Abstract
Streptococcus suis type 2 is a major swine pathogen and a zoonotic agent, causing meningitis in both swine and humans. S. suis infects the host through the respiratory route, reaches the bloodstream, and persists until breaching into the central nervous system. The capsular polysaccharide (CPS) of S. suis type 2 is considered a key virulence factor of the bacteria. Though CPS allows S. suis to adhere to the membrane of cells of the immune system, it provides protection against phagocytosis. In fact, nonencapsulated mutants are easily internalized and killed by macrophages and dendritic cells. The objective of this work was to study the molecular mechanisms by which the CPS of S. suis prevents phagocytosis. By using latex beads covalently linked with purified CPS, it was shown that CPS itself was sufficient to inhibit entry of both latex beads and bystander fluorescent beads into macrophages. Upon contact with macrophages, encapsulated S. suis was shown to destabilize lipid microdomains at the cell surface, to block nitric oxide (NO) production during infection, and to prevent lactosylceramide accumulation at the phagocytic cup during infection. In contrast, the nonencapsulated mutant was easily internalized via lipid rafts, in a filipin-sensitive manner, leading to lactosylceramide recruitment and strong NO production. This is the first report to identify a role for CPS in lipid microdomain stability and to recognize an interaction between S. suis and lactosylceramide in phagocytes.
INTRODUCTION
Streptococcus suis is one of the most important swine pathogens worldwide and a zoonotic agent able to induce septicemia with sudden death, meningitis, endocarditis, pneumonia, and arthritis (19). A total of 35 serotypes of S. suis have been described, with type 2 being the most commonly isolated type from diseased animals and humans. Until recently, S. suis disease in humans was considered rare, mostly affecting people in close contact with swine or pork by-products. However, S. suis is now emerging as an important threat to human health, especially in Asian countries. In fact, S. suis has been identified as the leading cause of adult meningitis in Vietnam, the second in Thailand, and the third in Hong Kong (18, 19). Moreover, in 2005, an important outbreak in China resulted in more than 200 human cases with a fatality rate nearing 20% (50). Patients presented symptoms associated with streptococcal toxic shock-like syndrome, such as high fever, malaise, nausea, and vomiting, followed by subcutaneous hemorrhage and coma in severe cases (50).
It has been suggested that S. suis infects pigs via the respiratory tract and remains localized in palatine tonsils, whereas in human skin abrasions represent the main route of entry (17, 18). In certain cases, bacteria reach the bloodstream and persist, causing either a rapid septic shock or delayed specific infections, depending on the targeted tissue (17). Different theories have been put forward to explain the ability of S. suis to survive within the host. The “Trojan horse” theory (bacteria traveling inside monocytes) has been suggested (48) although it is unlikely as it has been demonstrated that S. suis severely avoids phagocytosis by monocytes, macrophages, microglial cells, neutrophils, and dendritic cells (3, 5, 6, 27, 28, 35–37, 41).
S. suis is an encapsulated Gram-positive bacterium which possesses many suggested virulence factors, such as the capsular polysaccharide (CPS) (6, 41), opacity serum factor, and hemolysin (suilysin) as well as many other putative virulence factors (2). The CPS has been clearly shown to be critical for the pathogenesis of S. suis infections and has been proven to grant bacteria the ability to resist phagocytosis as nonencapsulated mutants of S. suis type 2 are efficiently internalized and killed by different types of phagocytes (3, 5, 17, 27, 28, 41). We have recently reported the structure of S. suis type 2 CPS (44), which is formed by the monosaccharides glucose, galactose, N-acetylglucosamine, and rhamnose into a unique repeating unit that contains a side chain terminated by sialic acid α2,6 linked to galactose.
The importance of CPS has been largely appreciated as a physical barrier that protects bacteria from the immune system (26), and for several streptococcal species, such as Streptococcus pneumoniae, Streptococcus pyogenes, Streptococcus uberis, and Streptococcus equi, these capsules are related to phagocytosis resistance (9, 42, 47). In the case of S. suis, the CPS, including the sialic acid moiety, has been shown to mediate bacterial attachment to the surface of phagocytes, but this attachment does not progress into bacterial internalization (36). In contrast, group B Streptococcus (GBS), which also possesses a sialic acid-rich CPS, is easily internalized by phagocytes (8, 35, 43). Thus, the old dogma stating that the antiphagocytic effect of CPSs is due to their net electrostatic charge may be questionable, and, thus, other mechanisms are probably more important overall in the antiphagocytic effect of CPS. Although certain signaling cascades have been shown to be activated or inhibited upon S. suis adherence to phagocytes (37), the exact molecular components and membrane receptors involved in S. suis resistance to phagocytosis are presently unknown.
Cellular membranes, mainly composed of phospholipids, cholesterol, and several types of membrane-associated proteins, can form localized regions with physical properties that differ from those of the rest of the membrane (45, 52). Specific sphingolipid and cholesterol-rich domains form distinct microdomains, called lipid rafts, and have been shown to function as signal transduction platforms (29, 31, 45, 51, 52). For example, lactosylceramide (LacCer), a neutral glycosphingolipid, forms microdomains that mediate, among other functions, phagocytosis by neutrophils (31, 51). In fact, it has been shown that LacCer binds specifically to several types of microorganisms such as Escherichia coli, Bordetella pertussis, and Candida albicans, among others (25, 33, 51). Although there is evidence suggesting a particular importance of these lipid microdomains during infection by well-encapsulated bacteria (16), no clear mechanisms have been identified hitherto, and no studies have focused on the interactions between S. suis CPS and lipid microdomains.
In the present study, the role of CPS in the inhibition of phagocytosis by macrophages was investigated by using latex beads covalently linked to S. suis purified CPS type 2. In addition, the role of CPS in the modulation of lipid microdomain-dependent phagocytosis was evaluated by comparing the interactions of a well-encapsulated S. suis type 2 strain with its nonencapsulated mutant strain. We show for the first time that CPS adherence to macrophage destabilizes lipid microdomains and prevents pattern recognition receptors (PRRs), such as LacCer, from binding efficiently to S. suis and activating signal transduction.
MATERIALS AND METHODS
Bacteria and cell culture.
The J774.A1 (J774) murine (BALB/c) macrophage cell line (ATCC TIB67; American Type Culture Collection, Rockville, MD) was used in the present study and cultured as previously described (36). For comparison purposes, bone marrow-derived dendritic cells were generated from female C57BL/6 mice (Charles River Laboratories, Pointe-Claire, Quebec, Canada) as previously described (27). The S. suis type 2 wild-type (WT) virulent strain 31533, isolated from a case of porcine meningitis, and its isogenic nonencapsulated mutant B218 (capsular polysaccharide deficient [CPS−]) were used. These strains have largely been used in previous studies of our laboratory (14, 27, 28). Strains were grown as described previously (14) in Todd-Hewitt broth (THB; Becton Dickinson, MD) or on sheep blood agar plates at 37°C. To perform S. suis macrophage interaction studies, isolated colonies were cultured in THB, followed by 8 h of incubation at 37°C with shaking. Working cultures were obtained by inoculating 10 μl of a 10−3 dilution of these cultures in 30 ml of THB and incubating them for 16 h at 37°C with shaking. Bacteria were washed twice in phosphate-buffered saline (PBS; pH 7.3) and diluted in complete cell culture medium for the experiments. The number of CFU/ml of the final suspension was determined by plating samples onto Todd-Hewitt agar, and colonies were counted using an Autoplate 4000 (Spiral Biotech, Norwood, MA). Bacteria were always used at nontoxic levels for cells, as measured by the lactate dehydrogenase assay for cytotoxicity (CytoTox 96; Promega, Madison, WI), as previously described (5).
Capsular polysaccharide purification.
CPS purification was carried out as previously described (44). Briefly, S. suis type 2 strain S735 (ATCC 43765) was cultured in 50 ml of THB at 37°C for 18 h, diluted in 2 liters of fresh THB, and grown to an optical density (540 nm) of 0.8. Cells were then centrifuged and resuspended in 33 mM PBS, pH 8.0, and chilled. The cell suspension was autoclaved at 121°C for 75 min, and the supernatant containing crude CPS was recovered by centrifugation at 9,000 × g for 50 min. Extraction with an equal volume of chloroform eliminated lipids, whereas nucleic acids were removed by precipitation by adding CaCl2 to 0.1 M and ethanol to 25% (vol/vol), followed by centrifugation at 7,200 × g for 30 min at room temperature. The concentration of ethanol in the supernatant was increased to 80% (vol/vol) to precipitate the CPS. The suspension was kept overnight at 4°C and then centrifuged at 9,100 × g for 30 min at 4°C. Pellets were dissolved in 50 mM NH4HCO3 (40 ml), dialyzed against the same solution for 48 h, and freeze-dried. The CPS was further purified by gel filtration chromatography on an XK26-100 column filled with Sephacryl S-400 (GE Healthcare, Uppsala, Sweden) as described previously (44). Fractions were collected and assayed for CPS by dot blot with a monoclonal antibody directed against CPS type 2-specific epitope (7). Fractions giving a positive response with the antibody but no absorption at 280 and 254 nm were pooled and freeze-dried. Purified CPS was analyzed by nuclear magnetic resonance (NMR) spectroscopy. Preservation of sialic acid was verified by gas chromatography (GC) after methanolysis and acetylation and by NMR. Lack of protein and RNA/DNA contamination was verified by the Lowry method and by spectrophotometry, respectively. All of these quality controls for the purified CPS were done as previously described (44).
