Abstract
The most recently discovered secretion pathway in Gram-negative bacteria, the type VI secretion system (T6SS), is present in many species and is considered important for the survival of non-O1 non-O139 Vibrio cholerae in aquatic environments. Until now, it was not known whether there is a functionally active T6SS in wild-type V. cholerae O1 strains, the cause of cholera disease in humans. Here, we demonstrate the presence of a functionally active T6SS in wild-type V. cholerae O1 strains, as evidenced by the secretion of the T6SS substrate Hcp, which required several gene products encoded within the putative vas gene cluster. Our analyses showed that the T6SS of wild-type V. cholerae O1 strain A1552 was functionally activated when the bacteria were grown under high-osmolarity conditions. The T6SS was also active when the bacteria were grown under low temperature (23°C), suggesting that the system may be important for the survival of the bacterium in the environment. A test of the interbacterial virulence of V. cholerae strain A1552 against an Escherichia coli K-12 strain showed that it was strongly enhanced under high osmolarity and that it depended on the hcp genes. Interestingly, we found that the newly recognized osmoregulatory protein OscR plays a role in the regulation of T6SS gene expression and secretion of Hcp from V. cholerae O1 strains.
INTRODUCTION
The severe diarrheal disease cholera is caused by Vibrio cholerae bacteria of serogroups O1 and O139 that carry the ctxAB and tcp genes, encoding cholera toxin and toxin-coregulated pili, respectively (22). These bacteria have been the cause of seven pandemics since 1817 (12, 38, 42). Non-O1, non-O139 V. cholerae (NOVC) constitutes a large group of V. cholerae bacteria found as environmental isolates. Typically, they lack the ctx and tcp genes and do not cause cholera but have recently been emerging as potential extraintestinal pathogens. NOVC isolates possess a protein secretion system, the type VI secretion system (T6SS), which appears to play an important role in the bacterium's environmental survival by promoting killing of predator organisms, like amoebas (37).
The T6SS is present in several Gram-negative bacterial species ranging from environmental to pathogenic bacteria and is involved in a variety of cellular processes (3–5, 10, 13, 43). It can secrete certain effector proteins into the extracellular milieu and/or translocate them into the eukaryotic host cell cytoplasm (31, 32, 36, 37). Intriguingly, a role for T6SS in interbacterial competition was recently described in Pseudomonas aeruginosa, Burkholderia thailandensis, and a NOVC isolate (19, 24, 44). Although little is known about the contribution of the T6SS to virulence in general, expression of T6SS loci is precisely modulated to adapt T6SS production to the specific needs of and conditions encountered by individual bacteria (3, 4). Two T6SS-associated proteins, hemolysin-coregulated protein (Hcp) and valine-glycine repeat protein G (VgrG), have recently been characterized in NOVC isolates and in P. aeruginosa (16, 36). However, the conditions that result in activation of the T6SS of wild-type isolates of the O1 serotype V. cholerae strains have not been identified so far, and therefore, this T6SS cluster was considered to be nonfunctional (37). Despite the notion of an inactive T6SS, our recent studies showed that serotype O1 strains of V. cholerae in fact do produce Hcp when the bacteria are cultivated under standard laboratory conditions (20). Furthermore, we observed that expression of hcp genes was positively and negatively regulated by the quorum-sensing regulators HapR and LuxO, respectively, thereby also including the RNA binding protein Hfq in the regulation (20). In addition, Hcp expression in the serotype O1 strain required the alternative sigma factor RpoN and the cyclic AMP-cyclic AMP receptor protein (cAMP-CRP) global regulatory complex (20). Subsequently, using a luxO mutant V. cholerae O1 serotype strain and transposon insertion mutagenesis, a gene denoted tsrA was identified as important for Hcp expression and secretion (56). These findings prompted us to carry out further analysis of the potential Hcp secretion from the wild-type O1 V. cholerae strain A1552, with the aim of identifying the environmental condition(s) that would allow its T6SS-dependent transport. Our results demonstrate that the T6SS of wild-type V. cholerae O1 strains is functional and that its expression is controlled by specific environmental conditions in a pathoadaptive fashion.
MATERIALS AND METHODS
Bacterial strains, culture conditions and plasmids.
The bacterial strains and plasmids used in this study are listed in Table 1. Bacterial strains were grown at 37°C or 23°C with shaking in Luria-Bertani (LB) broth (pH 7.4) supplemented, as appropriate, with reagents as mentioned for each experiment.
Table 1.
Bacterial strains and plasmids in this study
| Strain/plasmid | Relevant genotype/phenotype | Reference/source |
|---|---|---|
| Bacteria | ||
| E. coli | ||
| DH5α | λ− φ80dlacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17(rK− mK−) supE44 thi-1 gyrA relA1 | 18 |
| SM10λpir | thi thr leu tonA lacY supE recA::RP4-2 Tc::Mu Km λpir | 28 |
| TOP10 | F−mcrA Δ(mrr-hsdRMS-mcrBC) ϕ80lacZΔM15 ΔlacX74 nupG recA1 araD139 Δ(ara-leu)7697 galE15 galK16 rpsL(StrR) endA1 λ− | Invitrogen |
| MC4100 | F−araD139 Δ(argF-lac)U169 rpsL150(strR) relA1 deoC1 rbsR fthD5301 fruA25 λ− | 7 |
| V. cholerae | ||
| A1552 | O1 El Tor Inaba; Rifr | 55 |
| E7946 | O1 El Tor Ogawa | 26 |
| 93Ag49 | O1 El Tor Inaba | Argentina, 1993 |
| V:6/04 | O9 serotype | Clinical isolate (Sweden, 2005) |
| V52 | O37 serotype | 57 |
| Δvca0107 | Δvca0107 derivative of A1552 | This study |
| Δvca0115 | Δvca0115 derivative of A1552 | This study |
| Δvca0120 | Δvca0120 derivative of A1552 | This study |
| Δvca0121 | Δvca0121 derivative of A1552 | This study |
| ΔvgrG-3 | ΔvgrG-3 derivative of A1552 | This study |
| Δhcp | Δhcp derivative of A1552 | 20 |
| ΔoscR | ΔoscR derivative of A1552 | This study |
| ΔompR | ΔompR derivative of A1552 | This study |
| ΔenvZ | ΔenvZ derivative of A1552 | This study |
| Plasmids | ||
| pGEM-T Easy | TA cloning vector plasmid; Apr | Promega |
| pCR4-TOPO | TA cloning vector, Kmr; Apr | Invitrogen |
| pMMB66EH | Ptac expression vector; Apr | 14 |
| pBAD18 | Arabinose-inducible cloning vector; Apr | 15 |
| pBR322 | Cloning vector plasmid; Apr | 6 |
| pCVD442 | Apr positive-selection suicide vector plasmid | 11 |
| pJEB642 | pMMB66EH carrying vca0107; Apr | This study |
| pBAD18-vgrG-3 | pBAD18 carrying vgrG-3; Apr | This study |
| pBAD18-oscR | pBAD18 carrying oscR; Apr | This study |
| pΔvca0107 | pCVD442-based suicide plasmid for generating Δvca0107; Apr | This study |
| pΔvca0115 | pCVD442-based suicide plasmid for generating Δvca0115; Apr | This study |
| pΔvca0120 | pCVD442-based suicide plasmid for generating Δvca0120; Apr | This study |
| pΔvca0121 | pCVD442-based suicide plasmid for generating Δvca0121; Apr | This study |
| pΔvgrG-3 | pCVD442-based suicide plasmid for generating ΔvgrG-3; Apr | This study |
| pΔoscR | pCVD442-based suicide plasmid for generating ΔoscR; Apr | This study |
| pΔenvZ | pCVD442-based suicide plasmid for generating ΔenvZ; Apr | This study |
| pΔompR | pCVD442-based suicide plasmid for generating ΔompR; Apr | This study |
Construction of expression plasmids.
