | 8 | P | 32 | 3 | 3 | |
| M | 24–97 | 3 | 1 | 2 | ||
| 398 (M) | 6 | P | 63 | 3 | 3 | |
| M | 23–91 | 2 | 1 | 1 | ||
| 400 (F) | 7 | P | 63 | 3 | 1 | 2 |
| M | 23–91 | 3 | 3 | |||
Volunteers 395, 396, and 397 were inoculated in the first group; volunteers 398 and 400 were inoculated in the second group. F, female; M, male.
P, parent strain 35000HP; M, mutant strain 35000HP-RSM208.
Doses are indicated as follows: 24–97, one dose each of 24, 48, and 97 CFU; 23–91, one dose each of 23, 46, and 91 CFU.
At least one positive surface culture for H. ducreyi was obtained during follow-up visits from 20% of the parent-inoculated and 7% of the mutant-inoculated sites. All colonies recovered from the surface cultures of parent sites (n = 93) and mutant sites (n = 30) had the expected kanamycin resistance phenotype. Of 3 biopsy specimens cultured from parent-inoculated sites, 2 yielded H. ducreyi. Of 3 biopsy specimens cultured from mutant-inoculated sites, 2 yielded H. ducreyi. All colonies recovered from biopsy specimens of parent (n = 24) and mutant (n = 75) sites had the expected kanamycin resistance phenotype, as did all colonies tested from the parent (n = 72) and mutant (n = 69) inocula. Thus, there was no evidence of cross-contamination between mutant-inoculated and parent-inoculated sites.
Two subjects (395 and 398) developed pustules at both mutant-inoculated and parent-inoculated sites. One mutant- and one parent-inoculated site were biopsied from each subject and stained with hematoxylin-eosin and anti-CD3 antibodies as described previously (64). Both samples contained micropustules in the epidermis and a dermal infiltrate of perivascular CD3+ cells and were indistinguishable (data not shown). Taken together, the data indicate that the inability of H. ducreyi to incorporate Neu5Ac into its LOS did not impair its virulence in humans.
The neuA mutant does not sialylate its LOS in media containing CMP-Neu5Ac.
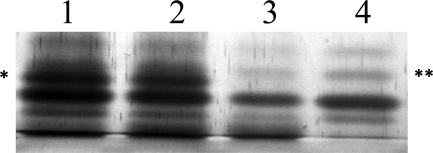
Some bacterial pathogens scavenge CMP-Neu5Ac from the host and sialylate their LOS using an extracellular sialyltransferase (60). To exclude the possibility that the neuA mutant could sialylate its LOS by this mechanism, we analyzed LOS prepared from 35000HP and the neuA mutant grown in broth with and without CMP-Neu5Ac supplementation. LOS purified from 35000HP contained the Neu5Ac a-branch; supplementation with CMP-Neu5Ac did not increase the level of expression of the a-branch (Fig. 1). Under both growth conditions, LOS purified from the neuA mutant lacked the a-branch and contained the lactosamine b-branch (Fig. 1). Thus, it is unlikely that the neuA mutant can utilize host-derived CMP-Neu5Ac to sialylate its LOS.
Fig 1.
Silver-stained gel of LOS isolated from 35000HP (lanes 1 and 2) and the neuA mutant (lanes 3 and 4). The bacteria were grown in broth without (lanes 1 and 3) and with (lanes 2 and 4) CMP-Neu5Ac supplementation. Note that under both growth conditions LOS derived from 35000HP contains the a-branch (∗), while LOS derived from the neuA mutant contains only the b-branch (∗∗).
Sialylation of LOS does not affect the serum susceptibility of H. ducreyi.
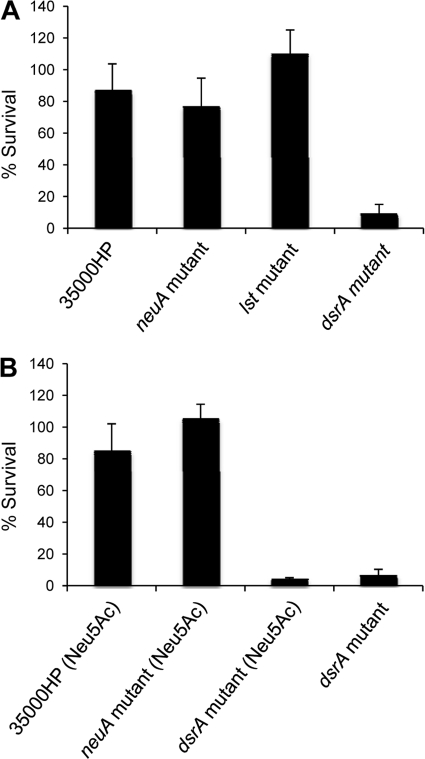
Resistance to the killing of normal human serum plays a major role in H. ducreyi pathogenesis and is mediated primarily by the outer membrane protein DsrA (10, 28). Sialylation of LOS affects the ability of several organisms to resist serum killing (30, 61). Thus, we compared the survival rates of 35000HP and its isogenic neuA, lst, and dsrA mutants in 50% NHS (6). When the bacteria were grown without Neu5Ac supplementation, the mean ± SD percent survival rates were 86% ± 17% for 35000HP, 76% ± 18% for the neuA mutant, 110% ± 15% for the lst mutant, and 9% ± 7% for the dsrA mutant (Fig. 2A). Only the percent survival of the dsrA mutant was significantly different from results for the other 3 strains (Fig. 2A). In the next set of experiments, we compared the survival rates of 35000HP, the neuA mutant, and the dsrA mutant grown in media supplemented with 1 mM Neu5Ac with that of the dsrA mutant grown in the absence of supplementation. The mean ± SD percent survival rates were 87% ± 19% for 35000HP, 105% ± 11% for the neuA mutant, 4% ± 1% for the dsrA mutant grown with Neu5Ac supplementation, and 7% ± 4% for the dsrA mutant grown without Neu5Ac supplementation (Fig. 2B). Only the percent survival of the dsrA mutant grown with and without Neu5Ac supplementation was significantly different from results for the other 2 strains (Fig. 2B). Thus, the ability of H. ducreyi to sialylate its LOS has no effect on serum susceptibility.
