Abstract
The intestinal immune system is crucial for the maintenance of mucosal homeostasis and has evolved under the dual pressure of protecting the host from pathogenic infection and coexisting with the dense and diverse commensal organisms in the lumen. Intestinal intraepithelial lymphocytes (iIELs) are the first element of the host T cell compartment available to respond to oral infection by pathogens. This study demonstrated that oral infection by Salmonella enterica serovar Typhimurium promoted the expansion of iIELs, particularly CD8+ TCRγδ+ IELs, enhanced expression of NKG2D on iIELs, increased expression of MULT1, and decreased expression of Qa-1 by intestinal epithelial cells (IECs), leading to activation of, particularly, CD8+ TCRγδ+ iIELs and cytolytic activity against S. Typhimurium-infected IECs. Blockade of NKG2D recognition or depletion of TCRγδ+ cells using a depleting monoclonal antibody significantly attenuated the clearance of S. Typhimurium in the intestine and other tissues. This study suggests that iIELs, particularly CD8+ TCRγδ+ iIELs, play important roles in the detection of pathogenic bacteria and eradication of infected epithelial cells and, thus, provide protection against invading pathogens. These data further our understanding of the mechanisms by which the immune system of the intestinal mucosa discriminates between pathogenic and commensal organisms.
INTRODUCTION
The mucosal surface of the mammalian intestine interfaces with a dense and diverse community of microbes. The intestinal immune system is crucial for maintenance of mucosal homeostasis and has developed under the dual pressure of protecting the host from pathogenic infections and coexisting with the myriad commensal organisms in the lumen. The mechanisms by which the intestinal immune system discriminates between commensal flora and pathogenic microbes are poorly defined. Immune cells reside not only in gut-associated lymphoid tissues (GALT) but also widely within the intestinal epithelium and the underlying lamina propria (17). Intestinal intraepithelial lymphocytes (iIELs), forming a highly specialized lymphoid compartment in the intestinal epithelium, are considered to play an important role in the regulation of mucosal immune responses. The majority of iIELs are CD8+ IELs, with subpopulations characterized by the expression of the CD8αα homodimer and the αβ T cell receptor (TCRαβ) or TCRγδ or by expression of the CD8αβ heterodimer and the TCRαβ. CD8αβ IELs bear the hallmarks of adaptive immune cells, while the CD8αα iIELs exhibit many “unconventional” features and are considered to function as part of the innate immune system (5, 8, 25).
iIELs exhibit cytotoxic activity, including NK cell-like cytotoxicity, and express NK cell receptors, which play major roles in the recognition and protection of the host from pathogenic infections (8, 19, 25). NK cell receptors, including stimulatory receptors and inhibitory receptors, are important receptors in the innate immune system. NKG2D is an activating costimulatory receptor on NK cells, NKT cells, activated CD8+ T cells, and γδ T cells, which respond to cellular stress, such as inflammation, transformation, and infection. It is also found to be expressed on iIELs, and its ligands, including retinoic acid early inducible 1 (RAE-1), H60, and murine ULib-binding protein (ULBP)-like transcript 1 (MULT1), are expressed on infected, transformed, or otherwise stressed cells (23). The inhibitory receptors, such as NKG2A and Ly49E/F, on iIELs seem important in the maintenance of immune homeostasis within the intestine (8, 12).
Salmonella is a Gram-negative, intracellular bacterium which enters the host via the intestinal epithelium. It is known to cause a spectrum of diseases ranging from self-limited gastrointestinal infections to systemic infections with high mortality (24). This study aimed to explore the role and the possible mechanism of action of the intestinal immune system in a pathogenic infection based on a model of oral infection of the intestine by a virulent Salmonella enterica serotype Typhimurium strain. Changes in the frequency of small intestinal IEL subpopulations and their associated NK cell-like cytotoxicity identified the subsets of iIELs important in the defense against pathogenic infection. Such information is beneficial in gaining an understanding of how immune responses and immunopathologies develop during intestinal Salmonella infection.
MATERIALS AND METHODS
Cell lines and cell culture.
The murine T cell lymphoma line YAC-1 and the murine colon adenocarcinoma cell line MCA-38 were cultured in RPMI 1640 medium (Gibco/BRL, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS) at 37°C in a humidified 5% CO2 atmosphere.
Mice.
Male C57BL/6 mice (6 to 8 weeks old) were purchased from the Shanghai Experimental Animal Center (Shanghai, China) and maintained under specific-pathogen-free conditions. All animal studies were approved by the Institute Animal Care and Use Committee of Shandong University. Mice were handled and experiments were conducted in accordance with guidelines for experimental animals from Shandong University. All animal manipulations were performed in class II biological safety cabinets.
Antibodies.
The following monoclonal antibodies (MAbs) were used in this study. Purified anti-Qa-1b, PercpCy5.5–anti-CD3ε (clone 145-2c11), and phycoerythrin (PE)–anti-gamma interferon (IFN-γ) (clone XMG1.2) were purchased from BD Bioscience (Franklin Lakes, NJ). Fluorescein isothiocyanate (FITC)–anti-γδ T cell receptor (TCRγδ) (clone GL3), PercpCy5.5–anti-CD8α (clone 53-6.7), FITC–anti-CD8β (clone 53.5.8), FITC–anti-TCRαβ (clone H57-597), PE–anti-CD69 (clone H1.2F3), purified anti-CD16/CD32 (clone2.4G2), allophycocyanin (APC)–anti-NKG2D (clone CX5), APC–anti-Ly49E/F (clone CM4), PE–anti-CD95 ligand (clone MFL3), APC–anti-tumor necrosis factor alpha (TNF-α) (clone MP6-XT22), and PE–anti-MULT1 (clone 5D10) were purchased from eBioscience (San Diego, CA). FITC–anti-Rae1 (clone 186107), PE–anti-H60 (clone 205326), and purified anti-mouse NKG2D-neutralizing MAbs were purchased from R&D Systems (Minneapolis, MN). Streptavidin-conjugated PE obtained from BD Bioscience (San Diego, CA) was used as a secondary reagent to identify biotinylated primary antibodies. Functional-grade purified anti-mouse TCRγδ MAb (UC7-13D5) obtained from eBioscience was used to eliminate TCRγδ+ iIELs. Each antibody was titrated to determine the optimal staining concentration for maximal signal.
Bacterial strains and growth conditions.
A virulent S. Typhimurium strain (CMCC, 50222) was grown in LB (10 g of tryptone, 5 g of yeast extract, and 10 g of NaCl/liter) broth or on LB agar plates. Bacteria were cultured overnight at 37°C, and the following day, bacterial cultures at saturation density were diluted (1/100) and cultured to the mid-logarithmic growth phase (optical density at 600 nm [OD600], 0.5 to 0.6). Bacterial cultures were centrifuged and washed in LB broth twice before use. The number of bacteria was calculated from a standard curve relating CFU to OD600 and verified by growing aliquots of serial dilutions on LB agar plates.