The PCR primers used for obtaining the plasmid clones with a wild-type locus allowing complementation tests of the vca0107, vgrG-3, and oscR mutant are listed in Table 2. The DNA fragment containing the vca0107, vgrG-3, and oscR genes was obtained after amplification by PCR using wild-type V. cholerae chromosomal DNA as a template. The PCR product was purified from an agarose gel and ligated into the TA cloning vector pCR4-TOPO or the pGEM-T Easy vector. After amplification in the Escherichia coli strain TOP10 or DH5α, the plasmid was isolated with a Qiaprep Spin Miniprep kit (Qiagen). The vca0107+ clone in pMMB66EH and the vgrG-3+ or oscR+ clone in pBAD18 were electroporated into Δvca0107, ΔvgrG-3, and ΔoscR mutants, respectively. As a negative control, the expression vectors pMMB66EH and pBAD18 without an insert were introduced into the mutant strains.
Table 2.
Primers used in this study
| Primer | Sequence | Source |
|---|---|---|
| Primers for deletion mutant construction | ||
| vca0107-A | 5′CGCTCTAGACGATCGCTTGAGTCATGTCTA3′ | This study |
| vca0107-B | 5′CCCATCCACTATAAACTAACAGGGAGCTACACTTCCTTCTTT3′ | This study |
| vca0107-C | 5′TGTTAGTTTATAGTGGATGGGCTGCTCAGTGGTCAAGAAGAG3′ | This study |
| vca0107-D | 5′CGCTCTAGAGGTTCGCCACCAAATTGACCA3′ | This study |
| vca0115-A | 5′GTTCCATTCTTTCAGCAGC3′ | This study |
| vca0115-B | 5′CCCATCCACTATAAACTAACACACATCGTCGAACAACAAAC3′ | This study |
| vca0115-C | 5′TGTTAGTTTATAGTGGATGGGGAAGGCAAATACCGTGTG3′ | This study |
| vca0115-D | 5′CGCTCTAGACACCTGATCGACTTCCAG3′ | This study |
| vca0120-A | 5′CGCTCTAGACGCGTCAAGGATTACCAAGA3′ | This study |
| vca0120-B | 5′CCCATCCACTATAAACTAACACCACCAAATGGCAACGTTCA3′ | This study |
| vca0120-C | 5′TGTTAGTTTATAGTGGATGGGGCGAGTCAAACGTCGGTTGAT3′ | This study |
| vca0120-D | 5′CGCTCTAGACCCGAGTACACCTAGAGTGAA3′ | This study |
| vca0121-A | 5′CGCTCTAGACGTGAGGCTTTCTTCAACC3′ | This study |
| vca0121-B | 5′CCCATCCACTATAAACTAACAGGACATCACAACTTCGTC3′ | This study |
| vca0121-C | 5′TGTTAGTTTATAGTGGATGGGGAGCAAACCGCGCTGTTC3′ | This study |
| vca0121-D | 5′CGCTCTAGACTGTAACCTTGCCATGCTG3′ | This study |
| vgrG3-A | 5′CGCTCTAGATCATTAGAAGAGTTCGCGGTC3′ | This study |
| vgrG3-B | 5′CCCATCCACTATAAACTAACAGCGTACGACGAGGGATTCAT3′ | This study |
| vgrG3-C | 5′TGTTAGTTTATAGTGGATGGGCATCATGGTGGAGTGATTTA3′ | This study |
| vgrG3-D | 5′CGCTCTAGACCGTAGTAAACCCAAAGCATT3′ | This study |
| oscR-A | 5′CGCTCTAGAGTATGGAAAGACCTCGGT3′ | This study |
| oscR-B | 5′CCCATCCACTATAAACTAACACATCAGCAATTGCAGTGA3′ | This study |
| oscR-C | 5′TGTTAGTTTATAGTGGATGGGCATGTATTGCAAGCCGC3′ | This study |
| oscR-D | 5′CGCTCTAGACGCGATAAAGCAAAGTGA3′ | This study |
| envZ-A | 5′CGCTCTAGATGCCGATGATTACCTGCCTAA3′ | This study |
| envZ-B | 5′CCCATCCACTATAAACTAACATCGCATAAGGCACCTAAATGT3′ | This study |
| envZ-C | 5′TGTTAGTTTATAGTGGATGGGCAGCAAAAGGGCTAACCTAAG3′ | This study |
| envZ-D | 5′CGCTCTAGAAACAGCTTTCCAAACTCCACC3′ | This study |
| ompR-A | 5′CGCTCTAGAATTGATCGTCGTGTACGCTTC3′ | This study |
| ompR-B | 5′CCCATCCACTATAAACTAACACACCATGATCCCACCTAACT3′ | This study |
| ompR-C | 5′TGTTAGTTTATAGTGGATGGGAACTAACTTCTTTTAGGCACG3′ | This study |
| ompR-D | 5′CGCTCTAGATGAGAGTGGAATACGCAGCA3′ | This study |
| Primers for cloning wild-type allelesa | ||
| vca0107 | F: 5′GAATTCATGTCTAAAGAAGGAAGTGTA3′ (EcoRI) | This study |
| R: 5′GGATCCTTACGCTTGTGGCTCTTCTTG3′ (BamHI) | ||
| vgrG-3 | F: 5′GCGGTACCCGAACCATTTTCATCGAACT3′ (KpmI) | This study |
| R: 5′GCTCTAGATTATTTTATATCAACCTCCAAACCG3′ (XbaI) | ||
| oscR | F: 5′GCGAATTCACTCATTGGGAATGCAATCC3′ (EcoRI) | This study |
| R: 5′GCTCTAGACTGGATGTACGATGTAAACG3′ (XbaI) | ||
| Primers for qRT-PCR | ||
| vca0107-1 | 5′AGAAGGAAGTGTAGCTCC3′ | This study |
| vca0107-2 | 5′ACTGTTGCACGCTCTTC3′ | This study |
| vca0108-1 | 5′ATGTCTACGACTGAAAAGG3′ | This study |
| vca0108-2 | 5′AGTGTTGTGAACCCATAAG3′ | This study |
| vca0115-1 | 5′CCACGACCAAGACTACTGG3′ | This study |
| vca0115-2 | 5′GATCTCTTCAATCGTCTGA3′ | This study |
| vca0120-1 | 5′GTTGGAATAGTAAGGCGG3′ | This study |
| vca0120-2 | 5′CCATGATCGACTCCAGC3′ | This study |
| vca0121-1 | 5′TGCTACCGATTAACCAATG3′ | This study |
| vca0121-2 | 5′GTTACAGGCCAGTTGTTC3′ | This study |
| vgrG1-1 | 5′GCGACATTAGCGTACAGC3′ | This study |
| vgrG1-2 | 5′GCCAGTTGCACTTCGTAG3′ | This study |
| vgrG2-1 | 5′GACATTAGCGTACAGCATTG3′ | This study |
| vgrG2-2 | 5′GATACCCGACTCGCCAGT3′ | This study |
| hcp-1 | 5′TCTCTATCGAAGGCCAAAC3′ | This study |
| hcp-2 | 5′GATTGTGGGTCAGTCGGTA3′ | This study |
| oscR-1 | 5′CAGCAAACCAAGTTAATGA3′ | This study |
| oscR-2 | 5′GTTCCATTCTTTCAGCAGC3′ | This study |
| ompT-F | 5′CTGATGCACTACACGATTCTC3′ | This study |
| ompT-R | 5′CCAAATGTTGCACCTACATACA3′ | This study |
| tmRNA-F | 5′TCGCAAACGACGAAAACTACG3′ | 49 |
| tmRNA-R | 5′TAGATCTCGCGCTTCATCCC3′ | 49 |
F, forward; R, reverse. In each sequence, the restriction site for the restriction enzyme in parentheses is italicized.