Fig 2.
Bactericidal assays. (A) Percent survival of 35000HP, 35000HP-RSM208 (neuA mutant), 35000HP-RSM203 (lst mutant), and FX517 (dsrA mutant) in 50% NHS, calculated as follows: [(geometric mean CFU in active NHS/geometric mean CFU in heat-inactivated NHS) × 100]. P values are as follows: for 35000HP versus 35000HP-RSM208, P = 0.22; for 35000HP versus 35000HP-RSM203, P = 0.25; for 35000HP versus FX517, P = 0.001; for 35000HP-RSM208 versus 35000HP-RSM203, P = 0.057; for 35000HP-RSM208 versus FX517, P = 0.0004; and for 35000HP-RSM203 versus FX517, P = 0.0002. (B) Percent survival of 35000HP, 35000HP-RSM208, and FX517 grown in media supplemented with 1 mM Neu5Ac and FX517 grown without Neu5Ac supplementation in 50% NHS, calculated as described for panel A. P values are as follows: for 35000HP versus 35000HP-RSM208, P = 0.059; for 35000HP versus FX517 grown with Neu5Ac, P = 0.006; for 35000HP versus FX517 grown without Neu5Ac, P = 0.0001; for 35000HP-RSM208 versus FX517 grown with Neu5Ac, P < 0.0001; for 35000HP-RSM208 versus FX517 without Neu5Ac, P < 0.0001; and for FX517 grown with Neu5Ac versus FX517 grown without Neu5Ac, P = 0.16. For both panels, values are means ± SDs from 5 independent experiments.
Sialylation of LOS does not modulate H. ducreyi association with or uptake by macrophages.
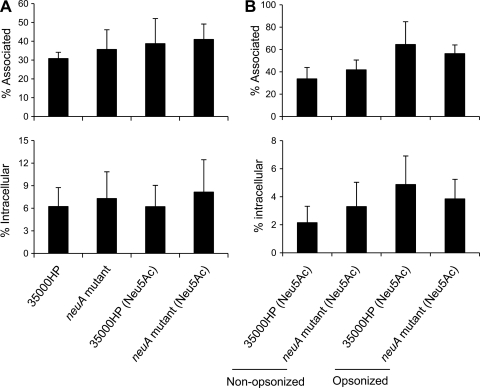
Human and murine macrophages express a sialoadhesin (Sn, siglec-1) that promotes the uptake of sialylated viruses and bacteria (29, 58). H. ducreyi resists phagocytosis by human macrophage-like cell lines and primary human MDM (52, 57, 62). We examined whether sialylation of H. ducreyi LOS affects the ability of the bacterium to associate with or be taken up by macrophages. 35000HP and the neuA mutant were grown with and without Neu5Ac supplementation and used to infect primary human MDM. After 30 min of infection, approximately 30 to 40% of the nonopsonized bacteria were associated with the MDM; under both growth conditions, there were no significant differences in the percentages of associated bacteria between the wild type and the neuA mutant strain (Fig. 3A, top). In gentamicin protection experiments, under both growth conditions there were also no significant differences in the percentages of ingested bacteria between the nonopsonized wild-type and mutant strains (Fig. 3A, bottom). Next, we grew 35000HP and the neuA mutant with sialic acid supplementation and compared uptake with and without opsonization with 100% autologous complement-replete serum. Again, we found no differences in adherence (Fig. 3B, top) or uptake (Fig. 3B, bottom) of the strains under opsonized and nonopsonized conditions. Thus, the ability to incorporate Neu5Ac into LOS did not affect the ability of opsonized and nonopsonized H. ducreyi to associate with or be taken up by macrophages.
Fig 3.
