Abstract
Live recombinant attenuated Salmonella vaccine (RASV) strains have great potential to induce protective immunity against Mycobacterium tuberculosis by delivering M. tuberculosis antigens. Recently, we reported that, in orally immunized mice, RASV strains delivering the M. tuberculosis early secreted antigenic target 6-kDa (ESAT-6) protein and culture filtrate protein 10 (CFP-10) antigens via the Salmonella type III secretion system (SopE amino-terminal region residues 1 to 80 with two copies of ESAT-6 and one copy of CFP-10 [SopENt80-E2C]) afforded protection against aerosol challenge with M. tuberculosis. Here, we constructed and evaluated an improved Salmonella vaccine against M. tuberculosis. We constructed translational fusions for the synthesis of two copies of ESAT-6 plus CFP-10 fused to the OmpC signal sequence (OmpCSS-E2C) and amino acids 44 to 338 of antigen 85A (Ag85A294) flanked by the signal sequence (SS) and C-terminal peptide (CT) of β-lactamase (BlaSS-Ag85A294-BlaCT) to enable delivery via the Salmonella type II secretion system. The genes expressing these proteins were cloned as an operon transcribed from Ptrc into isogenic Asd+/MurA+ pYA3681 lysis vector derivatives with different replication origins (pBR, p15A, pSC101), resulting in pYA4890, pYA4891, and pYA4892 for SopENt80-E2C/Ag85A294 synthesis and pYA4893 and pYA4894 for OmpCSS-E2C/Ag85A294 synthesis. Mice orally immunized with the RASV χ11021 strain engineered to display regulated delayed lysis and regulated delayed antigen synthesis in vivo and harboring pYA4891, pYA4893, or pYA4894 elicited significantly greater humoral and cellular immune responses, and the RASV χ11021 strain afforded a greater degree of protection against M. tuberculosis aerosol challenge in mice than RASVs harboring any other Asd+/MurA+ lysis plasmid and immunization with M. bovis BCG, demonstrating that RASV strains displaying regulated delayed lysis with delayed antigen synthesis resulted in highly immunogenic delivery vectors for oral vaccination against M. tuberculosis infection.
INTRODUCTION
Tuberculosis (TB) is one of the three major infectious diseases, along with AIDS and malaria, that are serious global health threats. Approximately 8 million new cases of TB are diagnosed every year throughout the world, and approximately 2 million people die of this disease each year (72). Although there are antibiotics for effectively treating TB, strains of Mycobacterium tuberculosis resistant to multiple drugs are increasing annually, compromising our ability to treat TB (5). The only available vaccine, an attenuated strain of Mycobacterium bovis Bacille Calmette-Guérin (BCG), is effective in preventing serious complications of TB in infants and small children, but this vaccine does not confer long-lasting immunity to infection (6, 29, 69), its efficacy in preventing TB in adults is variable, and the vaccine can cause disseminated disease in immunocompromised individuals (64).
Recombinant attenuated Salmonella vaccines (RASVs) offer an attractive alternative to BCG and its recombinant derivatives for delivering M. tuberculosis antigens to elicit long-lasting protective immunity.