SDS-PAGE and immunoblot analyses.
To determine the levels of protein production and secretion, bacterial strains were grown in LB medium containing 85 mM or 340 mM NaCl. The bacterial strains were grown to an optical density at 600 nm (OD600) of 2.0. Total bacterial viable-cell counts at OD600 were similar (A1552, 1.41 × 109 CFU/ml; V6, 1.49 × 109 CFU/ml; V52, 1.12 × 109 CFU/ml). One-millilliter bacterial samples were taken and centrifuged at 18,000 × g for 2 min. The pellets were suspended with 200 μl of sample buffer containing 10% glycerol, 0.05% bromophenol blue, 2% SDS, 5% 2-mercaptoethanol, and 10 mM Tris-HCl, pH 6.8, and the suspension was heat treated in a boiling-water bath for 5 min. Of the boiled 200-μl suspension, 2.5 μl was used as a whole-cell sample for SDS-PAGE immunoblot analysis; 500 μl of the culture supernatant fluid was precipitated with 10% trichloroacetic acid (TCA). The precipitates were resuspended in 40 μl of the same sample buffer and boiled for 5 min; 10 μl out of the 40-μl sample preparation was used as a supernatant sample for SDS-PAGE immunoblot analysis. The protein samples were separated by SDS-PAGE (23). Western blot analyses were performed as described previously (51, 53) using anti-Hcp polyclonal antiserum (20), anti-Crp polyclonal antiserum (2), and anti-β-lactamase polyclonal antiserum (33).
RNA extraction and qRT-PCR.
RNA extraction and quantitative reverse transcription (qRT)-PCR were performed as described previously (53). Three independent samples were tested in triplicate. For each sample, the mean cycle threshold of the test transcript was normalized to that of transfer-messenger RNA. In each case, the level of the wild type under low osmolarity (85 mM NaCl) was set to 1. Transcription of the ompT gene was used as a control representing gene expression affected by osmolarity. The recorded values representing transcription of T6SS genes ranged from 0.019 to 0.065 in low osmolarity and from 0.027 to 0.073 in high osmolarity, respectively. The values were generally lower than the corresponding values (0.07 and 0.107, respectively) recorded for the osmoregulated (1.5-fold) ompT gene. The oligonucleotide primers used for qRT-PCR are listed in Table 2.
Bacterial killing assay of interbacterial virulence.
V. cholerae strains grown to an OD of 2.0 in LB medium were mixed with E. coli strain MC4100 grown to an OD of 0.20 in LB medium at a ratio of 1:3 (vol/vol). One hundred microliters of this mixture was inoculated onto a 0.22-μm nitrocellulose membrane (Millipore) placed on Luria-Bertani agar (LA) plates with different NaCl concentrations (85 and 340 mM). After 5 h of incubation at 37°C, bacterial cells were harvested from the filter, serial dilutions were spread on LA plates with streptomycin (50 μg/ml) to monitor surviving E. coli bacteria, and their colony-forming ability (CFU/ml) was quantified.
Genome database searches.
Genome database accession number searches were done for the complete whole-genome sequence of V. cholerae O1 biovar El Tor strain N16961 chromosomes I and II at the NCBI website (http://www.ncbi.nlm.nih.gov).
In-frame deletion mutant construction.
In-frame deletion mutants were constructed by procedures described previously (52, 53). The primers used are summarized in Table 2.
Serotyping.
NOVC strain V6 was serotyped using the somatic antisera prepared against the collection of 206 different V. cholerae strains (46).
RESULTS
Expression and secretion of Hcp in V. cholerae O1 and NOVC strains.
Recently, we found that in the V. cholerae wild-type O1 strain A1552, expression of the T6SS substrate Hcp was controlled by quorum sensing and the global regulators Crp and RpoN (20). To test if Hcp of a V. cholerae O1 strain can be secreted into the culture medium, culture supernatants of strain A1552 were examined by immunoblot analyses using polyclonal anti-Hcp antiserum. The NOVC strains V52 and V6 were used as controls, because Hcp is known to be efficiently secreted from these strains (37). Although a large amount of Hcp was detected in the whole-cell lysates (Fig. 1), Hcp was not detected in supernatants from V. cholerae O1 strain A1552, in contrast to those of the NOVC strains V52 and V6 (Fig. 1). Importantly, the absence of the cytosolic Crp protein in the culture supernatants indicated that the appearance of Hcp in this fraction was not a consequence of bacterial cell lysis (Fig. 1). This control was routinely used in the subsequent analyses of Hcp secretion.
Fig 1.
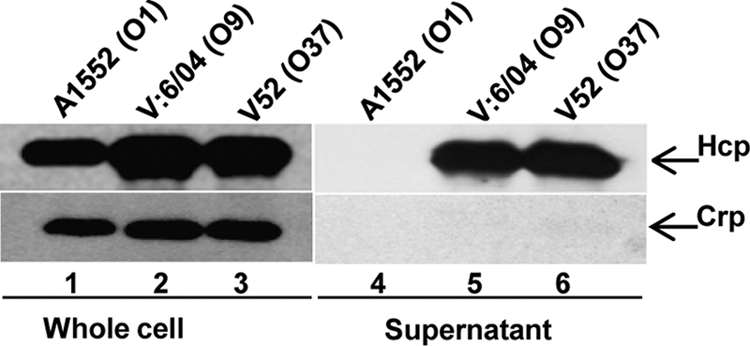
Immunoblot analyses of Hcp in whole-cell lysates and supernatants from V. cholerae strains. The bacterial strains were grown at 37°C in normal LB broth to an OD of 2.0. Lanes 1 to 3, whole-cell lysates; lanes 4 to 6, culture supernatants of V. cholerae O1 strain A1552, NOVC strain V6, and NOVC strain V52, respectively. The arrows indicate immunoblot reactivity to Hcp and Crp. The Crp protein was not detected in the culture supernatants, indicating that the secretion of Hcp was not a consequence of cell lysis.