In vivo infection with S. Typhimurium.
Mice (aged 8 to 10 weeks) were inoculated orally with suspensions (0.2 ml) of wild-type S. Typhimurium (5 × 105 bacteria), using a standard gastric intubation needle. An equal volume of a sterile LB broth vehicle was administered as a mock-infected control.
TCRγδ+ cell depletion experiments.
C57BL/6 mice were injected intraperitoneally (i.p.) with 200 μg of anti-mouse TCRγδ MAb (UC7-13D5) 3 days before S. Typhimurium infection to eliminate TCRγδ+ iIELs. The elimination of TCRγδ+ iIELs in mice was confirmed by flow cytometry.
Neutralization assay in vivo and in vitro.
For in vivo neutralization, C57BL/6 mice were pretreated with anti-NKG2D (CX5)-neutralizing MAb or control IgG (intravenously [i.v.]) at 400 μg per mouse 24 h before S. Typhimurium infection. For in vitro neutralization, purified TCRγδ+ iIELs were cocultured with saturating concentrations of anti-NKG2D Ab (20 μg/ml) or isotype-matched control antibody for 1 h and then washed for use as effector cells.
Bacterial enumeration.
The small intestine, Peyer's patches (PP), liver, and spleen were collected separately at various time points and homogenized with a tissue homogenizer (Polytron MR 21; Kinematica). Serial dilutions of the tissue homogenates were plated on bismuth sulfite (BS) agar plates. Plates were incubated at 37°C, and CFU were enumerated after 16 h.
Preparation of iIELs and intestinal epithelial cells (IECs).
iIELs were isolated from the small intestine as previously described (22). Briefly, the small intestine was everted, divided into four pieces, and washed twice in phosphate-buffered saline (PBS) containing 100 U/ml penicillin-streptomycin. The specimens were then incubated with stirring at 37°C in prewarmed Ca2+- and Mg2+-free Hanks' solution containing 100 U/ml penicillin-streptomycin, 5% fetal calf serum (FCS), 2 mM dithiothreitol (DTT), and 5 mM EDTA for 30 min, followed by vigorous shaking for 30 s. The supernatants were passed over two nylon wool columns to remove undigested tissue debris. The lymphocytes obtained were pooled and enriched on a discontinuous (40% and 70%) Percoll density gradient (Pharmacia Biosciences). Cells at the interface between the 40% and 70% fractions (iIELs) were collected. This population of iIELs was shown to be >90% CD3+ cells by flow cytometry analysis.
IECs were isolated as previously described (19). Peyer's patches were excised, and the small intestine was opened longitudinally and washed in PBS containing 100 U/ml penicillin-streptomycin. The tissue was then cut into 5-mm-long fragments and incubated for 15 min at 22°C on a shaker platform in PBS containing 0.02 mg/ml dispase I (Sigma-Aldrich), 60 U/ml collagenase XIa (Sigma-Aldrich), and 2% bovine serum albumin (BSA). Cells and small sheets of intestinal epithelium were separated from the intestinal fragments by harvesting supernatants after a 2-min stay in medium. Cells were centrifuged three times (800 rpm for 3 min) in Dulbecco's modified Eagle's medium (DMEM) plus 2% sorbitol. Supernatants were discarded. The remaining pellet consisted mainly of cells in intact crypts and small sheets of intestinal epithelium. This population of cells was confirmed to be >90% IECs by immunocytoplasmic staining with anti-cytokeratin 18 monoclonal antibody (28), which was obtained from Wuhan Boster Bio-engineering Limited Company, China.
Immunofluorescence staining for surface markers.
Nonspecific MAb binding to the recovered iIEL population and IECs was prevented by incubation with anti-CD16/CD32. All iIEL and IEC samples were stained with various combinations of directly or indirectly fluorochrome-conjugated MAbs. Relevant isotype-matched Abs were used as controls for nonspecific binding. Labeled cells were acquired using a FACSCalibur flow cytometer (BD Bioscience) and analyzed using WinMDI 2.9 software.
Intracellular cytokine staining.
Intestinal intraepithelial lymphocytes were cultured in 6-well plates with a volume of 2 ml of RPMI 1640 medium supplemented with glutamine, 2-mercaptoethanol (2-ME), gentamicin, and 10% heat-inactivated FCS (complete medium) and in the presence of brefeldin A (10 μg/ml; Sigma-Aldrich) for 4 h at 37°C in a humidified CO2 incubator. Cultured cells were washed and incubated (15 min) with rat serum and anti-CD16/CD32 MAb to block nonspecific Ab binding. Subsequently, cells were stained with FITC–anti-TCRγδ and PercpCy5.5–anti-CD8α or with FITC–anti-CD8β and PercpCy5.5–anti-CD8α MAbs to gate the iIELs subpopulations in a 100-μl volume (30 min at 4°C), washed with PBS, and fixed with 1% paraformaldehyde for 30 min at room temperature. Cells were then washed with PBS and permeabilized with PBS containing 0.1% BSA and 0.3% saponin (Sigma-Aldrich) for 30 min. Following this, PE-conjugated anti-IFN-γ MAb or APC-conjugated anti-TNF-α MAb was added. After incubation for a further 30 min at 4°C, cells were washed and resuspended in PBS. Data were acquired using a FACSCalibur flow cytometer (BD Bioscience) and analyzed using WinMDI 2.9 software.
MACS sorting of cells.
CD8+ TCRγδ+ T cells were enriched (>90% purity) from the iIELs by magnetically activated cell sorting (MACS) (Miltenyi-Biotec, Auburn, CA) using a mouse TCRγδ+ T cell isolation kit according to the protocol provided by the manufacturer.
RT-PCR.
Gene expression was determined by reverse transcription-PCR (RT-PCR). Total RNA was extracted from purified iIELs or IECs using TRIzol (Invitrogen Life Technologies) reagent according to the protocol provided by the manufacturer. Total RNA was reverse transcribed to generate cDNA according to the protocol provided by the manufacturer (Promega, Madison, WI). An aliquot of cDNA (2 μl) was then subjected to PCR. Samples were initially denatured for 5 min at 94°C, followed by 30 cycles of the following PCR amplification program: denaturation for 1 min at 94°C and then annealing for 1 min at 58°C for IFN-γ or 55°C for NKG2A, NKG2D, and 4-1BB and elongation for 1 min at 72°C, with a final extension interval (10 min) following the last cycle. Primer sequences used are shown in Table S1 in the supplemental material.
Quantitative PCR.