Oral administration of Salmonella results in colonization of the Peyer's patches via M cells in mammalian intestinal tracts and colonization of the mesenteric lymph nodes, liver, and spleen, resulting in the generation of a range of humoral and cellular immune responses against Salmonella at local and distal sites (15). Live attenuated Salmonella strains have been especially useful as carrier systems for delivery of recombinant heterologous antigens from bacterial, parasitic, viral, and tumor sources (15, 50). The R. Curtiss group has designed and developed a series of systems to increase the safety, efficacy, tolerability, immunogenicity, and utility of Salmonella for delivery of recombinant heterologous antigens (reviewed in reference 24). For example, balanced-lethal host-vector systems that have been generated on the basis of complementation of chromosomal deletions of genes such as asdA or murA in the RASVs eliminate the need for drug resistance markers in these vaccine strains (20, 24, 31, 43, 57). The asdA and murA genes encode enzymes involved in the biosynthesis of the bacterial cell wall (8, 13), and the asdA deletion imposes an obligate requirement for diaminopimelic acid (DAP) in noncomplemented mutant strains. Curtiss et al. (22), Kong et al. (46), and Wang et al. (71) have also developed RASVs that, in vivo, display regulated delayed attenuation (22), regulated delayed antigen synthesis (71), and regulated delayed lysis (46), to aid in the induction of high levels of immunogenicity. The genetic mechanisms that make possible these particular phenotypes have been generated by inclusion of a collection of defined deletion or deletion-insertion mutations in specific genes implicated in either the metabolism or virulence of Salmonella that eliminate their gene products or regulate their expression by replacing their original promoters with the tightly arabinose-regulated araC PBAD activator promoter (Fig. 1A and B) (22, 24, 46). Regulated delayed in vivo synthesis of protective heterologous antigens has been engineered to enhance immune responses by reducing the adverse effects of high-level heterologous antigen synthesis on Salmonella growth at the time of vaccination. This system is based on the presence of a chromosomal lactose repressor gene (lacI) under the transcriptional control of the arabinose-regulated araC PBAD promoter by the inclusion of the ΔrelA198::araC PBAD lacI TT deletion-insertion mutation (where P stands for promoter and TT stands for transcriptional terminator). LacI negatively regulates the expression from Ptrc that drives the synthesis of heterologous antigens (Fig. 1C) (2). In animal tissues, where arabinose is unavailable, the concentration of LacI decreases with each bacterial cell division, thus allowing increased antigen synthesis (Fig. 1D) (reviewed in reference 24). Strategies to achieve regulated delayed in vivo attenuation have been previously described (20, 21, 22, 24, 25, 49).
Fig 1.
Regulated delayed lysis and regulated delayed synthesis of heterologous antigens. (A) Arabinose-regulated araC PBAD activator promoter. In the presence of arabinose, the AraC protein changes its conformation and forms a dimer that binds the I1 and I2 sites, and then Crp and RNA polymerase bind the complex and activate the transcription of the araBAD genes. (B) In the absence of arabinose, two distal AraC molecules interact and one of them binds the O2 site and the other the I1 site, which generates a DNA loop that represses the transcription from the araC PBAD promoter. (C) In the RASV strains with regulated delayed lysis and synthesis of heterologous antigens, in the presence of arabinose, the transcription of the genes under the araC PBAD promoter is induced on the chromosome and on the plasmid, for the biosynthesis of the cell wall and the synthesis of the repressor proteins C2 and LacI. LacI negatively represses the expression from the Ptrc promoter. (D) In the animal tissues, arabinose is not available, and the synthesized proteins are diluted during each cell division, resulting in the synthesis of the heterologous antigens and bacterial cell lysis.
The regulated delayed lysis system has been engineered in Salmonella to release protective heterologous antigens and confer biological containment (46). It consists of two components. The first component is the RASV strain, which contains the ΔasdA27::TT araC PBAD c2 deletion-insertion mutation for the elimination of DAP synthesis and the ΔPmurA25::TT araC PBAD murA deletion-insertion mutation for arabinose-regulated expression of the murA gene. The murA gene encodes the first enzyme in muramic acid synthesis. Both DAP and muramic acid are required for synthesis of peptidoglycan in the bacterial cell wall. The bacteriophage P22 C2 repressor protein is also synthesized in the presence of arabinose to repress the transcription from the P22 PR promoter (Fig. 1C) (66). The second component of this lysis system is the Asd-positive (Asd+)/MurA-positive (Asd+/MurA+) lysis plasmid vector pYA3681. It carries the nucleotide sequences of the Shine-Dalgarno (SD)-GTG-asdA and SD-GTG-murA genes with GTG start codons to reduce the translation efficiency of the asd and murA genes on multicopy plasmids (46). These genes are in an operon that is under the transcriptional control of the tightly regulated araC PBAD activator-promoter. Downstream from this operon and in the opposite direction is the C2-repressible P22 PR promoter, which in the absence of C2 synthesizes antisense asd and murA mRNAs to block translation of any residual asd and murA mRNA made in the absence of arabinose (Fig. 1C and D) (46). The growth of these mutant strains in the presence of arabinose leads to synthesis of Asd, MurA, and C2, which enable synthesis of peptidoglycan. After oral vaccination, these bacteria are able to colonize the effector lymphoid tissues to levels similar to those seen with the wild-type strain, prior to the decrease in the Asd, MurA, and C2 levels during cell division, because transcription from the PBAD promoter ceases in vivo due to the absence of available arabinose in the host (Fig. 1D) (46). The inability of the RASV strain to synthesize Asd and MurA in vivo results in lysis with release of the previously synthesized heterologous protective antigens for better processing and presentation to the host immune system (46).