Percentages of H. ducreyi that were associated with (top) or internalized by (bottom) macrophages. (A) 35000HP and 35000HP-RSM208 (neuA mutant) grown on chocolate agar plates with and without Neu5Ac supplementation were incubated with macrophages at an MOI of 10:1 for 30 min without opsonization, followed by 30 min of treatment with gentamicin. Percent association or internalization was calculated as the ratio of bacteria recovered without or with gentamicin treatment to initial CFU added. The values represent the means ± SDs from assays done with macrophages from 5 (top) or 6 (bottom) donors. There were no significant differences among the groups in either assay. (B) 35000HP and 35000HP-RSM208 were grown on chocolate agar plates with Neu5Ac supplementation and were either not opsonized or opsonized with 100% autologous serum prior to performance of the assays outlined for panel A. The values in both panels represent the means ± SDs from assays done with macrophages from 4 donors. There were no significant differences between the neuA mutant and the parent strain in either assay.
DC activation is not affected by H. ducreyi sialylation.
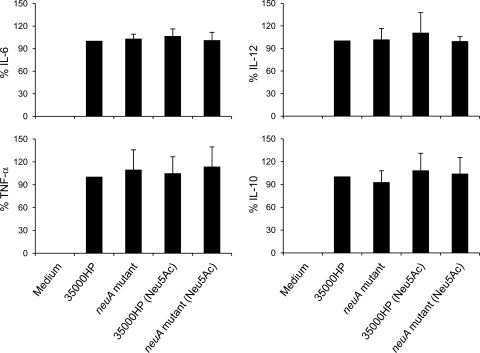
Sialylation of LOS can enhance the activation of DC as measured by surface markers and cytokine production (31). H. ducreyi infection of human monocyte-derived DC leads to secretion of both proinflammatory and anti-inflammatory cytokines and upregulation of several DC surface activation markers (7, 24). To examine whether sialylation of H. ducreyi LOS plays a role in modulating DC activation, 35000HP and the neuA mutant grown with and without Neu5Ac supplementation were used to infect DC for 24 h. Compared to uninfected DC, infected DC significantly upregulated the percent positive expression (Table 3) and mean fluorescence intensity (MFI) (Table 4) of the surface activation markers CD40, CD80, CD83, and CD86 and the MFI of HLA-DR (all P < 0.018). However, after adjustment for multiple comparisons, the levels of activation did not significantly differ between the parent and the mutant strains (Tables 3 and 4). H. ducreyi-infected DC secreted proinflammatory cytokines IL-6, IL-12, and TNF-α and the anti-inflammatory cytokine IL-10. However, the levels of cytokine production did not differ between the parent strain and the neuA mutant grown in the presence or absence of Neu5Ac supplementation (Fig. 4). Thus, we found little evidence that the ability of H. ducreyi to sialylate its LOS affected DC activation.
Table 3.
Effect of H. ducreyi sialylation on percent positive expression
| Treatmenta | Percent positive expressionb |
||||
|---|---|---|---|---|---|
| HLA-DR | CD40 | CD80 | CD83 | CD86 | |
| Media | 93.5 (4.0) | 11.1 (5.3) | 42.2 (7.9) | 1.3 (0.8) | 21.8 (14.4) |
| 35000HP | 94.5 (3.4) | 76.2 (7.1) | 87.2 (13.1) | 36.3 (10.7) | 83.7 (7.34) |
| 35000HP-RSM208 | 94.3 (2.1) | 74.4 (7.9) | 86.5 (12.4) | 38.12 (9.6) | 82.7 (6.9) |
| 35000HP (Neu5Ac)c | 94.0 (1.9) | 75.9 (6.5) | 87.4 (11.5) | 36.1 (11.9) | 81.9 (8.0) |
| 35000HP-RSM208 (Neu5Ac)c | 94.2 (1.9) | 74.7 (9.2) | 85.6 (12.2) | 36.4 (11.5) | 82.1 (7.9) |
Samples were incubated with media or with H. ducreyi strains for 24 h.
Values are means (SDs) of the percentages of cells expressing the surface markers over that of isotype-matched control antibodies and represent data from 6 donors.
Strains were grown with Neu5Ac supplementation.
Table 4.
Effect of H. ducreyi sialylation on MFI
| Treatmenta | Geometric MFIb |
||||
|---|---|---|---|---|---|
| HLA-DR | CD40 | CD80 | CD83 | CD86 | |
| Media | 27.9 (14.8) | 3.2 (2.2) | 3.9 (0.3) | 1.0 (0.1) | 2.8 (1.1) |
| 35000HP | 41.4 (17.3) | 10.2 (3.9) | 16.6 (6.5) | 3.1 (0.9) | 12.6 (3.4) |
| 35000HP-RSM208 | 39.0 (18.0) | 9.9 (3.2) | 16.9 (5.8) | 3.2 (1.0) | 12.7 (2.9) |
| 35000HP (Neu5Ac)c | 39.0 (18.0) | 9.8 (3.7) | 15.6 (5.2) | 3.0 (1.0) | 11.9 (3.1) |
| 35000HP-RSM208 (Neu5Ac)c | 40.0 (18.8) | 9.7 (3.6) | 16.1 (6.5) | 3.1 (1.1) | 12.0 (3.4) |
Samples were incubated with media or with H. ducreyi strains for 24 h.