The protocol used for total RNA extraction and cDNA synthesis was similar to that described for RT-PCR. cDNA was quantified by real-time PCR analysis. β-Actin was used as an internal control. Primer sequences used are shown in Table S1 in the supplemental material. PCRs were carried out using SYBR green mix (Toyobo, Osaka, Japan).
Lysis assay.
The cytolytic activity of iIELs and purified CD8+ TCRγδ+ iIELs was assessed by the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay. YAC-1 cells, which are the usual target for assay of NK cell cytotoxicity, were used as target cells. Target cells (YAC-1 or MCA-38) were resuspended in RPMI 1640 at a density of 3 × 105/ml and seeded into 96-well plates (100 μl/well). IEC target cells that were isolated from Salmonella- or mock-infected B6 mice were placed in 96-well plates (104/well) coated with rat tail collagen (Sigma-Aldrich) and incubated for 24 h in epithelial cell medium (ECM) (30). iIELs or CD8+ TCRγδ+ iIELs were isolated from Salmonella- or mock-infected B6 mice at day 5 after infection and added to target cells at effector/target (E:T) ratios of 25:1, 12.5:1, or 6.25:1. The assay was incubated for 12 h, and 20 μl MTT (5 mg/ml) was added for the final 4 h of the incubation period. The remaining formazan precipitate was completely dissolved in 200 μl dimethyl sulfoxide, and the absorbance at 570 or 630 nm (A570/630) of each sample was determined with a microplate autoreader (Bio-Rad). Cytotoxicity was calculated as follows: percent lysis = [1 − (A570/630 of cocultured cells − A570/630 of effector cells)/A570/630 of target cells] × 100%.
Cytotoxicity assay by CFSE and 7-AAD flow cytometry.
IEC target cells were isolated from Salmonella-infected B6 mice at day 5 after infection, incubated with 1 ml of carboxyfluorescein succinimidyl esther (CFSE; 2 mM) (Molecular Probes, Eugene, OR) for 10 min at 37°C, and then washed three times to stop the reaction. iIEL effector cells were isolated from Salmonella- or mock-infected B6 mice at day 5 and added to target cells at effector/target ratios of 25:1 at 37°C for 4 h. After the incubation, following a further wash, cells were labeled for 15 min with 7-aminoactinomycin D (7-AAD; optimized at 0.25 μg/ml; Sigma-Aldrich) to identify dead cells. After a final wash, cells were mixed thoroughly to interrupt cell-cell contact and resuspended at 300 μl/tube in fluorescence-activated cell sorting (FACS) buffer for immediate analysis. Cells doubly positive for CFSE and 7-AAD were the killed target cells, and the specific cytotoxicity was calculated as follows: percent lysis = (number of cells doubly positive for CFSE and 7-AAD/number of CFSE-positive cells) × 100%.
Histopathology.
Tissues from the small intestine and liver were excised at day 3, 5, or 7, fixed in 10% neutral buffered formalin, and embedded in paraffin. Four-micrometer sections were fixed to slides, deparaffinized, and stained with hematoxylin and eosin. For pathological scoring, five fields per sample were examined and scored as described previously to facilitate comparisons (4).
ELISA.
Mouse serum samples were stored at −80°C prior to cytokine measurement. Levels of IFN-γ were measured using commercially available enzyme-linked immunosorbent assay (ELISA) kits (Shanghai ExCell Biology, Inc.).
Statistical analysis.
Data were analyzed for statistical significance using one-way analysis of variance (ANOVA). Student's t tests were performed to compare values obtained from two groups. Results were considered statistically significant at a P of <0.05.
RESULTS
S. Typhimurium infection induces mouse enteritis.
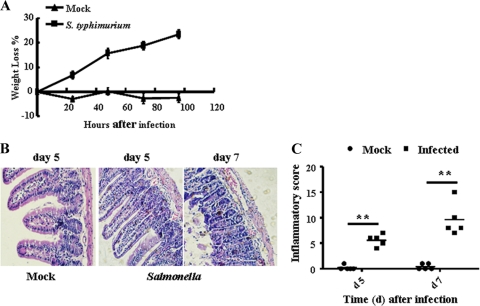
The intestinal immune system is crucial for the maintenance of mucosal homeostasis. It has the challenging task of protecting against food-borne pathogens while simultaneously maintaining tolerance to antigens from commensal microbes and nutrients. To explore the mechanisms that allow this discrimination, C57BL/6 mice were orally infected with S. Typhimurium. Infection with S. Typhimurium induced mouse enteritis, including weight loss (Fig. 1A) and mucosal erosion, which are caused by an infiltration consisting predominantly of mononuclear leukocytes (Fig. 1B). Figure 1C showed the results of histopathology scores. Finally, a systemic infection was established (see Fig. S1 in the supplemental material).
Fig 1.
Induction of mouse enteritis by S. Typhimurium infection. B6 mice were infected orally with wild-type S. Typhimurium (5 × 105 bcteria) or mock infected (LB broth). (A) Weight loss (referring to weight at 0 h) was evaluated at various time points postinfection (n = 5). (B) Representative photographs for hematoxylin and eosin-stained, paraffin-embedded sections from B6 mice (n = 5) at day 5 and day 7 after S. Typhimurium infection (original magnification, ×200). Note the presence of a diffuse enteritis associated with edema, which is caused by an infiltration that consists predominantly of mononuclear leukocytes in mouse small intestine infected with S. Typhimurium. (C) Histopathology scores in the small intestine from mice infected with S. Typhimurium. Data are aggregate scores of the pathology from five tissue sections from five individual mice per group. Each data point represents the result for an individual animal, and the scatter of data is shown, with horizontal lines representing the means. **, P < 0.01, compared with the mock-infected group.
S. Typhimurium infection augments the number of CD8+ TCRγδ+ iIELs and enhances the NK cell-like cytotoxicity of iIELs.
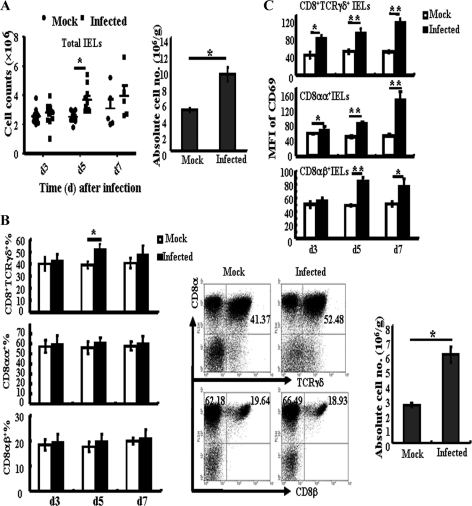
Intestinal intraepithelial lymphocytes are the first elements of the host T cell compartment available to respond to oral infection by pathogens. In this study, the role of iIELs during S. Typhimurium infection was explored. First, changes in iIEL subpopulations were examined. It was observed that the total numbers of iIELs (Fig. 2A, left) and the absolute numbers of iIELs (Fig. 2A, right) increased significantly. In particular, the percentage of CD8+ TCRγδ+ iIELs increased from 40.19% ± 3.57% in the mock-infected group to 53.71% ± 2.88% in the infected group at day 5 after S. Typhimurium infection (Fig. 2B, left and middle). The absolute numbers of CD8+ TCRγδ+ iIELs in the infected group were also much higher than those in the mock-infected group (Fig. 2B, right). However, the percentages of CD8αα+ iIELs and CD8αβ+ iIELs did not change. Further analysis of the expression of the activation marker CD69 by these iIEL subpopulations showed that infection with S. Typhimurium resulted in significant increases in CD69 expression by CD8+ TCRγδ+ iIELs and CD8αα+ iIELs from day 3 and by CD8αβ+ iIELs from day 5 after infection (Fig. 2C).