Mucosal immunity induced by oral vaccination with Salmonella vaccines is also effective in protecting the surfaces in the lung due to the networks of the mucosal immune system (37). Furthermore, live recombinant Salmonella vaccines induce gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) to augment the Th1 immune responses that are important in controlling infections and preventing diseases caused by intracellular pathogens such as M. tuberculosis (26, 52).
Among the T-cell antigens that have been reported to be protective against M. tuberculosis infection are the early secreted antigenic target 6-kDa (ESAT-6) protein and culture filtrate protein 10 (CFP-10) (3, 55, 60) and the antigen 85 (Ag85) complex, which includes the structurally related and secreted Ag85A, Ag85B, and Ag85C proteins (28, 41, 51). ESAT-6 and CFP-10 are virulence factors that are restricted to the organisms within the M. tuberculosis complex, while the Ag85 complex is conserved among mycobacterial species. These antigens are immunodominant in early phases of M. tuberculosis infection in mice. ESAT-6, Ag85A, and Ag85B possess multiple T-cell epitopes that are recognized by T cells of human TB patients with different HLA haplotypes, inducing stimulation of B- and T-cell proliferation and IFN-γ production (48, 61).
In this study, we designed and constructed RASVs displaying regulated delayed synthesis of antigens and the delayed lysis phenotype to deliver protective mycobacterial antigens into mice to evaluate the following: (i) the efficacy of oral immunization of mice with RASV strains synthesizing M. tuberculosis Ag85A alone or in combination with ESAT-6 and CFP-10 against aerosol challenge with M. tuberculosis; (ii) the efficient delivery and immunogenicity of Ag85A flanked in frame by the signal sequence and carboxy-terminal region of the β-lactamase (BlaSS and BlaCT, respectively) protein and ESAT-6 and CFP-10 fused to the OmpC signal sequence (both of these signal sequences allow the chimeric proteins to be secreted via the Salmonella type II secretion system [T2SS]); (iii) the delivery and immunogenicity of the BlaSS-Ag85-BlaCT protein in combination with ESAT-6 and CFP-10 fused to the SopE effector protein when the latter chimeric protein is translocated into the mammalian cell cytoplasm via the type III secretion system (T3SS); (iv) the effect of the copy number of isogenic Asd+/MurA+ plasmids (producing ESAT-6, CFP-10, and Ag85A), each containing a different replication origin such as pBR (9), p15A (18), or pSC101 (16), on immunogenicity and protection against aerosol challenge with M. tuberculosis; (v) the humoral and cellular immune responses elicited by combinations of the chimeric proteins delivered by the RASVs following oral immunization of mice; and (vi) the immunogenicity in vivo of RASV strain χ11021 displaying regulated delayed lysis (with Asd+/MurA+ lysis plasmids) compared to that of RASV strain χ9879 displaying regulated delayed attenuation and constitutively expressing fbpA to produce Ag85A.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
The bacterial strains and plasmids used in this study are listed in Table 1. Luria-Bertani (LB) broth (7) supplemented with 0.05% arabinose or supplemented with 0.2% arabinose and 0.2% mannose was used to grow the Salmonella strains for the immunization assays. LB broth, LB agar (1.5% agar), LB agar supplemented with 0.2% arabinose and 0.2% mannose, or MacConkey agar (Difco) was used for propagation and plating of Salmonella. For the growth of noncomplemented ΔasdA strains and plasmid stability tests, 50 μg/ml DAP was added to the growth medium (57). Middlebrook 7H9 broth and Middlebrook 7H11 agar (Difco) supplemented with 10% oleic acid-albumin-dextrose-catalase enrichment were used to grow M. tuberculosis.