Preparation of CPS-linked latex beads.
Aliphatic amine latex beads (2% [wt/vol], 2 μm) were from Molecular Probes (Eugene, OR), and reagents were from Sigma-Aldrich (St. Louis, MO). The CPS (8 mg) was oxidized with 0.8 ml of 5 mM sodium periodate in the dark at room temperature for 1 h. The unreacted periodate was depleted by adding 240 μl of ethylene glycol. The solution was dialyzed against 50 mM NH4HCO3 for 16 h and then lyophilized. For covalent coupling of oxidized CPS to amine latex beads, an aliquot of 500 μl of beads was settled by centrifugation at 9,000 × g for 3 min in a Sigma 113 centrifuge (Sigma, Osterode am Harz, Germany). The supernatant was removed, and beads were washed twice with 0.1 M PBS, pH 7.4. One milliliter of oxidized CPS (1 mg) in phosphate buffer and 10 μl of sodium cyanoborohydride (5 M) in NaOH (1 M) were added to the beads. Tubes were rotated slowly for 2 h at room temperature to keep the beads in suspension. Beads were then collected by centrifugation, and the supernatant was kept for further quantification of the unlinked oxidized CPS. A solution of 500 μl of phosphate buffer containing 5 mg of NaBH4 was added to the beads, and the reaction was allowed to proceed for 40 min at room temperature. The reaction was quenched with 100 μl of acetone, and beads were collected by centrifugation. Beads were washed four times with PBS, pH 7.4, containing 0.5% bovine serum albumin (BSA) to coat free binding sites. Finally, beads were resuspended in PBS containing 0.1% BSA and 0.02% NaN3 and stored at 4°C. The same experiment was performed without oxidized CPS, and these beads were used as controls. Residual NaN3 was removed by washing and dilution in cell culture medium prior to addition to cells.
The amount of linked CPS on the beads was determined by measuring the difference between the amount of CPS added at the beginning of the reaction and unreacted CPS remaining in the supernatant at the end of the coupling reaction. The unreacted oxidized CPS was quantified by the phenol-sulfuric method in microplates (15) using oxidized CPS as a standard. The presence of intact sialic acid and the antigenic properties of the CPS were evaluated by immunofluorescence using both a fluorescein isothiocyanate (FITC)-conjugated lectin isolated from Sambucus nigra (Vector Laboratories, Burlingame, CA), which binds preferably to sialic acid in an α2,6 linkage, and a rabbit anti-S. suis hyperimmune serum that recognizes CPS epitopes (22). Unlinked beads and CPS-linked beads were blocked for 10 min in PBS containing 2% BSA, followed by the addition of either S. nigra FITC-lectin or the anti-S. suis antibodies for 30 min. Beads were washed four times with PBS–1% BSA. When anti-S. suis antibodies were used, the secondary antibody Alexa-Fluor 488 goat anti-rabbit IgG was added to the bead preparation for 15 min for signal detection. Beads were washed four times in PBS–1% BSA and mounted on glass slides with Mowiol to be analyzed by confocal microscopy (see below).
In addition, the level of CPS oxidation obtained prior to bead linkage was evaluated by gas chromatography (GC) of the peracetylated derivatives. Briefly, oxidized CPS (0.9 mg) was reduced by adding 200 μl of NaBH4 (20 mg/ml) in NH4OH (2 M) for 24 h at room temperature. The reaction was quenched with 100 μl of acetone and evaporated to dryness using a stream of N2 at 40°C. The composition of the residue was determined by methanolysis: methanol (465 μl) and acetyl chloride (35 μl) were added to the residue. The solution was heated for 15 h at 75°C and evaporated to dryness, and 500 ml of tert-butanol was added and evaporated to dryness. The methyl glycosides were acetylated with 100 μl of pyridine and 100 μl of acetic anhydride at 100°C for 20 min. The cooled solution was partitioned with 5 ml of water and 2 ml of CH2Cl2. The organic layer containing the peracetylated methyl glycosides was analyzed by GC using flame ionization detection (GC-FID) or coupled to mass spectrometry (GC-MS). GC-FID analysis was done on a Hewlett-Packard model 7890 gas chromatograph equipped with a 30-m by 0.32-mm, (0.25-μm particle size) HP-5 capillary column (Agilent Technologies, Santa Clara, CA) using the following temperature program: 50°C for 2 min, an increase of 30°C/min to 150°C, then an increase of 3°C/min to 230°C, and a hold for 5 min. The temperatures of the injector and the flame ionization detector were 225°C and 250°C, respectively. Analysis by GC-MS was done with a Varian CP3800 gas chromatograph and a Saturn 2000 mass spectrometer equipped with a 30-m by 0.25-mm (0.25-μm particle size) DB-5MS capillary column (J&W Scientific, Folsom, CA) using the following temperature program: 120°C for 1.5 min, an increase of 4°C/min to 200°C, a hold for 8 min, and then an increase of 10°C/min to 230°C. The temperature of the injector was 250°C.
Phagocytosis assays.
For phagocytosis assays of CPS-linked latex beads, J774 macrophages (5 × 105) were plated on glass coverslips. CPS-linked latex beads or unlinked control latex beads were added at the same concentrations (1 × 106 beads/ml) to cells for 1 h and 2 h. Coverslips were then washed four times with PBS to remove noninternalized beads, and cells were fixed with methanol-acetone (80:20) for 20 min at −20°C, washed, and blocked for 10 min in 2% BSA and 0.2% gelatin PBS solution. Coverslips were incubated for 1 h with rabbit anti-S. suis antibodies and with rat anti-mouse lysosomal-associated membrane protein-1 (LAMP1) antibody (clone 1D4B; Developmental Studies Hybridoma Bank, Iowa City, IA). After coverslips were washed with PBS–1%, BSA they were incubated with Alexa-Fluor 488 goat anti-rabbit IgG and Alexa-Fluor 568 goat anti-rat IgG secondary antibodies (Invitrogen, Burlington, Ontario, Canada) for 30 min; samples were then washed and mounted on glass slides with Mowiol containing diazabicyclooctane (DABCO) and 4′,6′-diamidino-2-phenylindole (DAPI) to stain the nuclei. Samples were analyzed with an IX-80 confocal microscope integrated into the FV-1000 imagery system and analyzed using Fluoview software (Olympus, Markham, ON, Canada). Absence of cell toxicity induced by bead preparations was evaluated by measuring the release of lactate dehydrogenase (LDH) enzyme with the CytoTox 96 Non-Radioactive Cytotoxicity Assay (Promega, Madison, WI). LDH results showed that CPS-linked latex beads did not induce significant cytotoxicity (6.8% ± 3.4%) after 2 h of incubation with cells. Similar results were obtained with control beads (5.3% ± 2.5%).
Inhibition of phagocytosis capacity of macrophages.
The capacity of CPS to inhibit phagocytosis by macrophages was studied by fluorescence-activated cell sorting (FACS) and confocal microscopy. Macrophages were plated in six-well plates (1 × 106) for FACS analysis and on glass coverslips (5 × 105) for confocal studies. Either CPS-linked latex beads or unlinked control beads were added at the same concentration (1 × 106 beads/ml) to cells and centrifuged at 800 × g for 5 min to allow the beads to settle on the cells and cover their surfaces. After 30 min of bead pretreatment, red (580/605 nm) fluorescent unlinked latex beads of 1 μm (Invitrogen) were added at a final concentration of 5 × 105 beads/ml and again centrifuged at 800 × g for 5 min to allow the red beads to settle on the cells. After 30 min of incubation at 37°C, cells were washed with cold PBS to remove noninternalized beads and either fixed in 4% paraformaldehyde (PFA) for confocal analysis (glass coverslips) or harvested in cold PBS containing 1% fetal bovine serum followed by FACS analysis using a FACSCalibur instrument (BD Biosciences, Mississauga, ON, Canada). A total of 50,000 gated events were acquired per sample, and data analysis was performed using the CellQuest software. In additional experiments, different concentrations of beads and incubation times were also tested by FACS (Table 1).