Osmolarity-dependent secretion of Hcp from V. cholerae O1 strains.
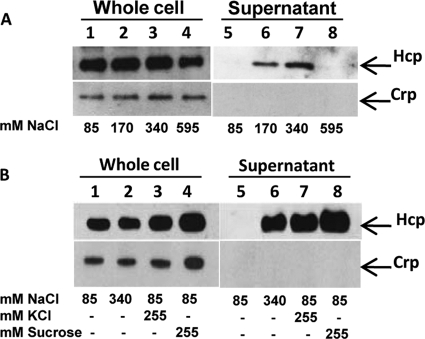
Next, we investigated if secretion of Hcp from V. cholerae O1 strains could be induced by growth under different environmental conditions. As V. cholerae normally resides in coastal and estuarine environments, we considered that salinity might influence the production and secretion of Hcp from V. cholerae O1 strains. We therefore compared Hcp expression and secretion after growth at 37°C in LB containing increasing concentrations of NaCl. In strain A1552, similar amounts of Hcp were produced in the cells regardless of salt concentrations (Fig. 2A, top, lanes 1 to 4). As shown (Fig. 2A, top, lanes 6 and 7), Hcp was readily detected in the culture supernatants of bacteria grown in LB supplemented with 170 or 340 mM NaCl. No Crp protein was detected in the culture supernatants (Fig. 2A, bottom, lanes 5 to 8), indicating that there was no cell lysis due to the increase in salinity. These results demonstrate that secretion of Hcp from the V. cholerae O1 strain A1552 occurred at 37°C in the presence of specific NaCl concentrations. We also tested if Hcp secretion could be stimulated by addition of KCl to the growth medium as an alternative anion supplement. As shown in Fig. 2B (top, lane 7), Hcp was also efficiently secreted into the culture supernatant after growth in KCl-supplemented LB.
Fig 2.
Effects of salinity and osmolarity on Hcp secretion from V. cholerae O1 strain A1552. (A) Bacterial samples from cultures grown at 37°C in LB containing different concentrations of NaCl were analyzed by SDS-PAGE and immunoblot analysis for Hcp and Crp in whole-cell lysates (lanes 1 to 4) and in culture supernatants (lanes 5 to 8). The arrows show the reaction bands of the Hcp and Crp proteins. (B) Analysis of samples from cultures grown at 37°C in LB supplemented with different amounts of salts and sucrose. Lanes 1 to 4, whole-cell lysates; lanes 5 to 8, culture supernatants.
Because we observed that Hcp secretion could be induced by high NaCl or KCl concentrations, we asked if secretion was dependent on osmolarity rather than salinity. To test this, A1552 was grown in LB supplemented with sucrose at a concentration of 255 mM to yield the same osmolarity as 340 mM NaCl. Immunoblot analysis revealed that Hcp was also efficiently secreted when the bacteria were grown at 37°C in LB containing 255 mM sucrose (Fig. 2B, top, lane 8). Our results suggest that the induction of Hcp secretion was not salinity dependent per se but was caused by the increased osmolarity.
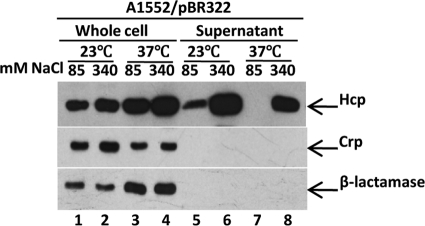
We also noted that temperature might influence the secretion of Hcp from V. cholerae O1. We compared Hcp expression and secretion after growth of strain A1552 at 23°C and 37°C in LB containing 340 mM NaCl (high osmolarity) or 85 mM NaCl (low osmolarity). Hcp was produced at both 23°C and 37°C, although slightly better at 37°C (Fig. 3, top, compare lanes 1 and 2 with lanes 3 and 4). Interestingly, in contrast to the case when bacteria were grown at 37°C, Hcp was also efficiently secreted from the V. cholerae O1 strain grown at 23°C in low osmolarity (Fig. 3, top, lanes 5 and 7). Moreover, under high-osmolarity conditions, we observed increased secretion of Hcp from bacteria grown at the lower temperature (Fig. 3, top, compare lanes 6 and 8). To be able to monitor any possible leakage of the periplasmic proteins into the supernatants, we used the V. cholerae O1 strain A1552 harboring the pBR322 plasmid expressing the periplasmic protein β-lactamase. As shown in Fig. 3 (bottom), no β-lactamase was detected in the culture supernatants, indicating that periplasmic proteins were not leaking into the culture supernatants under the specific conditions shown to induce secretion of Hcp.
Fig 3.
Effects of growth temperature and osmolarity on Hcp levels in whole-cell lysates and supernatants from V. cholerae O1 strain A1552. Bacteria were grown at 23°C or 37°C in LB containing 85 mM (the normal salinity concentration of LB medium) or in LB containing 340 mM NaCl (a high salinity concentration). SDS-PAGE and immunoblot analyses were performed using anti-Hcp, anti-Crp, and anti-β-lactamase antisera. Lanes 1 to 4, whole-cell lysates; lanes 5 to 8, culture supernatants. We used both a periplasmic marker (β-lactamase) and a cytoplasmic marker (Crp) as controls for the purity of the supernatant fractions, and since neither of these proteins was detected, we conclude that there was no significant periplasmic leakage or cell lysis.
To examine if the osmolarity-dependent secretion of Hcp was specific to the V. cholerae wild-type O1 strain A1552 or whether it might also occur for other V. cholerae O1 strains, we analyzed the secretion profiles from the V. cholerae O1 El Tor Ogawa strain E7946 and from the V. cholerae O1 El Tor Inaba strain 93Ag49. The results (see Fig. S1 in the supplemental material) showed that Hcp was efficiently secreted into the culture supernatants when the bacterial strains were grown in the presence of 340 mM NaCl, but not with 85 mM NaCl. Thus, we conclude that the secretion of Hcp under high-osmolarity conditions may be a feature common to V. cholerae wild-type O1 strains.
The T6SS in V. cholerae O1 strain A1552 plays a role in interbacterial virulence.
To test if the T6SS activity induced by high osmolarity might contribute to interbacterial virulence, we performed killing assays between the V. cholerae O1 strain A1552 and the E. coli K-12 strain MC4100. The results showed that the colony-forming ability of the E. coli strain was much reduced in the presence of V. cholerae under high-osmolarity conditions compared to low-osmolarity conditions (Fig. 4). The viability of E. coli was retained when an hcp double-deletion mutant derivative of V. cholerae strain A1552 was tested.
Fig 4.