Table 1.
Strains and plasmids used in this work
| Strain or plasmid | Derived, relevant genotype, or characteristica | Source or reference |
|---|---|---|
| Escherichia coli | ||
| χ6212 | ΔasdA4 | 43 |
| χ7213 | F−supE42 λ− T3rthi-1 thr-1 leuB6 supE44 tonA21 fhuA21 lacY1 recA1 RP4 2-Tc::Mu (λpir) ΔasdA4 Δ(zhf-2::Tn10) | 22 |
| LMG194 | F− ΔlacX74 galE thi rpsL ΔphoA(PvuII) Δara714 leu::Tn10 | Invitrogen |
| Salmonella enterica serovar Typhimurium | ||
| χ9879 | ΔasdA33 ΔPphoPQ176::TT araC PBADphoΔPcrp527::TT araC PBADcrp ΔaraBAD23 | 42 |
| χ11021 | ΔPmurA25::TT araC PBADmurA ΔasdA27::TT araC PBADc2ΔaraBAD23 Δ(gmd-fcl)-26 Δpmi-2426ΔrelA198::TT araC PBADlacI | This study |
| Mycobacterium | ||
| M. tuberculosis H37Rv | ATCC 25618 | |
| M. bovis BCG | Pasteur | ATCC 35734 |
| Plasmids | ||
| Suicide vectors | ||
| pYA3546 | pDMS197 Δpmi-2426 | 25 |
| pYA3629 | pMEG-375 Δ(gmd-fcl)-26 | 25 |
| pYA4064 | pRE112 ΔrelA198::TT araC PBADlacI | 34 |
| pYA4138 | pRE112 ΔasdA27::TT araC PBADc2 | 34 |
| pYA4280 | pRE112 Δ(araC PBAD)-5::PRaraBAD | 23 |
| pYA4343 | pRE112 ΔPphoPQ176::TT araC PBADphoPQ | 22 |
| pYA4686 | pRE112 ΔPmurA25::TT araC PBADmurA | Laboratory stock |
| Asd+ plasmids | ||
| pYA3342 | asdA+ vaccine vector, pBR ori | 43 |
| pYA3620 | asdA+ expression vector, Ptrc pBR ori blaSS-blaCT-T2SS signal sequence based | 44 |
| pYA3941 | pYA3620 blaSS-fbpA294 (optimized)-blaCT | This study |
| Asd+/MurA+ lysis plasmids | ||
| pYA3681 | asdA+murA+ expression vector, Ptrc pBR ori araC* PBADSD-GTG-asdA SD-GTG-murA P22 PR antisense mRNA | 46 |
| pYA4890 | pYA3681 sopENt80-esxA-esxA-esxB/(SD) blaSS-fbpA294-blaCT pBR ori | This study |
| pYA4893 | pYA3681 ompCSS-esxA-esxA-esxB/(SD) blaSS-fbpA294-blaCT pBR ori | This study |
| pYA4589 | asdA+murA+ expression vector, Ptrc p15A ori araC* PBADSD-GTG-asdA SD-GTG-murA P22 PR antisense mRNA | This study |
| pYA4891 | pYA4589 sopENt80-esxA-esxA-esxB/(SD) blaSS-fbpA294-blaCT p15A ori | This study |
| pYA4892 | pYA4595 sopENt80-esxA-esxA-esxB/(SD) blaSS-fbpA294-blaCT pSC101 ori | This study |
| pYA4894 | pYA4589 ompCSS-esxA-esxA-esxB/(SD) blaSS-fbpA294-blaCT p15A ori | This study |
| pYA4595 | asdA+murA+ expression vector, Ptrc pSC101 ori araC* PBADSD-GTG-asdA SD-GTG-murA P22 PR antisense mRNA | This study |
| pBK-CMV and its derivatives | ||
| pBK-CMV | Kmr, cloning vector | Stratagene |
| pYA3817 | pBK-CMV fbpA | This study |
| pYA3932 | pBK-CMV fbpA optimized | This study |
| Plasmids expressing 6×His-tagged recombinant proteins | ||
| pYA3814 | pBAD/His(B), 6×His-Ag85A | This study |
| pMRLB7 | Ampr, pET23+ ESAT-6–6×His | NIH-TB vaccine |
In the descriptions of the genotypes, TT is transcription terminator, P stands for promoter, and the subscripted number refers to a composite deletion and insertion of the indicated gene. Ampr, ampicillin resistance; Kmr, kanamycin resistance.