Fig 2.
Oral infection with S. Typhimurium causes changes in iIEL populations. At various time points after infection, the small intestine was removed and isolated iIELs were stained with fluorescently labeled MAbs for analysis by flow cytometry. (A) Total numbers of iIELs at different time points (left) and absolute numbers of iIELs at day 5 (right) after S. Typhimurium infection were determined by flow cytometry. (B) Statistical analysis of the percentage of CD8+ TCRγδ+ iIELs, CD8αα+ iIELs, and CD8αβ+ iIELs at days 3, 5, and 7 after S. Typhimurium infection (left), the distribution of CD8+ TCRγδ+ iIELs, CD8αα+ IELs, and CD8αβ+iIELs from the small intestine of mock-infected or S. Typhimurium-infected mice (middle), and the absolute numbers of CD8+ TCRγδ+ iIELs (right) at day 5 after oral infection. (C) Statistical analysis of CD69 expression (MFI, mean fluorescent intensity) by CD8αα+ iIELs, CD8αβ+ iIELs, and CD8+ TCRγδ+ iIELs. Data are expressed as the means ± standard deviations (SD) of results of at least three independent experiments. **, P < 0.01; *, P < 0.05, compared with the mock-infected group.
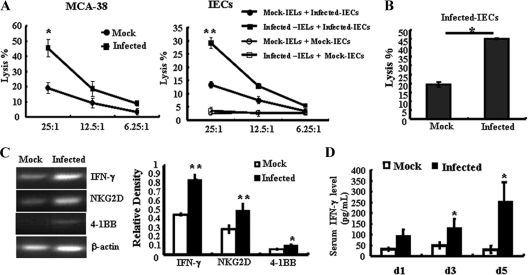
The NK cell-like cytotoxicity of S. Typhimurium-activated iIELs was then examined. As shown in Fig. 3A, iIELs isolated from S. Typhimurium-infected mice at day 5 exhibited much stronger cytotoxicity against the murine colon adenocarcinoma cell line MCA-38 (44.17% ± 6.75%) than iIELs isolated from mock-infected mice (19.01% ± 2.79%). Similar results were obtained from S. Typhimurium-infected iIELs against the S. Typhimurium-infected IECs (29.14% ± 2.03% in the infected group and 13.32% ± 1.84% in the mock-infected group). However, no cytotoxicity against mock-infected IECs was observed. We further detected the cytolysis of iIELs against S. Typhimurium-infected IECs by the CFSE/7-AAD double-staining method. As shown in Fig. 3B, the cytotoxicity of S. Typhimurium-infected iIELs against the S. Typhimurium-infected IECs was much higher than that of iIELs from control mice (Fig. 3B).
Fig 3.
S. Typhimurium infection enhances the NK cell-like cytotoxicity of iIELs. Total iIELs were isolated from mock-infected or Salmonella-infected B6 mice at day 5 after infection. (A) The cytotoxicity of iIELs against MCA-38 cells or S. Typhimurium-infected IECs was measured by MTT assay at various E:T ratios. (B) The cytotoxicity of iIELs against S. Typhimurium-infected IECs was measured by FACS assay with CFSE/7-AAD double staining at the E:T ratio of 25:1. (C) The mRNA levels of NKG2D, IFN-γ, and 4-1BB in Salmonella- or mock-infected iIELs were detected by RT-PCR. The expression of mRNA was normalized to that of β-actin. (D) Serum IFN-γ levels were detected by ELISA. Data are expressed as the means ± SD of results of at least three separate experiments. **, P < 0.01; *, P < 0.05, compared with the mock-infected group.
Changes in receptor-related gene expression in iIELs were detected by RT-PCR. Upregulation of 4-1BB, IFN-γ, and NKG2D mRNA levels in iIELs isolated from S. Typhimurium-infected mice was observed (Fig. 3C), and the serum levels of IFN-γ were also increased significantly (Fig. 3D). These results demonstrate that S. Typhimurium infection of the intestine was detected by iIELs, resulting in rapid activation and enhanced cytolytic activity. This response characterizes the role of these cells in immunosurveillance and protection against infection.
NKG2D expression on CD8+ TCRγδ+ iIELs is upregulated after S. Typhimurium infection.
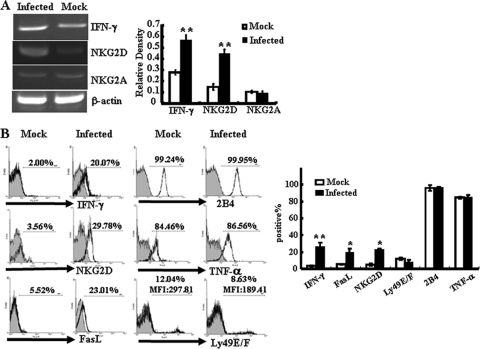
Changes in the proportions and levels of activation of the iIELs during S. Typhimurium infection were most notable in the CD8+ TCRγδ+ subpopulation. So cells of this phenotype were isolated from intestinal IELs by MACS (purity of up to 90% [data not shown]), and we further detected the changes in receptor-related gene expression in these cells by RT-PCR. As shown in Fig. 4A, NKG2D transcripts were upregulated in CD8+ TCRγδ+ iIELs isolated from S. Typhimurium-infected mice, while there was no change in the transcription of the inhibitory receptor NKG2A. Flow cytometric analysis confirmed that S. Typhimurium infection induced surface expression of NKG2D by CD8+ TCRγδ+ iIELs (Fig. 4B). Upregulation of the expression of IFN-γ and FasL was also observed. However, the expression levels of 2B4 and TNF-α remained unchanged (Fig. 4A and B). For murine inhibitory receptor Ly49E/F, although the percentage of Ly49E/F-positive cells did not change with infection, the mean fluorescent intensity (MFI) was reduced significantly, from 302.16 ± 7.38 in the mock-infected group to 191.42 ± 6.72 in the infected group (P < 0.05). The upregulation of NKG2D, IFN-γ, and FasL was also observed in the CD8αα+ iIEL subpopulation but not in the CD8αβ+ iIEL subpopulation (see Fig. S2 in the supplemental material).