Interbacterial virulence expressed by wild-type V. cholerae O1 strain A1552 toward E. coli strain MC4100 in a T6SS-dependent manner. Survival of the streptomycin-resistant E. coli strain MC4100 was determined by measuring CFU/ml following exposure to V. cholerae wild-type strain A1552 and the Δhcp derivative as described in Materials and Methods. The data represent three independent experiments. The error bars indicate standard deviations of the mean.
Analysis of gene expression from the putative T6SS cluster in V. cholerae O1 strain A1552.
In NOVC strains, the vas gene cluster-encoded T6SS mediates the extracellular secretion of four distinct proteins (Hcp, VgrG-1, VgrG-2, and VgrG-3) (37). To test and characterize the involvement of the putative T6SS gene cluster in the V. cholerae O1 strain A1552 for Hcp secretion, we constructed in-frame deletions in genes vca0107 (vipA) and vca0123 (vgrG-3) on the basis of the known sequence of the vas gene cluster in the genome of V. cholerae O1 strain N16961 (Fig. 5A). We analyzed Hcp secretion from the resulting mutant bacteria when grown under conditions permissive for Hcp secretion (high osmolarity, 37°C, or low osmolarity, 23°C). Under both of these conditions, secretion of Hcp was abolished in the Δvca0107 mutant (Fig. 5B; see Fig. S2A, top, lane 6, in the supplemental material), as well as the ΔvgrG-3 mutant (Fig. 5C; see Fig. S2B, top, lane 6, in the supplemental material). Secretion could be restored by introducing plasmids encoding wild-type vca0107 into the Δvca0107 mutant strain (Fig. 5B; see Fig. S2A, top, lane 7, in the supplemental material) and vgrG-3 into the ΔvgrG-3 mutant strain (Fig. 5C; see Fig. S2B, top, lane 7, in the supplemental material). To investigate the roles of other genes in the vas gene cluster in the expression and secretion of Hcp, we also constructed deletion mutant strains for vca0115 (vasF), vca0120 (vasK), and vca0121 (vasL), which are considered to be membrane-associated components of T6SS (47). As shown in Fig. 5D, the whole-cell level of Hcp appeared increased in the Δvca0115 mutant strain, whereas the level of secreted Hcp was reduced in comparison with the level in the wild-type strain. The Δvca0120 mutation totally abolished Hcp secretion. Hcp was secreted from the Δvca0121 mutant strain at a level similar to that from the wild-type strain.
Fig 5.
T6SS gene clusters on chromosomes I and II in V. cholerae O1. (A) The arrows indicate the presence and gene order of the vas cluster and the hcp-2 and vgrG-2 loci on chromosome II, as well as the hcp-1 and the vgrG-1 loci located on chromosome I. (B and C) Immunoblot analysis of Hcp secretion in Δvca0107 and ΔvgrG-3 mutants and the trans-complemented strains. Lanes 1 to 4, whole-cell lysates; lanes 5 to 8, culture supernatants. (D) Immunoblot analysis of Hcp production and secretion in some vas gene mutants compared with the wild-type strain. Lanes 1 to 4, whole cells; lanes 5 to 8, culture supernatants.
To investigate whether osmolarity can influence the expression of the putative T6SS genes in A1552, we performed qRT-PCR analyses. We used the transcription of the ompT gene as an osmolarity-responsive gene control of V. cholerae, since ompT expression is known to be regulated by the osmolarity of the culture medium (27). As expected, transcription of the ompT gene was increased under high-osmolarity conditions at 37°C (Fig. 6). As shown in Fig. 6, two selected T6SS genes at the beginning of the vas gene cluster (vca0107 and vca0108), as well as other genes representing the other end of the vas gene cluster (vca0115, vca0120, and vca0121) and the separately located hcp and vgrG clusters (vgrG-1, vgrG-2, and hcp), all showed increased levels of transcription when bacteria were grown at 37°C in LB containing 340 mM NaCl.
Fig 6.
qRT-PCR analyses of transcription from different T6SS genes, ompT, and oscR in V. cholerae O1 strain A1552 grown in LB with low or high osmolarity. qRT-PCR analyses were performed with samples from bacterial strains grown at 37°C. Data from triplicate samples were plotted to show the relative levels of transcripts, with the average level from low (85 mM NaCl) osmolarity samples in each case set to 1.0. The error bars indicate standard deviations of the mean.
The osmoregulator OscR controls T6SS activity in the V. cholerae O1 strain A1552 under low-osmolarity conditions.
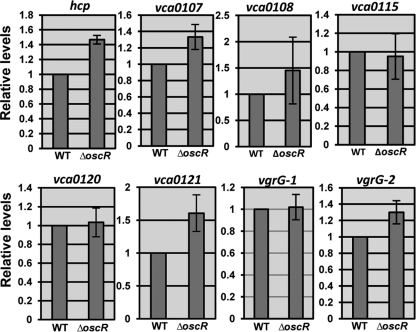
Bacteria like V. cholerae are known to have different sensing systems, making them capable of sensing modifications in their environment and generating appropriate responses. In general, signal transduction systems involving two-component sensor-regulator proteins constitute the basis of such sensing mechanisms. For example, the EnvZ-OmpR two-component system is an osmolarity-responsive signal transduction system that regulates gene expression in response to alterations in osmolarity in E. coli and several other eubacteria, including V. cholerae (21, 35, 50). Recently, a new osmoregulator, OscR, was described in the case of V. cholerae, and it was suggested to be a transcriptional regulator controlling an osmolarity adaptation response in a V. cholerae O1 strain (45). We therefore decided to test if any of the EnvZ-OmpR system and/or the OscR osmoregulator might affect the regulation of T6SS activity of V. cholerae. We constructed in-frame deletion mutations in the envZ, ompR, and oscR genes in V. cholerae strain A1552 and examined secretion of Hcp from each of the ΔenvZ, ΔompR, and ΔoscR mutant derivatives grown at 37°C under high-osmolarity conditions. The whole-cell level of Hcp (Fig. 7A) appeared somewhat upregulated in the ΔoscR mutant strain as estimated by our semiquantitative analysis (1.9-fold compared with the wild-type strain), whereas it was essentially unaltered in ΔenvZ and ΔompR strains (1.1-fold and 1.2-fold, respectively). In contrast to the parental strain, the ΔoscR mutant also showed secretion under nonpermissive (low-osmolarity; 37°C) conditions (Fig. 7B, compare lanes 5 and 6). However, Hcp was not detectably secreted from ΔenvZ and ΔompR mutant derivatives under these conditions (Fig. 7B, lanes 7 and 8). The apparent inhibitory effect of OscR on the T6SS of V. cholerae was restored by expressing oscR from pBAD18 (Fig. 7C, lanes 7 and 8). Furthermore, at 23°C, more Hcp was secreted from the ΔoscR derivatives than from the parental strain regardless of osmolarity (compare Fig. S3A and B, lanes 3 and 4, in the supplemental material). To investigate whether the oscR gene could influence the expression of the putative T6SS genes in A1552, we performed qRT-PCR analyses, and the transcription levels of some of the tested genes (vca0107, vca0108, vca121, vgrG-2, and hcp) were found to be higher in the ΔoscR mutant strain (Fig. 8). We also performed qRT-PCR analysis to compare the levels of oscR gene transcription under the different osmolarity conditions employed in the present study. As shown in Fig. 6, the transcription level of oscR was lower in bacteria grown under high-osmolarity conditions at 37°C. On the basis of these results, we suggest that osmolarity-dependent secretion of Hcp is due to negative regulation of the T6SS activity by the OscR osmoregulatory system in the V. cholerae O1 strain A1552.