Values are means (SDs) of the geometric mean fluorescence intensity (MFI) expressed as fold change over that of isotype-matched control antibodies and represent data from 6 donors.
Strains were grown with Neu5Ac supplementation.
Fig 4.
Cytokine production by DC. DC were uninfected or infected with 35000HP and 35000HP-RSM208 (neuA mutant) grown on chocolate agar plates with and without 1 mM Neu5Ac supplementation at an MOI of 10:1 for 24 h. Culture supernatants were assayed for IL-6 (n = 7), IL-12 (n = 4), TNF-α (n = 7), and IL-10 (n = 7) by ELISA. Due to donor-to-donor variation in cytokine production, cytokine levels were normalized to that of DC incubated with 35000HP grown in the absence of 1 mM Neu5Ac, which was set at 100%. Bars are means ± SDs. There were no significant differences in the levels of production of each cytokine among the groups in these assays.
DISCUSSION
Most clinical isolates of H. ducreyi belong to class I and express highly sialylated LOS (39, 45). In this study, we showed that a class I neuA mutant, which is incapable of LOS sialylation, is fully virulent in human volunteers. Even under growth conditions that favor maximal sialylation of the LOS in the wild type (18, 47), the neuA mutant and its parent had indistinguishable phenotypes in bactericidal, macrophage adherence and uptake, and DC activation assays. Taken together with the observation that the LOS of class II strains lack Neu5Ac (45), these data strongly suggest that sialylation of LOS is dispensable for H. ducreyi pathogenesis in humans.
The human challenge model has several limitations, including an artificial route of inoculation. Delivery of the bacteria by tines into the skin could mask the role of sialylation in an initial stage of pathogenesis, such as adherence. However, ncaA, fgbA, and flp-tad operon mutants are attenuated for pustule formation in human volunteers (9a, 16, 28a, 54), indicating that the model detects contributions of adherence factors to pathogenesis. Of the 28 mutants tested in the model, 9 are fully attenuated and 5 are partially attenuated for pustule formation (28, 28a, 32, 33, 42, 53); a partial role of sialylation in virulence should have been detected by the model. For safety reasons, the model is restricted to the papular and pustular stages of infection. Although the model cannot directly assess the contribution of a virulence determinant to the ulcerative stage, H. ducreyi maintains similar relationships with host cells in experimental and natural infection (8, 9). Neu5Ac is abundant in human tissues; it is likely that wild-type LOS become highly sialylated following inoculation. Thus, it seems unlikely that sialylation of LOS would contribute only to the ulcerative stage.
Sialylation of LOS can block the binding of antibodies directed at terminal LOS epitopes (36). We evaluated the ability of H. ducreyi to resist serum killing and uptake by macrophages using complement-replete sera obtained from uninfected donors. Antibody to H. ducreyi LOS is detectable in sera obtained from 88% and 98% of healthy individuals from areas where the related disease is nonendemic and endemic, respectively (15). Thus, serum from uninfected donors is appropriate to study potential effects of sialylation on serum resistance and phagocytosis.
Members of the Pasteurellaceae do not synthesize Neu5Ac and rely on ATP-dependent or ATP-independent transporter systems to scavenge sialic acid from the host (49). Disease-associated isolates of the bovine pathogen Histophilus somni express sialylated LOS, while commensal strains do not; LOS sialylation enhances the serum resistance of this organism (25). Addition of Neu5Ac into the LOS of nontypeable Haemophilus influenzae is required for serum resistance, biofilm formation, and virulence in experimental otitis media in animal models of infection (11, 30, 50). The role of sialylation of H. influenzae LOS in human disease is unknown. However, the data suggest that LOS sialylation may differ in importance among the human pathogens in this family.
In humans, the role of LOS sialylation has been studied most extensively in the pathogenesis of Neisseria gonorrhoeae infection in men. N. gonorrhoeae differs from H. ducreyi in that it lacks CMP-Neu5Ac synthetase, scavenges CMP-Neu5Ac from the host, and substitutes Neu5Ac on its LOS with an extracellular sialyltransferase encoded by lst (60). In urethral exudates obtained from naturally infected males, gonococci found within polymorphonuclear leukocytes (PMN) express LOS that are sialylated (5). However, N. gonorrhoeae grown in media supplemented with CMP-Neu5Ac sialylates its LOS and is impaired in its ability to invade urethral epithelial cells (19) and to infect male volunteers (48). In male volunteers, an lst mutant in the serum-resistant FA1090 background is as virulent as its parent; an lst mutant in the serum-sensitive MS11mkC background is infectious but trends to lower infectivity (22). In vivo, N. gonorrhoeae circulates between intracellular and extracellular compartments. Nonsialylated gonococci may be more efficient at invading urethral epithelial cells, while sialylated gonococci may have survival advantages within PMN or in extracellular compartments that contain serum transudates (22). Although the latter hypothesis has not been tested in humans, an MS11 lst mutant is impaired in its ability to resist killing by murine PMN and to survive in the murine model of female genital tract infection (63). In contrast to N. gonorrhoeae, H. ducreyi appears to reside only in an extracellular compartment; sialylation of its LOS may have a minor role in its ability to resist complement-mediated killing and phagocytic uptake, as discussed below.