Table 1.
Inhibition of phagocytosis capacity of macrophages by CPS-linked latex beads
| Beads | MFI ± SEM (% inhibition) by conditionc |
|||
|---|---|---|---|---|
| Coculture at the indicated bead ratioa |
Pretreatment at the indicated bead ratiob |
|||
| 2:1 | 1:1 | 2:1 | 1:1 | |
| Control beads + Fluo-Beads | ND | 308 ± 102 | 1,711 ± 205 | 1,586 ± 374 |
| Beads-CPS + Fluo-Beads | ND | 338 ± 127 (0) | 1,045 ± 124 (39) | 1,160 ± 293 (26) |
Either CPS-linked latex beads (Beads-CPS) or unlinked control beads were added together with red fluorescent latex beads (Fluo-Beads) at the same concentrations (5 × 105 beads/ml; ratio 1:1) to J774 macrophages and incubated for 30 min. ND, not done.
Either CPS-linked latex beads (Beads-CPS) or unlinked control beads were added at 1 × 106 beads/ml or 5 × 105 beads/ml to cells. After 30 min of bead pretreatment, Fluo-Beads were added at a final concentration of 5 × 105 beads/ml to obtain a ratio of 2:1 and 1:1 to Beads-CPS, respectively. After 30 min of incubation, phagocytosis of Fluo-Beads was analyzed by FACS.
MFI, mean fluorescence intensity. Percent inhibition is relative to the values obtained with control beads.
Immunofluorescence and bacterial infection assays.
For bacterial infection studies, J774 macrophages (5 × 105) were plated on glass coverslips and infected with S. suis at a multiplicity of infection (MOI) of 2:1 with either the encapsulated WT or its CPS− mutant strain. After 30 min or 1 h of bacteria-cell contact, coverslips were washed with PBS to remove nonassociated bacteria, and cells were fixed with methanol-acetone (80:20) for 20 min at −20°C, washed, and then blocked as described above for 10 min. Coverslips were incubated for 1 h with rabbit anti-S. suis antibodies and with rat anti-LAMP1 antibody. After coverslips were washed, they were incubated with the secondary antibodies, Alexa-Fluor 488 goat anti-rabbit IgG and Alexa-Fluor 568 goat anti-rat IgG (Invitrogen) for 30 min, washed, and then mounted on glass slides with Mowiol containing DABCO and DAPI to stain the nuclei. For labeling of lipid microdomains, cholera toxin–Alexa-Fluor 568 (Invitrogen) was used as described previously (11). Briefly, J774 macrophages were grown on coverslips and then infected as described above. After a washing step with PBS at the end of infection, cells were incubated for 10 min at 4°C with 5 μg/ml of cholera toxin to prevent its internalization. Finally, cells were washed and fixed. In selected experiments, macrophages were incubated with either CPS-linked latex beads or unlinked control latex beads (1 × 106 beads/ml) for 30 min, and cells were stained with cholera toxin as indicated above. Finally, for macrophage cell membrane staining, CellTracker CM-Dil (Invitrogen), a dye that covalently binds to cellular thiols, was used as recommended by the manufacturer. Samples were analyzed with an IX-80 confocal microscope as described above.
Isolation of lipid microdomains.
Lipid microdomains were isolated from J774 macrophages as described previously (11), with certain modifications. Briefly, cell pellets were resuspended in 0.3 ml of TNE-Triton buffer (25 mM Tris, 150 mM NaCl, 5 mM EDTA, pH 7.4, and 1% Triton X-100) supplemented with complete protease inhibitor (Boehringer Ingelheim, Burlington, Ontario, Canada), transferred to an Eppendorf tube, and shaken gently for 30 min at 4°C to solubilize membranes. The supernatant containing soluble and insoluble membrane components was mixed with 0.6 ml of 60% OptiPrep to obtain a final concentration of 40%, which was then poured in an Ultraclear centrifuge tube (Beckman Coulter, Mississauga, Ontario, Canada). Finally, 2.4 ml of 30% OptiPrep and 0.9 ml of TNE buffer (25 mM Tris, 150 mM NaCl, 5 mM EDTA, pH 7.4) with complete protease inhibitors were layered on top. After a 4-h centrifugation at 215,000 × g to float the insoluble rafts, 10 fractions of 1 ml were collected from the top. A total of 200 μl of each fraction was diluted in PBS and then loaded on a Minifold-1 dot blot system (Whatman International, Ltd., Sanford, ME) with a mounted nitrocellulose membrane. After each applied fraction was drained, the membrane was blocked in a solution of PBS containing 5% milk for 1 h and then incubated with cholera toxin B subunit conjugated to horseradish peroxidase (HRP; Sigma-Aldrich) for 1 h. The membrane was finally washed with PBS–0.02% Tween and then revealed.
Nitric oxide (NO) assay.
Due to bacterial toxic effects at long incubation times, suspensions of WT or CPS− S. suis strains were heat killed at 60°C for 45 min and then added to macrophages (5 × 105) previously plated on glass coverslips in 24-well plates. Bacteria were added at a concentration equivalent to 1 × 109 CFU (this dose represents approximately the equivalent concentration that would be reached by live bacteria at a 24-h time point). Contact with bacteria was allowed for 1 h, 6 h, or 24 h. Purified E. coli lipopolysaccharide (LPS; Sigma-Aldrich) was used as a positive control at a final concentration of 1 μg/ml and 24 h of incubation. After each time point, 10 μM NO probe DAF-FM (4-amino-5-methylamino-2′,7′-difluorofluorescein) diacetate (Invitrogen) was added to each well for 60 min. DAF-FM reacts with NO to form a fluorescent benzotriazole with excitation/emission maxima of 495/515 nm. Excess probe was removed by washing with PBS, and then fresh medium was added for 30 min. Cells were fixed in PFA for 10 min and then analyzed by confocal microscopy as described above.
LacCer assay.
Macrophages (5 × 105) were plated on glass coverslips in 24-well plates as described above. Cells were washed with PBS, and 5 μM LacCer-BODIPY (4,4-difluoro-4-bora-3a,4a-diaza-s-indacene; Invitrogen) was added to cells which were further incubated at 4°C for 30 min, allowing integration of LacCer within the plasma membrane. In parallel, cells were either treated or not with a final concentration of 5 μg/ml of cytochalasin D (CytoD). After 30 min, cells were washed to remove unincorporated LacCer, and macrophages were infected for 1 h with either S. suis WT strain or its CPS− mutant (MOI of 2:1). After the infection, cells were gently washed with PBS to remove noninternalized and nonadhered bacteria and then fixed either in PFA for cells that have incorporated LacCer or methanol-acetone (80:20) for cells requiring LAMP1 staining. Immunofluorescence staining was performed as described above to label S. suis. Fluoview (Olympus) was used to quantify the amount of pixels. A total of 30 cells were quantified, and average intensity was used to express the correlation between the increase in intracellular staining of LacCer and S. suis.
Filipin assay.
Macrophages (5 × 105) were plated on glass coverslips in 24-well plates as described above. Filipin was added for 15 min at a final concentration of 2 μg/ml. Cells were then infected for 1 h with the S. suis WT or CPS− mutant strain (MOI of 2:1), washed to remove bacteria that were not internalized or adhered, and then fixed with methanol-acetone (80:20). Immunofluorescence was performed as described above.
Data quantification and statistical analysis.
Using the Fluoview software, integrated fluorescence intensities for each fluorescence channel were analyzed. Briefly, 30 cells were randomly selected for each condition. Average integrated fluorescence was calculated by the software, and the obtained ratio was normalized according to DAPI (nuclei) channel variance between samples. Data were analyzed for significance using Student's unpaired t test. Dot blot results were analyzed by using Adobe Photoshop software. Semiquantitative measurement of each sample was performed and normalized to the preexisting blot background.