Fig 7.
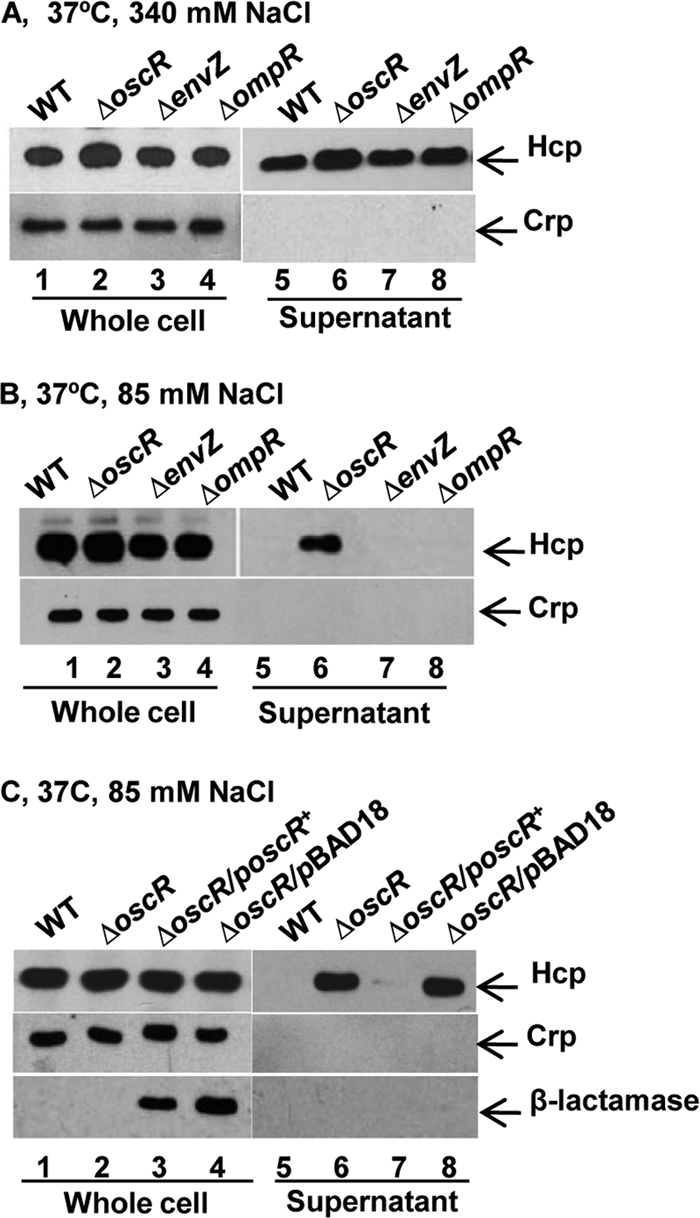
Immunoblot analysis of Hcp production and secretion by osmoregulatory mutants of V. cholerae O1 strain A1552. Shown are immunoblot analyses of whole-cell extracts and supernatants. Lanes 1 to 4, whole-cell lysates; lanes 5 to 8, culture supernatants. The arrows show reactivity to Hcp, Crp, and β-lactamase. (A) The indicated V. cholerae strains were grown in LB containing 340 mM NaCl at 37°C. (B) The indicated V. cholerae strains were grown in LB containing 85 mM NaCl at 37°C. (C) trans-complementation test of osmoregulated Hcp secretion in the ΔoscR mutant. Bacterial strains were grown in LB media containing 85 mM NaCl at 37°C.
Fig 8.
qRT-PCR analyses of transcription from some vas genes and hcp-vgrG loci in V. cholerae wild-type O1 strain A1552 and the ΔoscR derivative. The samples for qRT-PCR analysis were taken from the bacterial strains grown in LB with 85 mM NaCl at 37°C. Data from triplicate samples were plotted to show the relative levels of transcripts, with the wild-type level from low-osmolarity (85 mM NaCl) samples in each case set to 1.0. The error bars indicate standard deviations of the mean.
DISCUSSION
Our present findings establish that there is a fully functional T6SS in wild-type V. cholerae O1 strains, the etiological agents of human cholera. Expression of the T6SS was dependent on growth conditions that the V. cholerae bacteria are thought to experience outside the human host in the natural aquatic environments where temperature and salinity conditions may promote bacterial persistence, leading to outbreaks of cholera. Along with other environmental factors, salinity is thought to control the distribution of V. cholerae in aquatic environments and consequently might play a key role in the incidence and seasonal occurrence of the disease (8, 39, 40, 48). Our finding that activation of T6SS in the cholera bacterium depends on effects of salinity is therefore particularly intriguing because it is consistent with both the environmental persistence and outbreak potential of this waterborne pathogen and its ability to switch off expression of such properties upon entry into a mammalian host. Evidently, the regulatory system controlling activation of the T6SS in V. cholerae O1 strains has evolved to adapt to the salinity of aquatic environments (9, 48), so that effector proteins, like Hcp in V. cholerae O1 strain A1552, can promote bacterial survival. On the other hand, in contrast to the case of NOVC strains V6 and V52, the Hcp protein was not secreted from V. cholerae O1 strain A1552 when the bacteria were grown in ordinary (low-osmolarity) culture medium (LB) at 37°C (Fig. 1). Furthermore, we showed that Hcp secretion was strongly induced by low temperature. When strains were grown at 23°C, Hcp was efficiently secreted, similar to growth under higher osmolarity at 37°C. When we combined high-osmolarity and low-temperature conditions, we observed a seemingly additive effect on secretion of Hcp in comparison with either high-osmolarity or low-temperature conditions separately.
Our analysis established that expression of genes in the vas operon(s) was influenced by low-temperature and high-osmolarity conditions at the transcriptional level. The qRT-PCR analyses of the tested genes (vca0107, vca0108, vca0115, vca0120, vca0121, vgrG-1, vgrG-2, and hcp) showed increased levels of transcripts under high-osmolarity (340 mM NaCl) conditions (Fig. 6). Intriguingly, we found that transcription of genes in separate (vas, hcp, and vgrG) gene clusters were similarly upregulated under high-osmolarity conditions. However, it remains to be clarified if there is a direct relation between Hcp secretion and levels of transcription from these vas genes or how they may be joined in regulation.
Our investigation of the roles of several vas gene cluster genes in the expression and secretion of Hcp showed that there were different effects depending on the gene tested by construction of mutant derivatives (Fig. 5). The fact that Hcp was present at detectable levels irrespective of the osmolarity and temperature conditions presumably indicates that Hcp was readily produced but that the machine required for its assembly was not. It may highlight the fact that there could be a mechanism to tightly regulate the moment at which Hcp should be assembled.