Incorporation of Neu5Ac into the LOS of otherwise serum-susceptible strains of N. gonorrhoeae, such as MS11mkC, promotes the binding to the bacterium of factor H, an inhibitor of the alternative complement pathway, enabling the organism to become serum resistant (61). Other strains of N. gonorrhoeae, such as FA1090, are intrinsically serum resistant because their porin proteins bind factor H regardless of the sialylation state of their LOS (61). H. ducreyi is serum resistant primarily due to the expression of the outer membrane protein DsrA, which does not affect factor H binding but does prevent the binding of IgM and activation of the classical complement pathway (1). H. ducreyi also expresses two additional outer membrane proteins, MOMP and DltA, which have partial roles in serum resistance (21, 26, 34). Thus, sialylation of LOS likely does not affect the serum resistance of H. ducreyi due to the fact that the organism is intrinsically serum resistant.
Macrophages express the siglec Sn, which binds Neu5Ac in α2,3 or α2,8 linkages (58). Campylobacter jejuni strains that express sialylated LOS do not adhere to wild-type CHO cells but do adhere to CHO cells transfected with Sn (20). Meningococci that express sialylated LOS are ingested more efficiently by bone marrow-derived macrophages obtained from wild-type mice than by those from Sn-deficient (Sn−/−) mice (29). Although these data suggest that sialylation of LOS can facilitate bacterial adherence to and uptake by macrophages, sialylation of H. ducreyi LOS did not affect adherence to or uptake by macrophages. H. ducreyi secretes two proteins, LspA1 and LspA2, which are required for virulence in humans and inhibit the activation of Src family protein kinases and phagocytic signaling of macrophage-like cell lines (27, 41). Expression of these antiphagocytic proteins may interfere with the ability of sialylated H. ducreyi to be taken up by macrophages.
C. jejuni strains associated with the development of Guillain-Barré syndrome express sialylated LOS (20). Compared to unsialylated LOS, sialylated LOS from C. jejuni enhance DC activation through a Toll-like receptor 4 (TLR4)-dependent mechanism, which promotes B cell proliferation and may contribute to the development of antiganglioside antibodies (31). In contrast, sialylation of meningococcal LOS has little effect on the secretion of cytokines by DC (55), while sialylated LOS from H. somni induce less NF-κB activation and chemokine response from bone marrow-derived macrophages than desialylated LOS (23). We found little evidence that sialylated H. ducreyi LOS affected the expression of surface activation markers or cytokine production by DC compared to unsialylated LOS. Taken together, the data suggest that sialylation of LOS makes different contributions to activation of DC or macrophages in different organisms.
Some organisms, including Streptococcus pneumoniae and H. influenzae, utilize Neu5Ac as a carbon source, which aids in colonization of the host (37, 59). H. ducreyi contains homologues of several of the genes encoding proteins required for sialic acid catabolism but does not have an identified homologue of NanK (44). As the second step in the catabolism pathway, NanK phosphorylates N-acetylmannosamine; lack of a NanK homologue suggests that H. ducreyi may not be able to utilize sialic acid as a carbon source (44). As defined media for the growth of H. ducreyi are not available, it remains to be determined experimentally whether the organism utilizes Neu5Ac for growth in vitro or in vivo; this study does not preclude that possibility.
In summary, we conclude that sialylation of H. ducreyi LOS does not play a major role in the pathogenesis of chancroid. Addition of Neu5Ac to its LOS may initially have aided H. ducreyi in its adaptation to the human host as it diverged from its closest relatives, which are animal pathogens (17). The bulk of the evidence suggests that expression of other virulence determinants has supplanted potential roles for sialylated H. ducreyi LOS in vivo.
ACKNOWLEDGMENTS
This work was supported by grant AI059384 to S.M.S. from the National Institute of Allergy and Infectious Diseases (NIAID). The human challenge trials were supported by grants from NIAID (U19 AI31494) and the Indiana Clinical and Translational Sciences Institute and the Indiana Clinical Research Center (UL RR052761).
We have no relevant financial relationships to disclose.
We thank Shelia Ellinger for preparing the regulatory documents for the human trials, Byron Batteiger for his thoughtful criticism of the manuscript, and the volunteers who participated in the trial.