Fig 4.
S. Typhimurium infection increases the expression of NKG2D and cytotoxicity-related molecules on CD8+ TCRγδ+ iIELs. Total iIELs were isolated from mock-infected or Salmonella-infected B6 mice at day 5 after infection. TCRγδ+ iIELs were enriched to up to 90% purity from the iIELs by MACS. (A) The mRNA levels of NKG2D, IFN-γ, and NKG2A of TCRγδ+ iIELs were analyzed by RT-PCR. The expression levels of mRNA were normalized to that of β-actin. (B) The expression levels of IFN-γ, TNF-α, NKG2D, FasL, 2B4, and Ly49E/F of CD8+ TCRγδ+ iIELs after S. Typhimurium infection were examined by flow cytometry. Data shown are representative of three independent experiments (left) and of a statistical analysis of IFN-γ, TNF-α, NKG2D, FasL, 2B4, and Ly49E/F expression (right). Cells stained with isotype-matched control Ig demonstrated the specificity of MAb binding (filled histograms). **, P < 0.01; *, P < 0.05, compared with the mock-infected group. MFI, mean fluorescence intensity.
Expression of MULT1 on IECs is upregulated by S. Typhimurium infection, while Qa-1 expression is downregulated.
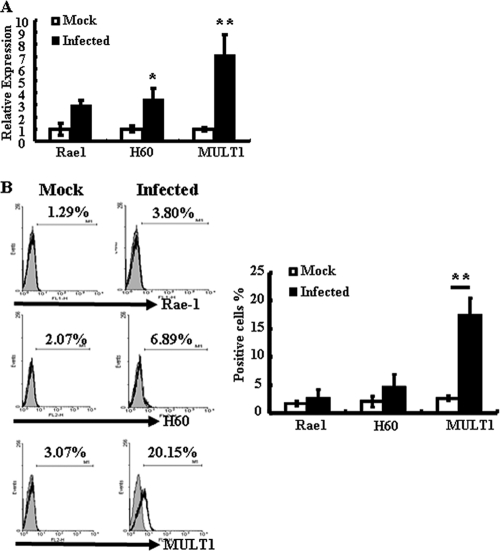
We next investigated whether S. Typhimurium induces NKG2D ligands' expression on IECs. Real-time PCR was used to detect IEC gene expression of all known NKG2D ligands (Rae1, H60, and MULT1). Analysis of these data revealed upregulation of H60 and MULT1 mRNA levels in IECs isolated from S. Typhimurium-infected mice (Fig. 5A). Flow cytometric analysis confirmed a significant increase in the surface expression of MULT1 in these cells (Fig. 5B). However, a slight, but not statistically significant, increase in the surface expression of H60 was detected.
Fig 5.
S. Typhimurium infection induces MULT1 expression on IECs. IECs were isolated from mock-infected or Salmonella-infected mice at day 5 after infection. (A) The mRNA levels of Rae1, H60, and MULT1 were determined by quantitative PCR. Results are shown as the increase in the expression of Rae1, H60, or MULT1 compared with that of β-actin. Gene expression values were then calculated based on the ΔΔCT method, using the mean of results for mock-infected IECs as a calibrator. Relative quantities (RQs) were determined using the equation RQ = 2−ΔΔCT. *, P < 0.05, compared with the mock-infected group. (B) The surface expression of Rae1, H60, and MULT1 on IECs was analyzed by flow cytometry. (Left) Data shown are representative of three independent experiments. (Right) A statistical analysis of Rae1, H60, and MULT1 expression is also displayed. **, P < 0.01, compared with the mock-infected group.
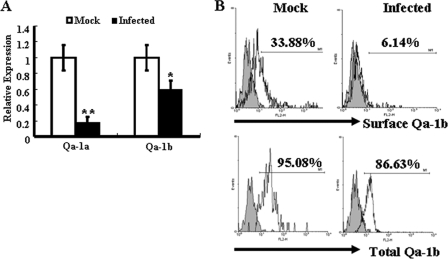
As shown in Fig. 4A, NKG2A transcripts were detected in control CD8+ TCRγδ+ iIELs and in S. Typhimurium-infected CD8+ TCRγδ+ iIELs, with no differences. Expression of Qa-1, the ligand for the inhibitory receptor CD94/NKG2A, has been shown in intestinal epithelial cells (6). Therefore, changes in Qa-1 expression were investigated in order to identify a possible role for this molecule in the regulation of the immune responses in intestine. Real-time PCR analysis showed that mRNA expression of Qa-1a and Qa-1b was downregulated significantly in IECs following S. Typhimurium infection (Fig. 6A). Flow cytometry analysis showed that total Qa-1b was highly expressed in both control IECs and S. Typhimurium-infected IECs. However, surface expression of Qa-1b by IECs following S. Typhimurium infection was noticeably downregulated (Fig. 6B).
Fig 6.
S. Typhimurium infection decreases Qa-1 expression on IECs. IECs were isolated from mock-infected or Salmonella-infected mice at day 5 after infection. (A) The mRNA levels of Qa-1a and Qa-1b were determined by quantitative PCR. Results are shown as the relative increase in the expression of Qa-1a or Qa-1b compared with the level of expression of β-actin. Gene expression values were then calculated based on the ΔΔCT method, using the mean of results with mock-infected IECs as a calibrator. Relative quantities (RQs) were determined using the equation RQ = 2−ΔΔCT. **, P < 0.01; *, P < 0.05, compared with the mock-infected group. (B) The surface expression and total expression of Qa-1b were analyzed by flow cytometry. The total expression of Qa-1b consisted of the surface and intracellular expression of Qa-1b. Data shown are representative of three independent experiments. *, P < 0.05, compared with the isotype-matched control group.
The blockade of NKG2D recognition inhibits the cytotoxicity of IELs against infected IECs and increases the number of S. Typhimurium bacteria in the small intestine.