We identified the newly recognized osmoregulatory protein OscR as a regulatory key factor in the osmoregulation of T6SS expression and secretion of Hcp from V. cholerae O1 strains. The results of our genetic analysis indicated that OscR mediates a negative regulatory effect on the T6SS under low osmolarity at 37°C. The oscR gene product is annotated as an IclR-type (isocitrate lyase) regulator (http://www.ncbi.nlm.nih.gov/gene/2612470). Members of the IclR family of transcriptional regulators are well represented and widely distributed in bacteria and can act as repressors, activators, or both (29). Proteins of the IclR family always have the helix-turn-helix DNA binding domain at their N termini (29). Although its structural and functional mechanisms have not been revealed, we hypothesize that OscR modulates the transcription of T6SS genes and thereby controls secretion of Hcp. Recently, Zheng et al. (56) obtained genetic evidence for a negative regulator (TsrA) of T6SS in a luxO mutant of V. cholerae O1 strain C6706, and the T6SS of V. cholerae appeared to be functionally active when tsrA was inactivated. Taken together, we propose that the T6SS of V. cholerae O1 strains is regulated in a pathoadaptive manner by alterations in osmolarity and temperature, whereas the T6SS seems to be constitutively active in NOVC strains. Our preliminary tests with the NOVC strain V52 indicated that expression and secretion of Hcp were not influenced by osmolarity (unpublished data). It remains to be elucidated whether the expression levels and/or activities of oscR and tsrA in NOVC strains are different from those of V. cholerae O1 strains or if other differences in regulatory networks make the T6SS constitutively active in NOVC strains. A summary of the regulators and environmental conditions that are proposed to regulate expression and secretion of the T6SS substrate Hcp in V. cholerae O1 strains is shown in Fig. 9.
Fig 9.
Schematic representation of the regulatory factors and pathways suggested to affect expression of V. cholerae T6SS genes.
Our findings show some resemblance to Yersinia pestis, in which the T6SS gene cluster is induced at low temperature and repressed at 37°C, suggesting that the T6SS in Y. pestis is probably more important for dissemination in the flea vector than in human host tissues (17, 30, 34, 41). The capacity to sense temperature changes and to respond by modifying the expression of genes encoding virulence factors is an important feature of many virulent bacteria. For example, in P. aeruginosa, the upregulation of genes associated with osmoprotectant synthesis, putative hydrophilins, and a type III protein secretion system (T3SS) after growth under steady-state hyperosmotic stress has been demonstrated (1). High-salt stress was also shown to be one of the environmental stimuli affecting expression of the Ysa T3SS in Yersinia enterocolitica (25, 54).
Our findings suggest that T6SS induction by these conditions is beneficial for V. cholerae and contributes to the fitness of O1 strains for survival in the natural environment. We hypothesize that the T6SS of V. cholerae O1 strains constitutes an important factor for competition with other microorganisms in the natural aquatic environment. Whereas the T6SS in the NOVC strains seemed to be active by default under most growth conditions, the V. cholerae O1 strains evidently have evolved a regulatory system, making T6SS expression conditional in a pathoadaptive fashion.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the Swedish Research Council, Carl Tryggers Stiftelse, the Swedish Foundation for International Cooperation in Research and Higher Education (STINT), and the Faculty of Medicine at Umeå University and was performed within the Umeå Centre for Microbial Research (UCMR) Linnaeus Program.
We thank Fitnat Yildiz for helpful suggestions and discussion on the OscR regulator and Amit Pal for V. cholerae strain V6 serotyping.
Footnotes
Published ahead of print 14 November 2011
Supplemental material for this article may be found at http://iai.asm.org/.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1. Aspedon A, Palmer K, Whiteley M. 2006. Microarray analysis of the osmotic stress response in Pseudomonas aeruginosa. J. Bacteriol. 188:2721–2725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Balsalobre C, et al. 2006. Release of the type I secreted alpha-haemolysin via outer membrane vesicles from Escherichia coli. Mol. Microbiol. 59:99–112 [DOI] [PubMed] [Google Scholar]
- 3. Bernard CS, Brunet YR, Gueguen E, Cascales E. 2010. Nooks and crannies in type VI secretion regulation. J. Bacteriol. 192:3850–3860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bingle LE, Bailey CM, Pallen MJ. 2008. Type VI secretion: a beginner's guide. Curr. Opin. Microbiol. 11:3–8 [DOI] [PubMed] [Google Scholar]
- 5. Bleves S, et al. 2010. Protein secretion systems in Pseudomonas aeruginosa: a wealth of pathogenic weapons. Int. J. Med. Microbiol. 300:534–543 [DOI] [PubMed] [Google Scholar]
- 6. Bolivar F, et al. 1977. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene 2:95–113 [PubMed] [Google Scholar]
- 7. Casadaban MJ. 1976. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J. Mol. Biol. 104:541–555 [DOI] [PubMed] [Google Scholar]
- 8. Colwell RR, Kaper J, Joseph SW. 1977. Vibrio cholerae, Vibrio parahaemolyticus, and other vibrios: occurrence and distribution in Chesapeake Bay. Science 198:394–396 [PubMed] [Google Scholar]
- 9. Constantin de Magny G, et al. 2009. Predicting the distribution of Vibrio spp. in the Chesapeake Bay: a Vibrio cholerae case study. EcoHealth 6:378–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Das S, Chaudhuri K. 2003. Identification of a unique IAHP (IcmF associated homologous proteins) cluster in Vibrio cholerae and other proteobacteria through in silico analysis. In Silico Biol. 3:287–300 [PubMed] [Google Scholar]
- 11. Donnenberg MS, Kaper JB. 1991. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect. Immun. 59:4310–4317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Faruque SM, Nair GB. 2002. Molecular ecology of toxigenic Vibrio cholerae. Microbiol. Immunol. 46:59–66 [DOI] [PubMed] [Google Scholar]
- 13. Filloux A, Hachani A, Bleves S. 2008. The bacterial type VI secretion machine: yet another player for protein transport across membranes. Microbiology 154:1570–1583 [DOI] [PubMed] [Google Scholar]
- 14. Furste JP, et al. 1986. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene 48:119–131 [DOI] [PubMed] [Google Scholar]
- 15. Guzman LM, Belin D, Carson MJ, Beckwith J. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121–4130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hachani A, et al. 2011. Type VI secretion system in Pseudomonas aeruginosa: secretion and multimerization of VgrG proteins. J. Biol. Chem. 286:12317–12327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Han Y, et al. 2004. Microarray analysis of temperature-induced transcriptome of Yersinia pestis. Microbiol. Immunol. 48:791–805 [DOI] [PubMed] [Google Scholar]
- 18. Hanahan D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557–580 [DOI] [PubMed] [Google Scholar]