Footnotes
Published ahead of print 5 December 2011
REFERENCES
- 1. Abdullah M, et al. 2005. Killing of dsrA mutants of Haemophilus ducreyi by normal human serum occurs via the classical complement pathway and is initiated by immunoglobulin M binding. Infect. Immun. 73:3431–3439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alfa MJ, Degagne P. 1997. Attachment of Haemophilus ducreyi to human foreskin fibroblasts involves LOS and fibronectin. Microb. Pathog. 22:39–46 [DOI] [PubMed] [Google Scholar]
- 3. Al-Tawfiq JA, et al. 1998. Standardization of the experimental model of Haemophilus ducreyi infection in human subjects. J. Infect. Dis. 178:1684–1687 [DOI] [PubMed] [Google Scholar]
- 4. Apicella MA, Griffiss JM, Schneider H. 1994. Isolation and characterization of lipopolysaccharides, lipooligosaccharides, and lipid A. Methods Enzymol. 235:242–252 [DOI] [PubMed] [Google Scholar]
- 5. Apicella MA, et al. 1990. Modification by sialic acid of Neisseria gonorrhoeae lipooligosaccharide epitope expression in human urethral exudates: an immunoelectron microscopic analysis. J. Infect. Dis. 162:506–512 [DOI] [PubMed] [Google Scholar]
- 6. Banks KE, et al. 2008. The enterobacterial common antigen-like gene cluster of Haemophilus ducreyi contributes to virulence in humans. J. Infect. Dis. 197:1531–1536 [DOI] [PubMed] [Google Scholar]
- 7. Banks KE, et al. 2007. Haemophilus ducreyi partially activates human myeloid dendritic cells. Infect. Immun. 75:5678–5685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bauer ME, Goheen MP, Townsend CA, Spinola SM. 2001. Haemophilus ducreyi associates with phagocytes, collagen, and fibrin and remains extracellular throughout infection of human volunteers. Infect. Immun. 69:2549–2557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bauer ME, Townsend CA, Ronald AR, Spinola SM. 2006. Localization of Haemophilus ducreyi in naturally acquired chancroidal ulcers. Microbe Infect. 8:2465–2468 [DOI] [PubMed] [Google Scholar]
- 9a. Bauer ME, et al. 2009. A fibrinogen-binding lipoprotein contributes to the virulence of Haemophilus ducreyi in humans. J. Infect. Dis. 199:684–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bong CTH, et al. 2001. A DsrA-deficient mutant of Haemophilus ducreyi is impaired in its ability to infect human volunteers. Infect. Immun. 69:1488–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bouchet V, et al. 2003. Host-derived sialic acid is incorporated into Haemophilus influenzae lipopolysaccharide and is a major virulence factor in experimental otitis media. Proc. Natl. Acad. Sci. U. S. A. 100:8898–8903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bozue JA, Tullius MV, Wang J, Gibson BW, Munson RS., Jr 1999. Haemophilus ducreyi produces a novel sialyltransferase: identification of the sialyltransferase gene and construction of mutants deficient in the production of the sialic acid-containing glycoform of the lipooligosaccharide. J. Biol. Chem. 274:4106–4114 [DOI] [PubMed] [Google Scholar]
- 13. Carlin AF, et al. 2009. Molecular mimicry of host sialylated glycans allows a bacterial pathogen to engage neutrophil Siglec-9 and dampen the innate immune response. Blood 113:3333–3336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Elkins C, Morrow KJ, Olsen B. 2000. Serum resistance in Haemophilus ducreyi requires outer membrane protein DsrA. Infect. Immun. 68:1608–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Frisk A, Ahmed H, Van Dyck E, Lagergard T. 1998. Antibodies specific to surface antigens are not effective in complement-mediated killing of Haemophilus ducreyi. Microb. Pathog. 25:67–75 [DOI] [PubMed] [Google Scholar]
- 16. Fulcher RA, et al. 2006. Expression of Haemophilus ducreyi collagen binding outer membrane protein NcaA is required for virulence in swine and human challenge models of chancroid. Infect. Immun. 74:2651–2658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gioia J, et al. 2006. The genome sequence of Mannheimia haemolytica A1: insights into virulence, natural competence, and Pasteurellaceae phylogeny. J. Bacteriol. 188:7257–7266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goon S, Schilling B, Tullius MV, Gibson BW, Bertozzi CR. 2003. Metabolic incorporation of unnatural sialic acids into Haemophilus ducreyi lipooligosaccharides. Proc. Natl. Acad. Sci. U. S. A. 100:3089–3094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harvey HA, Jennings MP, Campbell CA, Williams R, Apicella MA. 2001. Receptor-mediated endocytosis of Neisseria gonorrhoeae into primary human urethral epithelial cells: the role of the asialoglycoprotein receptor. Mol. Microbiol. 42:659–672 [DOI] [PubMed] [Google Scholar]