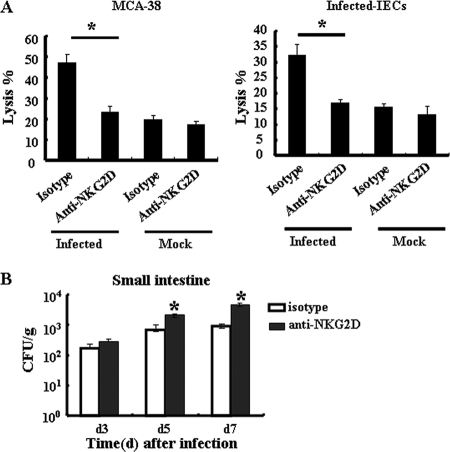
Analysis of the cytolytic potential of CD8+ TCRγδ+ iIELs against MCA-38 cells or IECs showed that CD8+ TCRγδ+ iIELs isolated from S. Typhimurium-infected mice exhibited much stronger cytotoxicity against MCA-38 cells or S. Typhimurium-infected IECs than CD8+ TCRγδ+ iIELs isolated from mock-infected mice (Fig. 7A). We further investigated whether NKG2D recognition is involved in the cytotoxicity of CD8+ TCRγδ+ iIELs against S. Typhimurium-infected IECs and bacterial clearance. As shown in Fig. 7A, blockade of NKG2D by addition of saturating concentrations of anti-NKG2D Ab attenuated the cytotoxicity of CD8+ TCRγδ+ iIELs against S. Typhimurium-infected IECs or MCA-38 cells significantly (P < 0.05, compared with an isotype-matched control), indicating the involvement of NKG2D recognition in this process. Furthermore, the blockade of NKG2D recognition in vivo resulted in an increase in numbers of S. Typhimurium bacteria in the small intestine at day 5 and day 7 after infection (Fig. 7B). Taken together, these results indicated that NKG2D recognition is involved in resistance to S. Typhimurium infection in the small intestine.
Fig 7.
Blockade of NKG2D recognition inhibits the cytotoxicity of IELs against infected IECs and increases the numbers of S. Typhimurium bacteria in the small intestine. (A) TCRγδ+ iIELs were enriched to up to 90% purity from the iIELs by MACS. The cytotoxicity of TCRγδ+ iIELs against MCA-38 cells or S. Typhimurium-infected IECs was detected at an E:T ratio of 25:1 by MTT assay. Purified TCRγδ+ iIELs were cocultured with saturating concentrations of anti-NKG2D Ab (20 μg/ml) or isotype-matched control antibody and then washed for use as effector cells. (B) C57BL/6 mice (n = 3) were pretreated with anti-NKG2D MAb or control IgG MAb (400 μg i.v.) per mouse at 24 h before S. Typhimurium infection. At the indicated time points after infection, the small intestines, with PP excised, were removed and homogenized and numbers of CFU per gram of organ were determined. Data are expressed as the means ± SD of results from at least three separate experiments. *, P < 0.05, compared with the isotype-matched control group.
TCRγδ+ cell-depleted mice display accelerated bacterial growth following oral infection with S. Typhimurium.
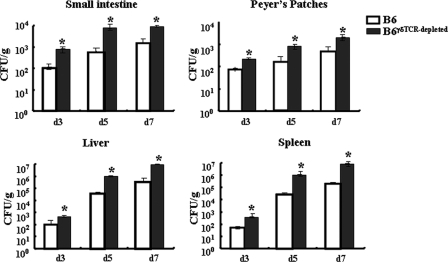
To further examine the role of TCRγδ+ cells in controlling early stages of S. Typhimurium infection, we depleted TCRγδ+ cells from wild-type B6 mice using purified anti-mouse TCRγδ MAb (UC7-13D5) and measured the bacterial loads in the small intestine, PP, liver, and spleen following oral infection. FACS analysis of iIELs showed an expected number of TCRγδ+ iIELs (43.2%) from B6 mice and a reduction of this proportion to 10.1% in TCRγδ+ cell-depleted mice (see Fig S3 in the supplemental material). As shown in Fig. 8, viable bacteria can be detected as early as day 3 postinfection in the small intestine and PP of B6 mice and TCRγδ+ cell-depleted mice. In TCRγδ+ cell-depleted mice, bacterial loads were consistently higher than in B6 mice. Similar observations were made when bacterial loads in liver and spleen were measured. These results indicated that TCRγδ+ iIELs indeed play important roles in protection from S. Typhimurium infection.
Fig 8.
In vivo S. Typhimurium growth following oral infection. C57BL/6 mice (n = 3) were pretreated with anti-TCRγδ Ab or control IgG Ab (i.p.) at 200 μg per mouse 3 days before S. Typhimurium infection. At the indicated time points after infection, the small intestine, PP, spleens, and livers were removed and homogenized and numbers of CFU per gram of organ were determined. Data are expressed as the means ± SD of results from three separate experiments. *, P < 0.05, compared with the isotype-matched control group.
DISCUSSION
This study provided a phenotypic analysis of iIEL perturbations induced by a food-borne pathogen, Salmonella, and the involvement of CD8+ TCRγδ+ iIELs in the eradication of infected epithelial cells. The prevalent route of Salmonella infection is through the ingestion of contaminated food; therefore, this issue was investigated in a mouse model of oral infection. Total iIELs and CD8+ TCRγδ+ iIELs increased in the small intestine following oral infection with S. Typhimurium. Furthermore, S. Typhimurium infection stimulated the activation of CD8+ TCRγδ+ iIELs, CD8αα+ iIELs, and CD8αβ+ iIELs, and the NK cell-like cytotoxicity of iIELs, particularly CD8+ TCRγδ+ iIELs, against the tumor cell line MCA-38 and infected IECs was augmented. These observations suggest that iIELs, particularly CD8+ TCRγδ+ iIELs, are critical for the detection of pathogenic bacteria and are involved in the eradication of infected epithelial cells, thus providing protection against invading pathogens.
Although they constitute a small part of the circulating lymphocyte population, γδ T cells are found in high abundance on mucosal and epithelial surfaces and form the first line of defense against pathogens. TCRγδ+ iIELs have been reported to be involved in the repair of damage to epithelial cells of the intestine elicited during protective immune responses, possibly by production of keratinocyte growth factors, thereby promoting epithelial growth and limiting further pathogen spread (3, 8, 9). They have also been found to regulate protective mucosal immune responses exerted by CD8αβ IELs (2). In the current study, S. Typhimurium infection stimulated the activation of CD8+ TCRγδ+ iIELs, increased the percentage and absolute numbers of CD8+ TCRγδ+ iIELs, and enhanced the cytolytic activity of CD8+ TCRγδ+ iIELs against MCA-38 cells or S. Typhimurium-infected IECs. Furthermore, TCRγδ+ cell depletion accelerated bacterial loads in small intestine as well as in liver and spleen following oral infection with S. Typhimurium. These observations indicate the important roles of TCRγδ+ iIELs in protecting against Salmonella infection and in limiting further pathogen spread. The percentage and absolute numbers of CD8αα+ iIELs and CD8αβ+ iIELs were not changed following Salmonella infection. However, activation of CD8αα+ iIELs and CD8αβ+ iIELs (Fig. 2C), in addition to increased expression of NKG2D, FasL, and IFN-γ by CD8αα+ IELs, was detected (Fig. S2). These results indicated the involvement of CD8αα+ iIELs in the clearance of S. Typhimurium. Owing to the predominant expression of CD8αα by TCRγδ+ iIELs, the overlap in CD8+ TCRγδ+ iIEL and CD8αα+ iIEL subsets might account for these results. These results are consistent with those from other studies, demonstrating the role of γδ T cells in defending against and clearing Salmonella infection (6, 20) and other intracellular pathogens, especially Listeria monocytogenes (10, 21, 29). In agreement with this, γδ T cells were also found to eradicate infected or transformed epithelial cells, thereby preventing systemic dissemination of infectious agents or malignant cells (8).