RESULTS
S. suis type 2 CPS can be covalently linked to latex beads.
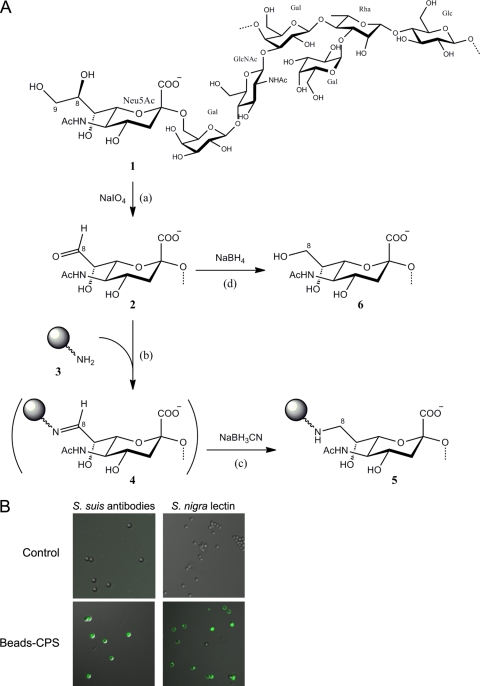
CPS is a critical virulence factor, and it plays an important role in the pathogenesis of various bacteria, such as S. suis type 2 (2, 17). To better understand its role and influence during phagocytosis, we covalently linked type 2 CPS to latex beads. Adapted from a technique that covalently links CPS to protein carriers (21), reductive amination was used to treat and bind purified S. suis type 2 CPS to latex beads functionalized with amine groups (Fig. 1A). For every 1 mg of CPS used in the assay, quantities of CPS were measured to determine the coupling efficiency. Approximately 30% (326 μg) of oxidized CPS was found to be covalently linked to latex beads. It is important that the level of CPS oxidation was 26%, meaning that 74% of sialic acid residues were conserved. This is an important feature to allow evaluation of the immunomodulatory properties of CPS.
Fig 1.
S. suis CPS can be covalently linked to latex beads. (A) Scheme of reaction for the preparation of CPS-linked latex beads. Step a shows limited periodate (IO4−) oxidation of the CPS (1) in which a portion of the sialic acid residues (Neu5Ac) were converted to the 8-carbon analog (2). Steps b and c show coupling by reductive amination, using sodium cyanoborohydride (NaBH3CN), of the oxidized sialic acid residues (2) with amine-modified latex beads (3) to yield, via the Schiff-base intermediate (4), the stable amine derivative (5). Step d shows the borohydride (BH4−) reduction of the excess oxidized sialic acid residues (2) to the nonreactive alcohol (6). (B) Latex beads (2 μm) linked or not to CPS were stained (green) with an anti-S. suis polyclonal serum to detect CPS or with S. nigra lectin to detect sialic acid. Unlinked control beads show no reaction (negative staining).
Once the CPS was linked to the latex beads, the quality of the preparation was assayed to determine if bound CPS preserved its antigenic properties, including intact sialic acid-dependent epitopes. An immunofluorescence test with anti-CPS serum showed the presence of CPS on the surface of the CPS-linked latex beads (Fig. 1B, lower left panel), whereas unlinked beads were nonreactive to the anti-S. suis antibodies (Fig. 1B, upper left panel). We then examined covalently bound CPS to latex beads to verify that the sialic acid group was intact and still attached α2,6 to galactose. Results showed a positive reaction with S. nigra lectin, indicating that sialic acid is preserved in CPS linked to latex beads (Fig. 1B, lower right panel), whereas unlinked beads were nonreactive to this lectin (Fig. 1B, upper right panel).
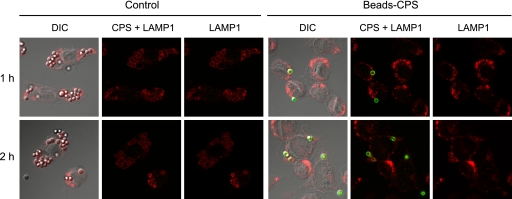
S. suis type 2 CPS blocks the internalization of latex beads by macrophages.
After covalently linking latex beads with CPS from S. suis type 2, we performed a phagocytosis study with macrophages. The same quantity of latex beads either unlinked or CPS-linked was added to macrophage cultures for 1 h and 2 h, and cells were stained with both anti-S. suis antibodies and a LAMP1 antibody (a marker for an endosome/lysosome membrane protein). Results showed that unlinked latex beads (negative for anti-S. suis antibody staining) were easily internalized by macrophages at both incubation times as revealed by a positive staining with the LAMP1 antibody, indicating their intracellular location (Fig. 2, control). CPS-linked beads, positive for anti-S. suis antibody staining, clearly resisted phagocytosis as only few beads were found associated to phagocytes (Fig. 2). Interestingly, this phenomenon was not strictly related to the cell type since results obtained with bone marrow-derived dendritic cells showed that they were also unable to internalize CPS-linked beads (see Fig. S1 in the supplemental material).
Fig 2.
S. suis CPS blocks the internalization of latex beads by macrophages. J774 macrophages were incubated with latex beads covalently linked to CPS (Beads-CPS) or unlinked beads (Control) for 1 h and 2 h. Cells were stained with rabbit anti-S. suis antibodies to detect CPS-linked beads (green) and with rat anti-LAMP1 antibody to show that beads were located inside the cells (red). Compared to control beads, CPS-linked beads are poorly internalized by macrophages. DIC, differential interference contrast.
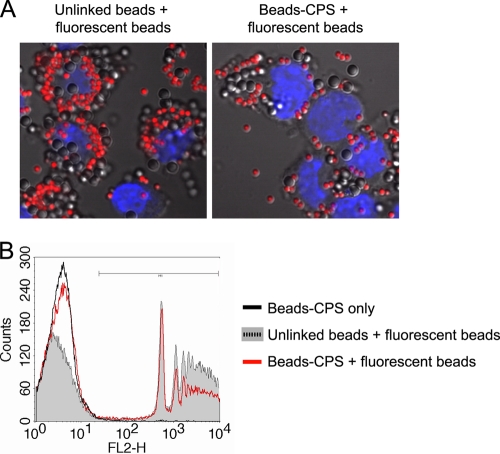
Latex beads covalently linked to S. suis type 2 CPS inhibit the phagocytic capacity of macrophages.
As drastic inhibition of macrophage phagocytosis of latex beads covalently linked to CPS was observed, we decided to investigate whether this was a localized effect of the CPS on the phagocyte membrane or whether CPS-linked beads could exert a more general effect on phagocytosis. To this end, macrophages were pretreated with either CPS-linked beads or with unlinked control beads for 30 min (Fig. 3A, uncolored beads) prior to addition of unlinked fluorescent beads (Fig. 3A, red beads) for an additional 30 min. Phagocytosis was then analyzed by confocal microscopy and FACS. Unlike results with control beads, pretreatment of macrophages with CPS-linked beads reduced the number of intracellular red fluorescent beads subsequently added to cultures, indicating that CPS is able to reduce the general phagocytosis capacity of macrophages (Fig. 3A). Confirming this observation, FACS analysis showed that the phagocytic ability of macrophages was reduced by 38.9% in cells that had been in contact with CPS-linked beads (Fig. 3B and Table 1). To further validate the specificity of the CPS effect on phagocytosis of fluorescent beads, we compared two different ratios of CPS-linked beads and fluorescent beads. As shown in Table 1, the capacity of latex beads covalently linked to S. suis type 2 CPS to inhibit the phagocytic machinery of macrophages was dose dependent. Furthermore, this effect was abrogated when CPS-linked beads and fluorescent beads were added at the same time (coculture) to macrophages, suggesting that pretreatment was essential to the observed effect. Taken together, these results suggest that S. suis type 2 CPS-linked beads interact with the macrophage membrane and have a bystander effect on mechanisms involved in phagocytosis.
Fig 3.
Latex beads covalently linked to CPS inhibit the phagocytic capacity of macrophages. J774 macrophages were pretreated with either unlinked control beads or CPS-linked beads (Beads-CPS) for 30 min. Then, fluorescent latex beads were added for an additional 30 min. (A) Confocal analysis showing significant less internalized fluorescent latex beads when macrophages were pretreated with CPS-linked beads. (B) FACS analysis of the quantity of internalized fluorescent latex beads. Macrophages incubated with CPS-linked beads only were used as controls and showed no fluorescence shift on FL2. Cells pretreated with unlinked control beads showed high levels of fluorescent bead internalization. A significant reduction in mean fluorescence intensity was observed when cells were pretreated with CPS-linked beads. Histograms are representative of three independent experiments.