- 20. Heikema AP, et al. 2010. Characterization of the specific interaction between sialoadhesin and sialylated Campylobacter jejuni lipooligosaccharides. Infect. Immun. 78:3237–3246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hiltke TJ, et al. 1999. Effect of normal and immune sera on Haemophilus ducreyi 35000HP and its isogenic MOMP and LOS mutants. Microb. Pathog. 26:93–102 [DOI] [PubMed] [Google Scholar]
- 22. Hobbs MM, et al. 2011. Experimental gonococcal infection in male volunteers: cumulative experience with Neisseria gonorrhoeae strains FA1090 and MS11mkC. Front. Microbiol. 2:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Howard MD, et al. 2011. Genetics and molecular specificity of sialylation of Histophilus somni lipooligosaccharide (LOS) and the effect of LOS sialylation on Toll-like receptor-4 signaling. Vet. Microbiol. 153:163–172 [DOI] [PubMed] [Google Scholar]
- 24. Humphreys T, et al. 2007. Dysregulated immune profiles for skin and dendritic cells are associated with increased host susceptibility to Haemophilus ducreyi infection in human volunteers. Infect. Immun. 75:5686–5697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Inzana TJ, Glindemann G, Cox AD, Wakarchuk W, Howard MD. 2002. Incorporation of N-acetylneuraminic acid into Haemophilus somnus lipooligosaccharide (LOS): enhancement of resistance to serum and reduction of LOS antibody binding. Infect. Immun. 70:4870–4879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Janowicz D, et al. 2006. A DltA mutant of Haemophilus ducreyi is partially attenuated in its ability to cause pustules in human volunteers. Infect. Immun. 74:1394–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Janowicz DM, et al. 2004. Expression of the LspA1 and LspA2 proteins by Haemophilus ducreyi is required for virulence in human volunteers. Infect. Immun. 72:4528–4533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Janowicz DM, Ofner S, Katz BP, Spinola SM. 2009. Experimental infection of human volunteers with Haemophilus ducreyi: 15 years of clinical data and experience. J. Infect. Dis. 199:1671–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28a. Janowicz DM, et al. 2011. Expression of the flp proteins by Haemophilus ducreyi is necessary for virulence in human volunteers. BMC Microbiol. 11:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jones C, Virji M, Crocker PR. 2003. Recognition of sialylated meningococcal lipopolysaccharide by siglecs expressed on myeloid cells leads to enhanced bacterial uptake. Mol. Microbiol. 49:1213–1225 [DOI] [PubMed] [Google Scholar]
- 30. Jurcisek J, et al. 2005. Role of sialic acid and complex carbohydrate biosynthesis in biofilm formation by nontypeable Haemophilus influenzae in the chinchilla middle ear. Infect. Immun. 73:3210–3218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kuijf ML, et al. 2010. TLR4-mediated sensing of Campylobacter jejuni by dendritic cells is determined by sialylation. J. Immunol. 185:748–755 [DOI] [PubMed] [Google Scholar]
- 32. Labandeira-Rey M, et al. 2011. A Haemophilus ducreyi cpxR deletion mutant is virulent in human volunteers. J. Infect. Dis. 203:1859–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Labandeira-Rey M, et al. 2009. Inactivation of the Haemophilus ducreyi luxS gene affects the virulence of this pathogen in human subjects. J. Infect. Dis. 200:409–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Leduc I, Richards P, Davis C, Schilling B, Elkins C. 2004. A novel lectin, DltA, is required for expression of a full serum resistance phenotype in Haemophilus ducreyi. Infect. Immun. 72:3418–3428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li Y, et al. 2007. The Hd0053 gene of Haemophilus ducreyi encodes an alpha2,3-sialyltransferase. Biochem. Biophys. Res. Commun. 361:555–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mandrell RE, et al. 1990. In vitro and in vivo modification of Neisseria gonorrhoeae lipooligosaccharide epitope structure by sialylation. J. Exp. Med. 171:1649–1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Marion C, Burnaugh AM, Woodiga SA, King SJ. 2011. Sialic acid transport contributes to pneumococcal colonization. Infect. Immun. 79:1262–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McBride WJ, Hannah RC, Le Cornec GM, Bletchly C. 2008. Cutaneous chancroid in a visitor from Vanuatu. Australas. J. Dermatol. 49:98–99 [DOI] [PubMed] [Google Scholar]
- 39. Melaugh W, Campagnari AA, Gibson BW. 1996. The lipooligosaccharides of Haemophilus ducreyi are highly sialylated. J. Bacteriol. 178:564–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mizanur RM, Pohl NL. 2008. Bacterial CMP-sialic acid synthetases: production, properties, and applications. Appl. Microbiol. Biotechnol. 80:757–765 [DOI] [PubMed] [Google Scholar]