DNA procedures.
DNA manipulations were carried out using standard procedures (63). Plasmid DNA was isolated using a QIAprep Spin miniprep kit (Qiagen Inc., Valencia, CA). Restriction enzymes were used as recommended by the manufacturer (New England BioLabs, Ipswich, MA). Plasmid constructs were verified by DNA sequencing (Arizona State University facilities).
Construction of plasmids pYA3817 and pYA3932.
A DNA fragment containing the nucleotide sequence of the fbpA gene (Rv3804c), encoding the Ag85A protein, was PCR amplified from M. tuberculosis H37Rv chromosomal DNA. The PCR product of 1,111 bp was digested with XbaI and EcoRI and cloned into XbaI-EcoRI-digested pBK-CMV (Stratagene) to generate pYA3817. To optimize the expression of fbpA in Salmonella, pYA3817 was used as the template to replace 24 codons of fbpA with the codons most frequently found in Salmonella using a QuikChange site-directed mutagenesis kit (Stratagene) with appropriate primers. fbpA codons 62 (TCC to TCT), 63 (CGG to CGT), 66 (TTG to CTG), 88 (AGT to AGC), 94 (CCC to CCG), 136 (TCA to TCT), 145 (CCC to CCG), 173 (AGG to CGT), 177 (CCC to CCG), 179 (GGA to GGT), 200 (CCC to CCG), 207 (GGA to GGT), 213 (TTG to CTG), 215 (CCC to CCG), 221 (CCC to CCG), 255 (TTG to CTG), 258 (GGG to GGT), 294 (CGG to CGT), 336 (CCC to CCG), 240 (CGG to CGT), 346 (CCC to CCG), 349 (GGG to GGT), 250 (CCC to CCG), and 252 (CCC to CCG) were substituted. The resulting recombinant plasmid containing all of the optimized sequences of fbpA was named pYA3932 (Table 1).
Construction of Asd+ plasmid pYA3941.
A 900-bp DNA fragment containing the nucleotide sequence of codon-optimized M. tuberculosis fbpA, encoding amino acid residues 44 to 338 of the mature Ag85A protein, was PCR amplified from pYA3932. This PCR product was digested with EcoRI and SalI and was cloned into EcoRI-SalI-digested pYA3620 to generate pYA3941. Plasmid pYA3620 is an Asd+ vector containing the pBR replication origin (ori) and Ptrc to drive the expression of the gene of interest, which is flanked by the signal sequence (SS) and carboxy-terminal region (CT) of the β-lactamase gene in order to direct the secretion of the resulting protein into the periplasm and supernatant (25). Thus, plasmid pYA3941 carries the nucleotide sequence blaSS-fbpA294-blaCT (where fbpA294 is the gene encoding recombinant Ag85A from amino acids 44 to 338 [Ag85A294]). Both β-lactamase regions are required for the efficient secretion of the recombinant protein (12, 47).
Construction of Asd+/MurA+ lysis vectors pYA4890, pYA4891, and pYA4892 for expression of sopENt80-esxA-esxA-esxB/blaSS-fbpA294-blaCT.
An 1,135-bp DNA fragment containing the nucleotide sequence of sopE80-esxA-esxA-esxB, an operon fusion encoding the chimeric protein formed with the SopE secretion and translocation signal sequence in amino acids 1 to 80 (SopENt80), two copies of the ESAT-6 protein, and one copy of the CFP-10 protein (SopENt80-E2C) was PCR amplified from pYA4257 (42). The PCR product was digested with NcoI and XmaI and cloned into pYA3681 digested with the same enzymes (46). Plasmid pYA3681 is an Asd+/MurA+ lysis expression vector containing the pBR origin of replication (ori). The plasmid obtained was digested with NotI and XmaI, and an 1,120-bp NotI-XmaI-digested DNA fragment carrying the nucleotide sequence of blaSS-fbpA294-blaCT (PCR amplified from pYA3941) was cloned into the plasmid to generate pYA4890 (Table 1). Plasmid pYA4890 contains the sopENt80-esxA-esxA-esxB and SD sequence blaSS-fbpA294-blaCT in an operon transcribed from the Ptrc promoter. This operon, without Ptrc, was released from pYA4890 by digestion with NcoI and XmaI and subcloned into isogenic Asd+/MurA+ lysis vectors pYA4589 (p15A ori) and pYA4595 (pSC101 ori), which were digested with the same enzymes, generating pYA4891 and pYA4892, respectively (Table 1).