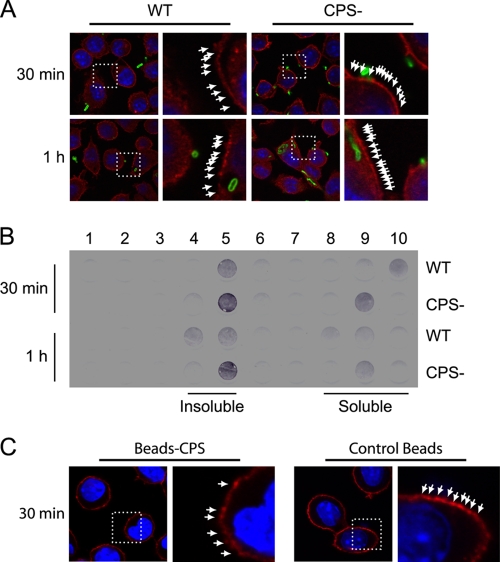
S. suis type 2 CPS disrupts lipid microdomains of macrophages.
As CPS covalently linked to an artificial support (latex beads) has an inhibitory effect on phagocytosis, we decided to use a live-bacteria model and investigated the effect of S. suis type 2 CPS on components of the plasma membranes of macrophages. To study this objective, we used a CPS− mutant of S. suis and compared it with its WT encapsulated strain. In previous studies, using quantitative techniques combined with confocal and electron microscopy, we demonstrated that well-encapsulated S. suis is poorly phagocytosed by macrophages, whereas nonencapsulated strains are rapidly ingested and killed by most phagocytes, including J774 macrophages (5, 14, 27, 28, 35). First, S. suis was allowed to interact with cells for either 30 min or 1 h, and then lipid microdomains were labeled with cholera toxin. Cholera toxin binds to GM1 ganglioside, typically giving a punctured pattern at the surface (11) (Fig. 4A, white arrows). Results showed that lipid microdomain patterns seemed to be more irregular and spread apart in macrophages cultured in the presence of WT S. suis than those in the CPS− S. suis mutant strain. In contrast, no differences in cell membrane staining were observed when CM-Dil was used to label cellular thiols (see Fig. S2 in the supplemental material), suggesting a localized effect of WT S. suis on lipid raft integrity. To validate these results, membranes from macrophages infected with either the S. suis WT strain or the CPS− mutant strain were purified. The insoluble lipid microdomains were then separated from soluble fractions by ultracentrifugation on an OptiPrep gradient; soluble lipids migrated to the bottom of the centrifugation tube (fractions 8 to 10), whereas insoluble lipids containing microdomains migrated to the top of the tube (fractions 4 and 5). By staining the GM1 ganglioside with cholera toxin-HRP, we were able to confirm the presence and quantify the amount of insoluble/soluble lipids from each sample and compare them to each other through dot blotting. Macrophages infected with the WT strain showed reduced amounts of GM1 ganglioside in insoluble fractions, corresponding to lipid microdomains, compared to those infected with the CPS− strain, which contained larger amounts of GM1 in the same fractions (Fig. 4B, insoluble). This reduction was observed at both 30 min and 1 h postinfection. Although GM1 staining of soluble fractions spread from fractions 8 to 10, their combination for quantification did not show a significant difference in the amount of GM1 in soluble fractions between WT-infected and CPS− mutant-infected macrophages (Fig. 4B, soluble). To further evaluate the effect of CPS in lipid microdomain integrity, macrophages were incubated with either CPS-linked beads or with unlinked control beads for 30 min, and then cells were labeled with cholera toxin. Similar to patterns observed with WT S. suis-infected cells, lipid microdomain patterns seemed to be diffuse and spread apart in macrophages cultured in the presence of CPS-linked beads compared to patterns with control beads (Fig. 4C). Taken together, these experiments (Fig. 4A to C) suggest that encapsulated S. suis has an effect on the stability of lipid microdomains in macrophages.
Fig 4.
CPS disrupts lipid microdomains during phagocytosis of S. suis by macrophages. J774 macrophages were infected with S. suis WT or its nonencapsulated mutant strain (CPS−) for 30 min and 1 h. (A) Confocal analysis of lipid microdomain distribution. Cells were stained with fluorescent cholera toxin (red), which binds to GM1 ganglioside in lipid microdomains, and with rabbit anti-S. suis antibodies (green) to stain bacteria. The pattern of staining of GM1 (arrows) is more diffuse at 30 min and 1 h when cells are infected with WT S. suis than when they are infected with the CPS− mutant. (B) Dot blot analysis of lipid microdomain distribution. Cells were infected as described above and lysed, and membranes were fractionated by ultracentrifugation on an OptiPrep gradient (1, top fraction; 10, bottom fraction). GM1 ganglioside was revealed by incubating with cholera toxin-HRP. Insoluble membrane fractions corresponding to lipid microdomains are shown in fractions 4 and 5. Soluble membrane fractions are shown in fractions 8, 9, and 10. Macrophages showed a higher GM1 label intensity in lipid microdomains when infected by the CPS− mutant than in cells infected by WT S. suis. (C) Confocal analysis of lipid microdomain distribution after macrophages were incubated with either unlinked control beads or CPS-linked beads (Beads-CPS) for 30 min. Cells were stained with fluorescent cholera toxin (red). The pattern of staining of GM1 (arrows) is more diffuse when cells are treated with CPS-linked beads than when they are treated with control beads.
CPS inhibits nitric oxide production in S. suis type 2-infected macrophages.
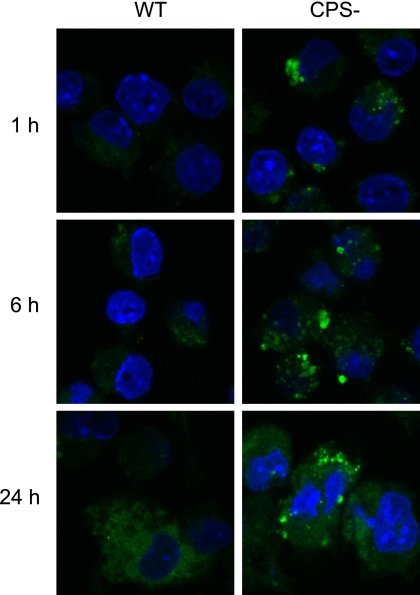
Since CPS from S. suis type 2 affected macrophage phagocytosis and promoted the distribution of lipid microdomains on the cell membrane, we decided to examine if cellular mechanisms and transduction pathways linked to these microdomains could be affected. As NO production has been shown to be activated by the Lyn kinase pathways and is linked to components of microdomains (31), we decided to follow its production by macrophages stimulated with either the WT strain or its CPS− mutant. NO production was evaluated after 1 h, 6 h, and 24 h of bacteria-cell contact (Fig. 5). NO production was visibly higher when cells were activated with the CPS− mutant, whereas relatively low NO production was observed with the WT strain, suggesting that signaling pathways involved in NO production are inhibited by the presence of the CPS.
Fig 5.
CPS inhibits nitric oxide production by macrophages. J774 macrophages were incubated with heat-killed S. suis WT or its nonencapsulated mutant strain (CPS−) for 1 h, 6 h, and 24 h. Nitric oxide production was detected by incubation with DAF-FM (green). Macrophages interacting with S. suis WT show a delayed and reduced nitric oxide response compared to that of cells incubated with the CPS− mutant.
S. suis type 2 CPS blocks pathogen pattern recognition by LacCer in lipid microdomains of macrophages.
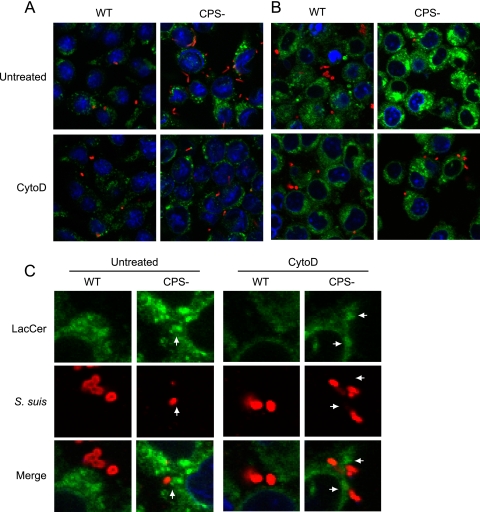
NO production is controlled by Lyn kinase, which can be activated by LacCer, present in the lipid microdomains of the membrane of immune cells (31). LacCer recognizes pathogen surface patterns although its interaction with S. suis has never been studied. To determine the possible role of LacCer in the phagocytosis of S. suis type 2, we performed a series of experiments using a fluorescent marker (LacCer linked to a BODIPY fluorophore) and an inhibitor of actin polymerization (cytochalasin D). Cytochalasin D is a potent inhibitor of phagocytosis which prevents pseudopod extension. In a first experiment, macrophages were infected with either the S. suis WT strain or the CPS− mutant S. suis (Fig. 6A, red). Bacteria and LAMP1 (Fig. 6A, green) were costained with specific antibodies, and average integrated fluorescence intensities were quantified. We observed an increase of 275% of internalized S. suis when CPS is absent (P < 0.05) (Fig. 6A, untreated). Treatment of macrophages with cytochalasin D, followed by costaining and quantification of the fluorescence intensities of S. suis and LAMP1, showed no increase of internalized S. suis (Fig. 6A, CytoD). However, we could still observe S. suis attached to the surface of macrophages even though pseudopod extension had been inhibited by cytochalasin D, which agrees with what has previously been reported for other bacteria (46).