Type b iIELs (CD8αα+ IELs and TCRγδ + IELs) exhibit innate immune properties. These cells are primed directly by epithelial cells rather than by recognition of antigen in the context of major histocompatibility complex (MHC) molecules on antigen-presenting cells. Furthermore, expression of NK cell-like receptors, such as NKG2D, and direct cytolytic activity are observed (8). In our former study, we reported the involvement of the interaction of NKG2D in CD8αα+ iIELs with Rae1 expressed on IECs in iIEL-mediated epithelial destruction by genomic rotavirus double-stranded RNA (dsRNA) (30). Here, NKG2D was shown to be involved in the clearance of S. Typhimurium-infected IECs by CD8+ TCRγδ+ iIELs. Expression of NKG2D and cytotoxicity-related molecules, such as FasL on CD8+ TCRγδ+ iIELs, was upregulated following S. Typhimurium infection, while NKG2D was minimally expressed on iIELs in normal mice. In contrast, S. Typhimurium infection promoted MULT1 expression on IECs. It can be speculated that the interaction of increased NKG2D on CD8+ TCRγδ+ iIELs and enhanced MULT1 on IECs enhances activation but that downregulation of Qa-1 on IECs attenuates the inhibitory signal, thus leading to the cytotoxicity of iIELs against infected IECs. The involvement of NKG2D and its ligands in the cytolysis of CD8+ TCRγδ+ iIELs was confirmed by neutralizing antibody blockade of these interactions both in vitro and in vivo (Fig. 7). This is in accordance with reports that intestinal epithelial γδ T cells recognize stress-induced major histocompatibility complex class I chain-related A and B (MICA and MICB, respectively), the human forms of NKG2D ligands, on IECs and serve as an immune surveillance mechanism for the detection of infected or transformed IECs (7, 11, 12). To our knowledge, this is the first description of murine NKG2D ligand MULT1 induction by an invading pathogen and the involvement of NKG2D and MULT1 in the clearance of infected IECs. In addition, IFN-γ was reported to play a critical role in intestinal immunity against Salmonella infection (1, 18). In accordance with this, we found that Salmonella infection increased serum IFN-γ levels (Fig. 3C) and IFN-γ production by iIELs (Fig. 3B), particularly CD8+ TCRγδ+ (Fig. 4B) and CD8αα+ (see Fig. S2 in the supplemental material) iIELs. We proposed that, in addition to iIELs being cytotoxic to infected IECs, the IFN-γ secreted by iIELs contributes to the clearance of S. Typhimurium.
The nonclassical MHC molecule Qa-1 is reported to be highly expressed by IECs and can be directly recognized by TCRγδ T cells. On the one hand, Qa-1, acting as an antigen-presenting molecule, binds and presents peptides derived from Salmonella to iIELs; on the other hand, it acts as a ligand of the inhibitory receptor CD94/NKG2A and transduces the inhibitory signal, thus limiting cell activation (14–16, 26). Although there is speculation that Qa-1 surface expression by intestinal epithelial cells is upregulated during S. Typhimurium infection, perhaps due to the increased supply of a relevant Qa-1 binding peptide (6), Davies et al. just showed the high expression of Qa-1 in intestine but did not display the results of the upregulation of surface Qa-1 after S. Typhimurium infection in their paper. In the current study, we found that S. Typhimurium infection downregulated the surface expression of Qa-1b but that the total Qa-1b level did not change, with high expression occurring in both control IECs and S. Typhimurium-infected IECs (Fig. 6). We speculate that S. Typhimurium infection might either induce the internalization of Qa-1b or inhibit the transportation of Qa-1b to the cell surface. Owing to the innate immune properties of CD8+ TCRγδ+ iIELs, their activation is at least partly determined by the balance between activating and inhibitory signals. As Qa-1 is the ligand of inhibitory receptor NKG2A, its downregulated expression on IECs may attenuate the inhibitory signal recognized by CD8+ TCRγδ+ iIELs, leading to a reduction in the activation threshold of iIELs. In accordance with this, the human counterpart of Qa-1, HLA-E, was found to participate in a negative-feedback loop on cytotoxic T lymphocyte (CTL) responses in which TCR stimulation upregulates NKG2A expression and, in turn, NKG2A downregulates TCR activation by modifying antigen activation thresholds (12, 13, 27). The decreased expression of Ly49E and Ly49F on CD8+ TCRγδ+ iIELs may also contribute to this effect. We proposed that infection by S. Typhimurium alters the homeostatic balance regulating the activation and cytolytic activity of iIELs, particularly CD8+ TCRγδ+ iIELs. The data presented here suggest that this occurs by induction of MULT1 expression and inhibition of Qa-1 on IECs, in addition to enhanced expression of NKG2D on IELs.
In summary, the results of the present study showed that CD8+ TCRγδ+ iIELs are important in immunosurveillance of the intestinal mucosa and eradication of infected or malignant epithelial cells. Activation of iIELs is at least partly determined by the balance between activating and inhibitory signals. Understanding the mechanisms by which the mucosal immune system detects and eradicates invading pathogens or malignancy while maintaining tolerance to commensal organisms will be beneficial for the development of new therapeutic interventions targeting intestinal infections and tumors.
Supplementary Material
ACKNOWLEDGMENTS
We thank Hong Meng from the Shandong Academy of Medical Sciences for generously providing the virulent S. Typhimurium strain (CMCC, accession no. 50222).
This work was supported by grants from the National Natural Science Foundation of China (90713033, 30901307, and 30772497), the National 973 Basic Research Program of China (2007CB815803), and the National 115 Key Project for HBV Research (2008ZX10002-008).
Footnotes
Published ahead of print 5 December 2011
Supplemental material for this article may be found at http://iai.asmusa.org/.