- 41. Mock JR, et al. 2005. Haemophilus ducreyi targets Src family protein tyrosine kinases to inhibit phagocytic signaling. Infect. Immun. 73:7808–7816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mount KL, et al. 2010. Haemophilus ducreyi SapA contributes to cathelicidin resistance and virulence in humans. Infect. Immun. 78:1176–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Peel TN, Bhatti D, De Boer JC, Stratov I, Spelman DW. 2010. Chronic cutaneous ulcers secondary to Haemophilus ducreyi infection. Med. J. Aust. 192:348–350 [DOI] [PubMed] [Google Scholar]
- 44. Post DM, Mungur R, Gibson BW, Munson RS., Jr 2005. Identification of a novel sialic acid transporter in Haemophilus ducreyi. Infect. Immun. 73:6727–6735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Post DMB, et al. 2007. Identification of genes involved in the expression of atypical lipooligosaccharide structures from a second class of Haemophilus ducreyi. Infect. Immun. 75:113–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ricotta EE, Wang N, Cutler R, Lawrence JG, Humphreys TL. 2011. Rapid divergence of two classes of Haemophilus ducreyi. J. Bacteriol. 193:2941–2947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schilling B, et al. 2001. Biosynthesis of sialylated lipooligosaccharides in Haemophilus ducreyi is dependent on exogenous sialic acid and not mannosamine. Incorporation studies using N-acylmannosamine analogues, N-glycolylneuraminic acid, and 13C-labeled N-acetylneuraminic acid. Biochemistry 40:12666–12677 [DOI] [PubMed] [Google Scholar]
- 48. Schneider H, et al. 1996. Sialylation lessens the infectivity of Neisseria gonorrhoeae MS11mkC. J. Infect. Dis. 173:1422–1427 [DOI] [PubMed] [Google Scholar]
- 49. Severi E, Hood DW, Thomas GH. 2007. Sialic acid utilization by bacterial pathogens. Microbiology 153:2817–2822 [DOI] [PubMed] [Google Scholar]
- 50. Severi E, et al. 2005. Sialic acid transport in Haemophilus influenzae is essential for lipopolysaccharide sialylation and serum resistance and is dependent on a novel tripartite ATP-independent periplasmic transporter. Mol. Microbiol. 58:1173–1185 [DOI] [PubMed] [Google Scholar]
- 51. Spinola SM. 2008. Chancroid and Haemophilus ducreyi, p 689–699 In Holmes KK, et al. (ed), Sexually transmitted diseases, 4th ed. McGraw-Hill, New York, NY [Google Scholar]
- 52. Spinola SM, et al. 2003. Differences in host susceptibility to disease progression in the human challenge model of Haemophilus ducreyi infection. Infect. Immun. 71:6658–6663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Spinola SM, et al. 2010. Activation of the CpxRA system by deletion of cpxA impairs the ability of Haemophilus ducreyi to infect humans. Infect. Immun. 78:3898–3904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Spinola SM, et al. 2003. Haemophilus ducreyi requires an intact flp gene cluster for virulence in humans. Infect. Immun. 71:7178–7182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Unkmeir A, et al. 2002. Lipooligosaccharide and polysaccharide capsule: virulence factors of Neisseria meningitidis that determine meningococcal interaction with human dendritic cells. Infect. Immun. 70:2454–2462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ussher JE, Wilson E, Campanella S, Taylor SL, Roberts SA. 2007. Haemophilus ducreyi causing chronic skin ulceration in children visiting Samoa. Clin. Infect. Dis. 44:e85–e87 [DOI] [PubMed] [Google Scholar]
- 57. Vakevainen M, Greenberg S, Hansen EJ. 2003. Inhibition of phagocytosis by Haemophilus ducreyi requires expression of the LspA1 and LspA2 proteins. Infect. Immun. 71:5994–6003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Varki A. 2010. Colloquium paper: uniquely human evolution of sialic acid genetics and biology. Proc. Natl. Acad. Sci. U. S. A. 107(Suppl 2):8939–8946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Vimr E, Lichtensteiger C, Steenbergen S. 2000. Sialic acid metabolism's dual function in Haemophilus influenzae. Mol. Microbiol. 36:1113–1123 [DOI] [PubMed] [Google Scholar]
- 60. Vimr ER, Kalivoda KA, Deszo EL, Steenbergen SM. 2004. Diversity of microbial sialic acid metabolism. Microbiol. Mol. Biol. Rev. 68:132–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Welsch JA, Ram S. 2008. Factor H and neisserial pathogenesis. Vaccine 26(Suppl 8):I40–I45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wood GE, Dutro SM, Totten PA. 2001. Haemophilus ducreyi inhibits phagocytosis by U-937 cells, a human macrophage-like cell line. Infect. Immun. 69:4726–4733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wu H, Jerse AE. 2006. α-2,3Sialytransferase enhances Neisseria gonorrhoeae survival during experimental murine genital tract infection. Infect. Immun. 74:4094–4103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Young RS, et al. 1999. Expression of sialylated or paragloboside-like lipooligosaccharides are not required for pustule formation by Haemophilus ducreyi in human volunteers. Infect. Immun. 67:6335–6340 [DOI] [PMC free article] [PubMed] [Google Scholar]