Fig 6.
S. suis CPS blocks pathogenic pattern recognition by LacCer in lipid microdomains of macrophages. J774 macrophages were either pretreated or not with CytoD and then infected with WT S. suis or its nonencapsulated mutant strain (CPS−) for 1 h. (A) Cells were stained with rat anti-LAMP1 antibody to show bacterial internalization (green) and with rabbit anti-S. suis antibodies (red) to stain bacteria. Compared to WT S. suis-infected macrophages, confocal analyses showed an increase of the intracellular CPS− mutant in untreated macrophages. CytoD blocked phagocytosis of S. suis by macrophages. (B) LacCer-BODIPY (green) was added to cells for 30 min, allowing integration of LacCer within the plasma membrane. In parallel, cells were either treated or not with CytoD. After 30 min, cells were washed to remove unincorporated LacCer, and macrophages were infected for 1 h with either S. suis WT strain or its CPS− mutant. Rabbit anti-S. suis antibodies (red) were used to stain bacteria. Untreated cells showed an increase in the intensity and size of LacCer-positive vesicles when macrophages were infected with the CPS− mutant. CytoD blocked LacCer label intensity by preventing internalization of S. suis by macrophages. (C) Closeup confocal analysis of the interaction of S. suis with LacCer at the plasma membrane of macrophages shown in panel B. Arrows indicate the phagocytic cup and close proximity of LacCer-positive membranes (green) with CPS− S. suis (red) adhered to the cell surface. WT S. suis shows no close proximity and less intensity of LacCer in the region directly adjacent to the bacteria. Inhibition of phagocytosis by CytoD reduced accumulation of LacCer at the phagocytic cup and prevented the increase of LacCer intensity in internal vacuoles.
In a second experiment, macrophages were pretreated for 30 min with LacCer-BODIPY (Fig. 6B, green) to incorporate fluorescent glycosphingolipid in the lipid microdomains of their plasma membranes, followed by infection with WT or CPS− S. suis as in the first experiment. In this case, cells were not permeabilized, so we could differentiate external from internalized bacteria. Hence, stained S. suis bacteria are at the surface of macrophages (Fig. 6B, red). Macrophages that were infected with the CPS− S. suis showed a 177% increase of quantity and intensity of LacCer-positive vacuoles (P < 0.05) (Fig. 6B, untreated). Inhibition of actin polymerization and phagocytosis by treatment with cytochalasin D abolished this increase, demonstrating an existing correlation between LacCer and the internalized CPS− S. suis mutant (Fig. 6B, CytoD).
The second experiment was analyzed by close-up magnification to verify the accumulation of LacCer at the phagocytic cup during internalization of CPS− S. suis (Fig. 6C). When macrophages were infected with CPS− S. suis, a clear accumulation of LacCer at the cup in various areas of the cells was observed as well as a morphological continuity between the noninternalized portion of the Streptococcus chain and the internalized one (Fig. 6C, untreated CPS−, arrow). WT S. suis shows neither such continuity nor clear accumulation of LacCer at bacteria-cell contact points (Fig. 6C, WT). Inhibition of phagocytosis by cytochalasin D reduces accumulation of LacCer at the phagocytic cup and prevents the increase of LacCer intensity in internal vacuoles (Fig. 6C, CytoD CPS−, arrow). This indicates that S. suis type 2 CPS could likely act as a shell to prevent pathogen patterns from being recognized and the accumulation of ligands, such as LacCer, in lipid microdomains.
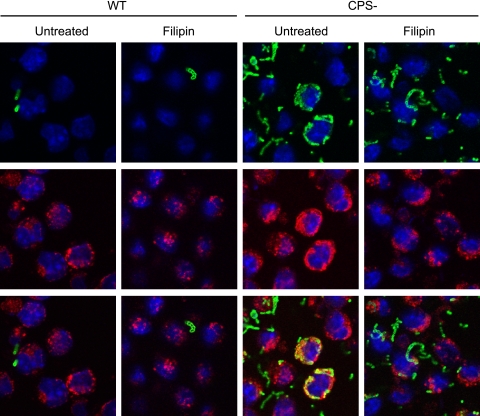
Filipin partially disrupts entry of CPS− S. suis in macrophages.
Since LacCer is a component of lipid microdomains and seems to be involved in the entry of CPS− S. suis in macrophages, the lipid microdomain inhibitor filipin was used. Filipin binds to cholesterol and disrupts lipid microdomain stability in the membrane, acting as an inhibitor of the raft/caveola endocytosis pathway (13, 34). Pretreatment of macrophages with filipin did not affect the weak capacity of macrophages to internalize S. suis WT (Fig. 7, left panels). Filipin treatment of macrophages partially prevented internalization of the CPS− mutant of S. suis (Fig. 7, right panels). Whereas in nontreated cells most CPS− S. suis bacteria were located in LAMP1-positive vacuoles, only a few CPS− S. suis bacteria were internalized when lipid microdomains were destabilized by filipin.
Fig 7.
Filipin partially disrupts entry of nonencapsulated S. suis in macrophages. J774 macrophages were either pretreated or not with filipin for 15 min and then infected with WT S. suis or with its nonencapsulated mutant strain (CPS−) for 1 h. Cells were stained with rat anti-LAMP1 antibody to show bacterial internalization (red) and with rabbit anti-S. suis antibodies (green) to stain bacteria. Filipin partially blocked CPS− S. suis phagocytosis by macrophages.
DISCUSSION
Gram-positive streptococci such as S. suis bacteria ensure their pathogenicity and survival through a collection of virulence factors that allow the pathogen to evade the immune system (2, 17). Among them, type 2 CPS plays a key role by protecting the pathogen against phagocytosis and by preventing bacterial surface antigen recognition by immune cell receptors (2, 14, 20, 37, 41). Surface polysaccharides can also display similarities to host antigens and are often poorly immunogenic (26, 39). The role of S. suis type 2 CPS as an antiphagocytic factor has already been shown by different research groups using isogenic mutants defective in CPS production (3, 6, 35, 41). Nevertheless, the mechanisms underlying the antiphagocytosis function of S. suis CPS have never been addressed. In the present study, specific pathways used by the type 2 CPS of S. suis to protect it against phagocytosis by macrophages were investigated.
Macrophages can efficiently internalize latex beads to form nascent phagosomes displaying all their properties, such as maturation protein markers like LAMP1 (12, 23). To obtain CPS-linked latex beads, we adapted a technique commonly used to conjugate a protein carrier to CPS (21), replacing the protein by a latex bead with amine groups at its surface, which covalently link purified CPS. We obtained an artificial particle about the size of a bacterium, which allowed us to discriminate biological effects on phagocytes directly related with CPS without interference from other virulence factors. Our results showed that linking S. suis type 2 CPS to latex beads is sufficient to impair their phagocytosis not only by macrophages but also by dendritic cells. This could be due to CPS acting as a shell that prevents contact between the latex beads and the surface of macrophages. However, results obtained in the present study demonstrate that S. suis type 2 CPS plays a more active and specific role at the macrophage surface than simply acting as a physical barrier. Indeed, S. suis CPS seems to actively disrupt essential cellular processes involved in phagocytosis. This is supported by the fact that S. suis type 2 CPS-linked beads possess the ability to inhibit not only their own entry into phagocytes but also that of unlinked latex beads. It was recently reported that phagocytosis of latex microspheres by various phagocytes, including J774 macrophages, was suppressed by treatment with cholesterol or sphingolipid depletion agents, suggesting that lipid rafts play a significant role in incorporation of latex microspheres through phagocytosis by macrophages (30). Based on these observations, we evaluated the role of lipid rafts in S. suis type 2 interactions with macrophages. By using a nonencapsulated mutant, we showed that WT S. suis causes disruption of lipid microdomains at the macrophage surface, a trend observed in other pathogens such as Leishmania donovani, which modifies the organization of microdomains through its repetitive carbohydrate moieties (11). However, unlike the results in Leishmania, disruption of lipid rafts by S. suis type 2 CPS prevents phagocytosis. Lipid rafts have been shown to play a crucial role in immunity and phagocytic functions by acting as signaling platforms on the cell membrane (31, 51). Lipid microdomains are also targeted by many bacterial pathogens as a way of entry into host cells or as a way to penetrate mucosal barriers (1, 29, 45, 52). Porphyromonas gingivalis capitalizes on the lipid raft structure to down-modulate innate defense mechanisms (10). Compared to the number of studies of Gram-negative bacteria, few studies are available on the interactions of Gram-positive bacteria with lipid rafts of phagocyte membranes or on the role of CPS on modulation of these microdomains (45). For instance, a study has shown the ability of CPS from Salmonella enterica serovar Typhi to target specific components of lipid microdomains to suppress the inflammatory response of epithelial cells (40).