Construction of Asd+/MurA+ lysis vectors pYA4893 and pYA4894 for expression of ompCSS-esxA-esxA-esxB/blaSS-fbpA294-blaCT.
A 97-bp DNA fragment encoding the Salmonella ompC signal sequence (ompCSS) flanked with sticky NcoI-XmaI ends was obtained by annealing two complementary single-stranded 97-b oligonucleotides, pPSOC-F1 and pPSOC-R1. The 97-bp NcoI-XmaI synthetic DNA was cloned into NcoI-XmaI-digested pYA3342 (39). Plasmid pYA3342 is an Asd+ expression vector with Ptrc and pBR ori (Table 1). The resulting plasmid was digested with EcoRI and XmaI, and a 906-bp DNA fragment containing the esxA-esxA-esxB fused genes (PCR amplified from pYA4257) and digested with the same enzymes was cloned into the plasmid. The resulting plasmid was digested with NotI and XmaI, and the 1,120-bp NotI-XmaI-digested DNA fragment encoding blaSS-fbpA294-blaCT was cloned into it. This plasmid was digested with NcoI and XmaI to release the 2,255-bp DNA fragment containing the ompCSS-esxA-esxA-esxB and SD blaSS-fbpA294-blaCT sequences, which were subcloned into the isogenic plasmids pYA3681 (pBR ori) and pYA4589 (p15A ori) to generate pYA4893 and pYA4894, respectively.
Construction of pYA3814 producing histidine-tagged Ag85A protein.
For production of the recombinant 6×His-tagged Ag85A protein (His6-Ag85A), a 950-bp DNA fragment containing the nucleotide sequence of the fbpA gene without its signal peptide was PCR amplified from M. tuberculosis H37Rv chromosomal DNA. The PCR product was digested with BglII and EcoRI and cloned into the BglII-EcoRI-digested pBAD/His(B) vector (Invitrogen) to obtain pYA3814 (Table 1).
Construction and phenotypic characterization of RASV strains.
The RASV strains used in this study, such as χ9879 (42) and χ11021, were designed to allow regulated delayed attenuation in vivo and regulated delayed lysis in vivo, respectively. Briefly, suicide vectors were used to construct strain χ11021 containing the nucleotide sequences designed to delete specific genes or introduce defined deletion-insertion mutations with modified promoter (P), SD, and start codon sequences (Table 1). RASV strain construction and characterization were performed as described previously (22, 34, 46, 49, 67, 71). Maps of the deletions and deletion-insertion mutations have been described previously for Δ(gmd-fcl)-26, Δpmi-2426, ΔrelA198::araC PBAD lacI TT, and Δasd27::TT araC PBAD c2 (49).
Determination of plasmid stability.
Stability of the recombinant plasmids was determined for approximately 50 generations of bacterial growth in LB medium under selective and nonselective (presence of DAP) conditions as described previously (22, 32, 57, 67).
Growth conditions for synthesis of recombinant M. tuberculosis Ag85A protein.
Escherichia coli strain LMG194 (Invitrogen) transformed with pYA3814 (His6-Ag85A) was grown in minimal salts medium (19) supplemented with 0.2% (vol/vol) glycerol, 2% Casamino Acids, 50 μg/ml thiamine, and 100 μg/ml ampicillin at 37°C to an optical density at 600 nm (OD600) of 0.5, and production of the recombinant His6-Ag85A protein was induced with 0.05% arabinose for 5 h.
Antigen preparation.