REFERENCES
- 1. Bao S, Beagley K, France M, Shen J, Husband A. 2000. Interferon-γ plays a critical role in intestinal immunity against Salmonella typhimurium infection. Immunology 99:464–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bhagat G, et al. 2008. Small intestinal CD8+ TCRγδ + NKG2A+ intraepithelial lymphocytes have attributes of regulatory cells in patients with celiac disease. J. Clin. Invest. 118:281–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carding SR, Egan PJ. 2002. γδT cells: functional plasticity and heterogeneity. Nat. Rev. Immunol. 2:336–345 [DOI] [PubMed] [Google Scholar]
- 4. Coburn B, Li Y, Owen D, Vallance BA, Finlay BB. 2005. Salmonella enterica serovar Typhimurium pathogenicity island 2 is necessary for complete virulence in a mouse model of infectious enterocolitis. Infect. Immun. 73:3219–3227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Das G, Janeway CA. 1999. Development of CD8α/α and CD8α/β T cells in major histocompatibility complex class I-deficient mice. J. Exp. Med. 190:881–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Davies A, et al. 2004. Infection-Induced Expansion of a MHC class Ib-dependent intestinal intraepithelial γδT cell subset. J. Immunol. 172:6828–6837 [DOI] [PubMed] [Google Scholar]
- 7. Groh V, Steinle A, Bauser S, Spies T. 1998. Recognition of stress-induced MHC molecules by intestine epithelial γδ T cells. Science 279:1737–1740 [DOI] [PubMed] [Google Scholar]
- 8. Hayday A, Theodoridis E, Ramsburg E, Shires J. 2001. Intraepithelial lymphocytes: exploring the third way in immunology. Nat. Immunol. 2:997–1003 [DOI] [PubMed] [Google Scholar]
- 9. Hayday A, Tigelaar R. 2003. Immunoregulation in the tissues by γδT cells. Nat. Rev. Immunol. 3:233–242 [DOI] [PubMed] [Google Scholar]
- 10. Hiromatsu K, et al. 1992. A protective role of gamma/delta T cells in primary infection with Listeria monocytogenes in mice. J. Exp. Med. 175:49–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hue S, et al. 2004. A direct role for NKG2D/MICA interaction in villous atrophy during celiac disease. Immunity 21:367–377 [DOI] [PubMed] [Google Scholar]
- 12. Jabri B, Ebert E. 2007. Human CD8+ intraepithelial lymphocytes: a unique model to study the regulation of effector cytotoxic T lymphocytes in tissue. Immunol. Rev. 215:202–214 [DOI] [PubMed] [Google Scholar]
- 13. Jabri B, et al. 2002. TCR specificity dictates CD94/NKG2A expression by human CTL. Immunity 17:487–499 [DOI] [PubMed] [Google Scholar]
- 14. Jensen PE, Sullivan BA, Reed-Loisel LM, Weber DA. 2004. Qa-1, a nonclassical class I histocompatibility molecule with roles in innate and adaptive immunity. Immunol. Res. 29:81–92 [DOI] [PubMed] [Google Scholar]
- 15. Jiang H. 2005. The Qa-1 dependent CD8+ T cell mediated regulatory pathway. Cell. Mol. Immunol. 2:161–167 [PubMed] [Google Scholar]
- 16. Jiang H, Chess L. 2009. How the immune system achieves self-nonself discrimination during adaptive immunity. Adv. Immunol. 102:95–133 [DOI] [PubMed] [Google Scholar]
- 17. Kunisawa J, Takahashi I, Kiyono H. 2007. Intraepithelial lymphocytes: their shared and divergent immunological behaviors in the small and large intestine. Immunol. Rev. 215:136–153 [DOI] [PubMed] [Google Scholar]
- 18. Liu X, Lu R, Xia Y, Sun J. 2010. Global analysis of the eukaryotic pathways and networks regulated by Salmonella typhimurium in mouse intestinal infection in vivo. BMC Genomics 11:722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Macartney KK, Baumgart DC, Carding SR, Brubaker JO, Offit PA. 2000. Primary murine small intestinal epithelial cells, maintained in long-term culture, are susceptible to rotavirus infection. J. Virol. 74:5597–5603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mixter P, Camerini V, Stone B, Miller V, Kronenberg M. 1994. Mouse T lymphocytes that express a gamma delta T-cell antigen receptor contribute to resistance to Salmonella infection in vivo. Infect. Immun. 62:4618–4621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mombaerts P, Arnoldi J, Russ F, Tonegawa S, Kaufmann SH. 1993. Different roles of alpha beta and gamma delta T cells in immunity against an intracellular bacterial pathogen. Nature 365:53–56 [DOI] [PubMed] [Google Scholar]
- 22. Montufar-Solis D, Klein JR. 2006. An improved method for isolating intraepithelial lymphocytes (IELs) from the murine small intestine with consistently high purity. J. Immunol. Methods 308:251–254 [DOI] [PubMed] [Google Scholar]
- 23. Raulet DH. 2003. Roles of the NKG2D immunoreceptor and its ligands. Nat. Rev. Immunol. 3:781–790 [DOI] [PubMed] [Google Scholar]
- 24. Saphra I, Winter J. 1957. Clinical manifestations of salmonellosis in man: an evaluation of 7779 human infections identified at the New York Salmonella Center. N. Engl. J. Med. 256:1128–1134 [DOI] [PubMed] [Google Scholar]
- 25. Shires J, Theodoridis E, Hayday AC, Bridge L. 2001. Biological insights into TCRγδ+and TCRαβ+ intraepithelial lymphocytes provided by serial analysis of gene expression (SAGE). Immunity 15:419–434 [DOI] [PubMed] [Google Scholar]
- 26. van Hall T, Oliveira CC, Joosten SA, Ottenhoff TH. 2010. The other Janus face of Qa-1 and HLA-E: diverse peptide repertoires in times of stress. Microbes Infect. 12:910–918 [DOI] [PubMed] [Google Scholar]
- 27. Varthaman A, et al. 2010. Control of T cell reactivation by regulatory Qa-1-restricted CD8+ T cells. J. Immunol. 184:6585–6591 [DOI] [PubMed] [Google Scholar]
- 28. Yamamoto M, Fujihashi K, Kawabata K, McGhee JR, Kiyono H. 1998. A mucosal intranet: intestinal epithelial cells down-regulate intraepithelial, but not peripheral, T lymphocytes. J. Immunol. 160:2188–2196 [PubMed] [Google Scholar]
- 29. Yamamoto S, Russ F, Teixeira HC, Conradt P, Kaufmann SH. 1993. Listeria monocytogenes-induced gamma interferon secretion by intestinal intraepithelial gamma/delta T lymphocytes. Infect. Immun. 61:2154–2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhou R, Wei H, Sun R, Zhang J, Tian Z. 2007. NKG2D recognition mediates Toll-like receptor 3 signaling-induced breakdown of epithelial homeostasis in the small intestines of mice. Proc. Natl. Acad. Sci. U. S. A. 104:7512–7515 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.