Other bacterial CPS being composed of diverse polysaccharide chains might have the ability to be easily recognized by macrophage lectins and regulate endocytosis as well as signaling patterns. Notably, the sialic acid-rich CPS of GBS interacts with Sia-binding immunoglobulin superfamily lectins (Siglecs) and impairs the bactericidal function of neutrophils (4). C-type lectin receptors (CTLs) are also recruited to lipid rafts upon activation, and integrity of lipid rafts is important for the signaling and cellular functions initiated by this class of innate receptors (49). There has been no report, however, on S. suis recognition via Siglecs or CTLs. It is interesting that in contrast to S. suis, GBS CPS seems to engage lipid rafts as a mechanism of entry to host cells (16) (M. Segura, presented at the XVIII Lancefield International Symposium, Palermo, Italy, 4 to 8 September 2011).
Insight into the disruption of lipid microdomains led us to investigate specific components involved in pathogen recognition that could be inhibited by S. suis type 2 CPS. Among the family of lipids composing the membrane microdomains, LacCer has been demonstrated to act as a PRR, binding various pathogens such as E. coli, B. pertussis, Bacillus dysenteriae, Propionibacterium freudenreichii, and C. albicans (25, 33, 51). Pathogen binding to LacCer induces superoxide production through Lyn and p38 mitogen-activated protein kinase (MAPK) (24, 31, 51). LacCer-enriched domains can also form supramolecular complexes with other PRRs for the phagocytosis of nonopsonized bodies (31). Here, we observed a clear inhibition of NO induction after encapsulated S. suis interaction with macrophages. Similarly, CPS also modulates inducible nitric oxide synthase expression and further NO production from S. suis-infected microglial cells (14). The possibility cannot be ruled out that reduced NO production by cells stimulated with encapsulated S. suis might be, in part, an indirect consequence arising from limited internalization of this strain compared to that of the CPS− mutant. However, previous experiments with cytochalasin-treated macrophages showed that the stimulation of cytokine production by S. suis is phagocytosis independent (38). As NO production has been linked to the cascade of LacCer and Lyn kinase (32), we examined whether LacCer accumulation on microdomains could be affected by the presence or absence of CPS on S. suis type 2. Our results show accumulation of LacCer in vesicles from macrophages interacting with the CPS− mutant of S. suis. In contrast, such accumulation was not observed with the well-encapsulated WT strain. The staining patterns of S. suis and LacCer show a clear proximity between this lipid and CPS− S. suis, which is in line with the described accumulation of LacCer at the phagocytic cup (31). The key role of LacCer as a PRR in immunity has been highlighted in recent years (25, 51). We showed in this study for the first time that CPS from S. suis type 2 prevents LacCer accumulation at the membrane of macrophages interacting with this bacterium. Moreover, inhibition of lipid microdomain-mediated entry in macrophage by filipin partially prevented CPS− S. suis from entering macrophages. These results suggest that encapsulated S. suis blocks its own phagocytosis by disrupting lipid raft-LacCer signaling pathways, whereas in the absence of CPS these pathways are engaged, resulting in CPS− mutant internalization. Phosphoinositide 3-kinase (PI3K)/Akt and p38 MAPK pathways are both known activators of phagocytic mechanisms and are recruited at the lipid raft-LacCer platform (51). Ligand binding to LacCer in LacCer-enriched microdomains induces activation of the Lyn/PI3K/p38 MAPK/protein kinase C (PKC) signal transduction pathway leading to phagocyte function activation (51). In this regard, previous work has shown high levels of Akt and PKC phosphorylation after infection of J774 macrophages with a nonencapsulated mutant of S. suis type 2, whereas the encapsulated strain showed reduced activation of the PI3K/Akt/PKC signaling pathway (37). In addition, p38 MAPK phosphorylation was impaired by the presence of CPS in S. suis-infected microglial cells (14).
Different studies have shown how CPS from S. suis type 2 can influence the outcome of the inflammatory response by hampering recognition of potential antigens of the bacterial cell wall by phagocytes (14, 20, 27, 28). Our data provide an additional function of S. suis type 2 CPS at the bacteria-cell interface. This would happen through destabilization of lipid microdomains, preventing accumulation of LacCer at the interaction points of S. suis with the membrane with consequent inhibition of key signaling pathways. CPS could also physically restrict proper access of LacCer to components of the bacterial cell wall, resulting in overall down-modulation of phagocyte innate functions.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by Natural Sciences and Engineering Research Council of Canada (NSERC) through a grant to M.S. (342150-07) and NSERC 154280 as well as Discovery Accelerator Supplement 380299 to M.G. M.S. is the recipient of a Fonds de recherche en santé du Québec (FRSQ) Career Award.
We are grateful to Damian Clarke (Université de Montréal, Canada) for reviewing the manuscript.
Footnotes
Published ahead of print 28 November 2011
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1. Abraham SN, Duncan MJ, Li G, Zaas D. 2005. Bacterial penetration of the mucosal barrier by targeting lipid rafts. J. Investig. Med. 53:318–321 [DOI] [PubMed] [Google Scholar]
- 2. Baums CG, Valentin-Weigand P. 2009. Surface-associated and secreted factors of Streptococcus suis in epidemiology, pathogenesis and vaccine development. Anim. Health Res. Rev. 10:65–83 [DOI] [PubMed] [Google Scholar]
- 3. Benga L, Fulde M, Neis C, Goethe R, Valentin-Weigand P. 2008. Polysaccharide capsule and suilysin contribute to extracellular survival of Streptococcus suis co-cultivated with primary porcine phagocytes. Vet. Microbiol. 132:211–219 [DOI] [PubMed] [Google Scholar]
- 4. Carlin AF, et al. 2009. Molecular mimicry of host sialylated glycans allows a bacterial pathogen to engage neutrophil Siglec-9 and dampen the innate immune response. Blood 113:3333–3336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chabot-Roy G, Willson P, Segura M, Lacouture S, Gottschalk M. 2006. Phagocytosis and killing of Streptococcus suis by porcine neutrophils. Microb. Pathog. 41:21–32 [DOI] [PubMed] [Google Scholar]
- 6. Charland N, Harel J, Kobisch M, Lacasse S, Gottschalk M. 1998. Streptococcus suis serotype 2 mutants deficient in capsular expression. Microbiology 144:325–332 [DOI] [PubMed] [Google Scholar]
- 7. Charland N, Jacques M, Lacouture S, Gottschalk M. 1997. Characterization and protective activity of a monoclonal antibody against a capsular epitope shared by Streptococcus suis serotypes 1, 2 and 1/2. Microbiology 143:3607–3614 [DOI] [PubMed] [Google Scholar]
- 8. Cornacchione P, et al. 1998. Group B streptococci persist inside macrophages. Immunology 93:86–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dale JB, Washburn RG, Marques MB, Wessels MR. 1996. Hyaluronate capsule and surface M protein in resistance to opsonization of group A streptococci. Infect. Immun. 64:1495–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Darveau RP. 2009. Bacteria modulate host-cell responses by capitalizing on the lipid raft structure. Future Microbiol. 4:155–157 [DOI] [PubMed] [Google Scholar]
- 11. Dermine JF, Goyette G, Houde M, Turco SJ, Desjardins M. 2005. Leishmania donovani lipophosphoglycan disrupts phagosome microdomains in J774 macrophages. Cell. Microbiol. 7:1263–1270 [DOI] [PubMed] [Google Scholar]
- 12. Desjardins M, Griffiths G. 2003. Phagocytosis: latex leads the way. Curr. Opin. Cell Biol. 15:498–503 [DOI] [PubMed] [Google Scholar]
- 13. Doherty GJ, McMahon HT. 2009. Mechanisms of endocytosis. Annu. Rev. Biochem. 78:857–902 [DOI] [PubMed] [Google Scholar]