- 19. Hood RD, et al. 2010. A type VI secretion system of Pseudomonas aeruginosa targets a toxin to bacteria. Cell Host Microbe 7:25–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ishikawa T, Rompikuntal PK, Lindmark B, Milton DL, Wai SN. 2009. Quorum sensing regulation of the two hcp alleles in Vibrio cholerae O1 strains. PLoS One 4:e6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jubelin G, et al. 2005. CpxR/OmpR interplay regulates curli gene expression in response to osmolarity in Escherichia coli. J. Bacteriol. 187:2038–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kaper JB, Morris JG, Jr, Levine MM. 1995. Cholera. Clin. Microbiol. Rev. 8:48–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- 24. MacIntyre DL, Miyata ST, Kitaoka M, Pukatzki S. 2010. The Vibrio cholerae type VI secretion system displays antimicrobial properties. Proc. Natl. Acad. Sci. U. S. A. 107:19520–19524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mildiner-Earley S, Walker KA, Miller VL. 2007. Environmental stimuli affecting expression of the Ysa type three secretion locus. Adv. Exp. Med. Biol. 603:211–216 [DOI] [PubMed] [Google Scholar]
- 26. Miller VL, DiRita VJ, Mekalanos JJ. 1989. Identification of toxS, a regulatory gene whose product enhances toxR-mediated activation of the cholera toxin promoter. J. Bacteriol. 171:1288–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Miller VL, Mekalanos JJ. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170:2575–2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Miller VL, Taylor RK, Mekalanos JJ. 1987. Cholera toxin transcriptional activator toxR is a transmembrane DNA binding protein. Cell 48:271–279 [DOI] [PubMed] [Google Scholar]
- 29. Molina-Henares AJ, Krell T, Eugenia Guazzaroni M, Segura A, Ramos JL. 2006. Members of the IclR family of bacterial transcriptional regulators function as activators and/or repressors. FEMS Microbiol. Rev. 30:157–186 [DOI] [PubMed] [Google Scholar]
- 30. Motin VL, et al. 2004. Temporal global changes in gene expression during temperature transition in Yersinia pestis. J. Bacteriol. 186:6298–6305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mougous JD, et al. 2006. A virulence locus of Pseudomonas aeruginosa encodes a protein secretion apparatus. Science 312:1526–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mougous JD, Gifford CA, Ramsdell TL, Mekalanos JJ. 2007. Threonine phosphorylation post-translationally regulates protein secretion in Pseudomonas aeruginosa. Nat. Cell Biol. 9:797–803 [DOI] [PubMed] [Google Scholar]
- 33. Oscarsson J, et al. 2002. Characterization of a pore-forming cytotoxin expressed by Salmonella enterica serovars Typhi and Paratyphi A. Infect. Immun. 70:5759–5769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pieper R, et al. 2009. Temperature and growth phase influence the outer-membrane proteome and the expression of a type VI secretion system in Yersinia pestis. Microbiology 155:498–512 [DOI] [PubMed] [Google Scholar]
- 35. Pratt LA, Hsing W, Gibson KE, Silhavy TJ. 1996. From acids to osmZ: multiple factors influence synthesis of the OmpF and OmpC porins in Escherichia coli. Mol. Microbiol. 20:911–917 [DOI] [PubMed] [Google Scholar]
- 36. Pukatzki S, Ma AT, Revel AT, Sturtevant D, Mekalanos JJ. 2007. Type VI secretion system translocates a phage tail spike-like protein into target cells where it cross-links actin. Proc. Natl. Acad. Sci. U. S. A. 104:15508–15513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pukatzki S, et al. 2006. Identification of a conserved bacterial protein secretion system in Vibrio cholerae using the Dictyostelium host model system. Proc. Natl. Acad. Sci. U. S. A. 103:1528–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ramamurthy T, Yamasaki S, Takeda Y, Nair GB. 2003. Vibrio cholerae O139 Bengal: odyssey of a fortuitous variant. Microbes Infect. 5:329–344 [DOI] [PubMed] [Google Scholar]
- 39. Rawlings TK, Ruiz GM, Colwell RR. 2007. Association of Vibrio cholerae O1 El Tor and O139 Bengal with the Copepods Acartia tonsa and Eurytemora affinis. Appl. Environ. Microbiol. 73:7926–7933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Reidl J, Klose KE. 2002. Vibrio cholerae and cholera: out of the water and into the host. FEMS Microbiol. Rev. 26:125–139 [DOI] [PubMed] [Google Scholar]
- 41. Robinson JB, et al. 2009. Evaluation of a Yersinia pestis mutant impaired in a thermoregulated type VI-like secretion system in flea, macrophage and murine models. Microb. Pathog. 47:243–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sack DA, Sack RB, Nair GB, Siddique AK. 2004. Cholera. Lancet 363:223–233 [DOI] [PubMed] [Google Scholar]
- 43. Schwarz S, Hood RD, Mougous JD. 2010. What is type VI secretion doing in all those bugs? Trends Microbiol. 18:531–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schwarz S, et al. 2010. Burkholderia type VI secretion systems have distinct roles in eukaryotic and bacterial cell interactions. PLoS Pathog. 6:e1001068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shikuma NJ, Yildiz FH. 2009. Identification and characterization of OscR, a transcriptional regulator involved in osmolarity adaptation in Vibrio cholerae. J. Bacteriol. 191:4082–4096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shimada T, Sakazaki R. 1988. A serogroup of non-O1 Vibrio cholerae possessing the Inaba antigen of Vibrio cholerae O1. J. Appl. Bacteriol. 64:141–144 [DOI] [PubMed] [Google Scholar]
- 47. Shrivastava S, Mande SS. 2008. Identification and functional characterization of gene components of type VI secretion system in bacterial genomes. PLoS One 3:e2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Singleton FL, Attwell R, Jangi S, Colwell RR. 1982. Effects of temperature and salinity on Vibrio cholerae growth. Appl. Environ. Microbiol. 44:1047–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Song T, et al. 2008. A new Vibrio cholerae sRNA modulates colonization and affects release of outer membrane vesicles. Mol. Microbiol. 70:100–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tow LA, Coyne VE. 1999. Cloning and characterisation of a novel ompB operon from Vibrio cholerae 569B. Biochim. Biophys. Acta 1444:269–275 [DOI] [PubMed] [Google Scholar]
- 51. Towbin H, Staehelin T, Gordon J. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. U. S. A. 76:4350–4354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vaitkevicius K, et al. 2006. A Vibrio cholerae protease needed for killing of Caenorhabditis elegans has a role in protection from natural predator grazing. Proc. Natl. Acad. Sci. U. S. A. 103:9280–9285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Valeru SP, et al. 2009. Role of melanin pigment in expression of Vibrio cholerae virulence factors. Infect. Immun. 77:935–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Walker KA, Miller VL. 2004. Regulation of the Ysa type III secretion system of Yersinia enterocolitica by YsaE/SycB and YsrS/YsrR. J. Bacteriol. 186:4056–4066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yildiz FH, Schoolnik GK. 1998. Role of rpoS in stress survival and virulence of Vibrio cholerae. J. Bacteriol. 180:773–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zheng J, Shin OS, Cameron DE, Mekalanos JJ. 2010. Quorum sensing and a global regulator TsrA control expression of type VI secretion and virulence in Vibrio cholerae. Proc. Natl. Acad. Sci. U. S. A. 107:21128–21133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zinnaka Y, Carpenter CC., Jr 1972. An enterotoxin produced by noncholera vibrios. Johns Hopkins Med. J. 131:403–411 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.