- 14. Dominguez-Punaro MC, et al. 2010. In vitro characterization of the microglial inflammatory response to Streptococcus suis, an important emerging zoonotic agent of meningitis. Infect. Immun. 78:5074–5085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fox JD, Robyt JF. 1991. Miniaturization of three carbohydrate analyses using a microsample plate reader. Anal. Biochem. 195:93–96 [DOI] [PubMed] [Google Scholar]
- 16. Goluszko P, Popov V, Wen J, Jones A, Yallampalli C. 2008. Group B Streptococcus exploits lipid rafts and phosphoinositide 3-kinase/Akt signaling pathway to invade human endometrial cells. Am. J. Obstet. Gynecol. 199:548.e1-548.e9 [DOI] [PubMed] [Google Scholar]
- 17. Gottschalk M, Segura M. 2000. The pathogenesis of the meningitis caused by Streptococcus suis: the unresolved questions. Vet. Microbiol. 76:259–272 [DOI] [PubMed] [Google Scholar]
- 18. Gottschalk M, Segura M, Xu J. 2007. Streptococcus suis infections in humans: the Chinese experience and the situation in North America. Anim. Health Res. Rev. 8:29–45 [DOI] [PubMed] [Google Scholar]
- 19. Gottschalk M, Xu J, Calzas C, Segura M. 2010. Streptococcus suis: a new emerging or an old neglected zoonotic pathogen? Future Microbiol. 5:371–391 [DOI] [PubMed] [Google Scholar]
- 20. Graveline R, Segura M, Radzioch D, Gottschalk M. 2007. TLR2-dependent recognition of Streptococcus suis is modulated by the presence of capsular polysaccharide which modifies macrophage responsiveness. Int. Immunol. 19:375–389 [DOI] [PubMed] [Google Scholar]
- 21. Guttormsen HK, et al. 2008. Rational chemical design of the carbohydrate in a glycoconjugate vaccine enhances IgM-to-IgG switching. Proc. Natl. Acad. Sci. U. S. A. 105:5903–5908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Higgins R, Gottschalk M. 1990. An update on Streptococcus suis identification. J. Vet. Diagn. Invest. 2:249–252 [DOI] [PubMed] [Google Scholar]
- 23. Houde M, et al. 2003. Phagosomes are competent organelles for antigen cross-presentation. Nature 425:402–406 [DOI] [PubMed] [Google Scholar]
- 24. Iwabuchi K, Nagaoka I. 2002. Lactosylceramide-enriched glycosphingolipid signaling domain mediates superoxide generation from human neutrophils. Blood 100:1454–1464 [PubMed] [Google Scholar]
- 25. Jansson L, Tobias J, Lebens M, Svennerholm AM, Teneberg S. 2006. The major subunit, CfaB, of colonization factor antigen i from enterotoxigenic Escherichia coli is a glycosphingolipid binding protein. Infect. Immun. 74:3488–3497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kasper DL. 1986. Bacterial capsule-old dogmas and new tricks. J. Infect. Dis. 153:407–415 [DOI] [PubMed] [Google Scholar]
- 27. Lecours MP, et al. 2011. Critical role for Streptococcus suis cell wall modifications and suilysin in resistance to complement-dependent killing by dendritic cells. J. Infect. Dis. 204:919–929 [DOI] [PubMed] [Google Scholar]
- 28. Lecours MP, et al. 2011. Characterization of porcine dendritic cell response to Streptococcus suis. Vet. Res. 42:72–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Manes S, del Real G, Martinez AC. 2003. Pathogens: raft hijackers. Nat. Rev. Immunol. 3:557–568 [DOI] [PubMed] [Google Scholar]
- 30. Nagao G, Ishii K, Hirota K, Makino K, Terada H. 2011. Role of lipid rafts in innate immunity and phagocytosis of polystyrene latex microspheres. Colloids Surf. B Biointerfaces 84:317–324 [DOI] [PubMed] [Google Scholar]
- 31. Nakayama H, et al. 2008. Lyn-coupled LacCer-enriched lipid rafts are required for CD11b/CD18-mediated neutrophil phagocytosis of nonopsonized microorganisms. J. Leukoc. Biol. 83:728–741 [DOI] [PubMed] [Google Scholar]
- 32. Pannu R, Won JS, Khan M, Singh AK, Singh I. 2004. A novel role of lactosylceramide in the regulation of lipopolysaccharide/interferon-gamma-mediated inducible nitric oxide synthase gene expression: implications for neuroinflammatory diseases. J. Neurosci. 24:5942–5954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Prasad SM, Yin Y, Rodzinski E, Tuomanen EI, Masure HR. 1993. Identification of a carbohydrate recognition domain in filamentous hemagglutinin from Bordetella pertussis. Infect. Immun. 61:2780–2785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rohde M, Muller E, Chhatwal GS, Talay SR. 2003. Host cell caveolae act as an entry-port for group A streptococci. Cell Microbiol. 5:323–342 [DOI] [PubMed] [Google Scholar]
- 35. Segura M, Cleroux P, Gottschalk M. 1998. Streptococcus suis and group B Streptococcus differ in their interactions with murine macrophages. FEMS Immunol. Med. Microbiol. 21:189–195 [DOI] [PubMed] [Google Scholar]
- 36. Segura M, Gottschalk M. 2002. Streptococcus suis interactions with the murine macrophage cell line J774: adhesion and cytotoxicity. Infect. Immun. 70:4312–4322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Segura M, Gottschalk M, Olivier M. 2004. Encapsulated Streptococcus suis inhibits activation of signaling pathways involved in phagocytosis. Infect. Immun. 72:5322–5330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Segura M, Stankova J, Gottschalk M. 1999. Heat-killed Streptococcus suis capsular type 2 strains stimulate tumor necrosis factor alpha and interleukin-6 production by murine macrophages. Infect. Immun. 67:4646–4654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Severi E, Hood DW, Thomas GH. 2007. Sialic acid utilization by bacterial pathogens. Microbiology 153:2817–2822 [DOI] [PubMed] [Google Scholar]
- 40. Sharma A, Qadri A. 2004. Vi polysaccharide of Salmonella Typhi targets the prohibitin family of molecules in intestinal epithelial cells and suppresses early inflammatory responses. Proc. Natl. Acad. Sci. U. S. A. 101:17492–17497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Smith HE, et al. 1999. Identification and characterization of the cps locus of Streptococcus suis serotype 2: the capsule protects against phagocytosis and is an important virulence factor. Infect. Immun. 67:1750–1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Timoney JF. 2004. The pathogenic equine streptococci. Vet. Res. 35:397–409 [DOI] [PubMed] [Google Scholar]
- 43. Valentin-Weigand P, Benkel P, Rohde M, Chhatwal GS. 1996. Entry and intracellular survival of group B streptococci in J774 macrophages. Infect. Immun. 64:2467–2473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Van Calsteren MR, Gagnon F, Lacouture S, Fittipaldi N, Gottschalk M. 2010. Structure determination of Streptococcus suis serotype 2 capsular polysaccharide. Biochem. Cell Biol. 88:513–525 [DOI] [PubMed] [Google Scholar]
- 45. Vieira FS, Correa G, Einicker-Lamas M, Coutinho-Silva R. 2010. Host-cell lipid rafts: a safe door for micro-organisms? Biol. Cell 102:391–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang M, Hajishengallis G. 2008. Lipid raft-dependent uptake, signalling and intracellular fate of Porphyromonas gingivalis in mouse macrophages. Cell. Microbiol. 10:2029–2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ward PN, Field TR, Ditcham WG, Maguin E, Leigh JA. 2001. Identification and disruption of two discrete loci encoding hyaluronic acid capsule biosynthesis genes hasA, hasB, and hasC in Streptococcus uberis. Infect. Immun. 69:392–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Williams AE, Blakemore WF. 1990. Pathogenesis of meningitis caused by Streptococcus suis type 2. J. Infect. Dis. 162:474–481 [DOI] [PubMed] [Google Scholar]
- 49. Xu S, Huo J, Gunawan M, Su IH, Lam KP. 2009. Activated dectin-1 localizes to lipid raft microdomains for signaling and activation of phagocytosis and cytokine production in dendritic cells. J. Biol. Chem. 284:22005–22011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ye C, et al. 2006. Streptococcus suis sequence type 7 outbreak, Sichuan, China. Emerg. Infect. Dis. 12:1203–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yoshizaki F, et al. 2008. Role of glycosphingolipid-enriched microdomains in innate immunity: microdomain-dependent phagocytic cell functions. Biochim. Biophys. Acta 1780:383–392 [DOI] [PubMed] [Google Scholar]
- 52. Zaas DW, Duncan M, Rae Wright J, Abraham SN. 2005. The role of lipid rafts in the pathogenesis of bacterial infections. Biochim. Biophys. Acta 1746:305–313 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.