His6-Ag85A protein was purified by nickel-nitrilotriacetic acid (Ni-NTA) agarose chromatography under native conditions. Expression and purification of the recombinant His6–ESAT-6 protein have been described previously (42). The purified recombinant protein His6-Ag85A was used for production of polyclonal rabbit antisera. For recombinant proteins used in enzyme-linked immunosorbent assays (ELISAs) and enzyme-linked immunospot (ELISPOT) assays, endotoxins were removed from the Ni-NTA-purified proteins using Detoxi-Gel endotoxin-removing gels (Pierce, Rockford, IL). The endotoxin content in the recombinant proteins was determined with the Limulus amebocyte lysate assay (Cambrex Bio Science Walkersville, Inc., Walkersville, MD), which was used according to the manufacturer's instructions. The amount of endotoxin detected was <0.01 endotoxin unit (EU) per μg of recombinant protein. The Salmonella outer membrane proteins (SOMPs) were obtained from Salmonella enterica serovar Typhimurium strain χ4746, using the sonication and Triton X-100 extraction procedure described elsewhere (43, 53).
Analysis of synthesis and secretion of the recombinant proteins.
To analyze the synthesis of recombinant Ag85A294, cultures of S. Typhimurium χ9879 transformed with pYA3941 (blaSS-fbpA294-blaCT) were grown to an OD600 of 0.8 in LB broth supplemented with 0.05% arabinose. For whole-cell lysates, 50 μl of culture was centrifuged and the pellet was resuspended with 10 μl of phosphate-buffered saline (PBS) and 15 μl of lithium dodecyl sulfate (LDS) sample buffer (Invitrogen). For the isolation of proteins released into the culture supernatant, 1 ml of supernatant was filtered using a 0.22-μm-pore-size filter (Corning Gilbert Inc., Glendale, AZ) and precipitated with 10% trichloroacetic acid (TCA). The pellet obtained by centrifugation was resuspended in 50 μl of LDS sample buffer. Then, 25 μl of each sample was boiled for 5 min, analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 4 to 20% gels (Bio-Rad), and immunoblotted. To analyze the production and secretion of the recombinant proteins by S. Typhimurium χ11021 strains independently harboring each Asd+/MurA+ lysis plasmid (pYA4890, pYA4891, and pYA4892, which synthesize the DNA construct that encodes the chimeric protein ESAT-6–ESAT-6–CFP-10 [E2C] fused to Salmonella SopE protein amino acids [aa] 1 to 80 [SopENt80] [SopENt80-E2C], SopENt80-E2C/Ag85A294, or pYA4893 and pYA4894, which synthesize OmpCSS-E2C/Ag85A294), cultures were grown at 37°C to an OD600 of 0.8 in LB broth supplemented with 0.2% arabinose and 0.2% mannose. Parallel cultures were grown to an OD600 of 0.5 in LB broth supplemented with 0.2% arabinose and 0.2% mannose. Then, the cultures were washed once with LB broth, the OD600 was adjusted to 0.5 in prewarmed LB broth supplemented with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG), and the cultures were grown to an OD600 of 0.8. Whole-cell lysates and supernatant fractions were obtained and analyzed as described above for strain χ9879(pYA3941).
Immunoblotting.
The recombinant proteins were identified by immunoblotting using rabbit anti-Ag85A (generated in this study) and anti-ESAT-6 sera (42), followed by alkaline phosphatase-conjugated goat anti-rabbit IgG (Sigma). All of these antibodies were used at a 1:5,000 dilution.
Immunization of mice.
Female 6- to 7-week-old C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA). The Arizona State University Animal Care and Use Committee approved all animal procedures. Mice were acclimated for 7 days before starting the experiments. A group of 6 mice was vaccinated subcutaneously with a single dose of 5 × 10 4 CFU of M. bovis BCG at day 0. For immunization with attenuated recombinant Salmonella strains, mice were deprived of food and water for 6 h before oral immunization. Cultures of Salmonella strains χ9879(pYA3620) vector control and χ9879(pYA3941) were grown at 37°C to an OD600 of 0.8 in LB broth with 0.05% arabinose. Cultures of S. Typhimurium χ11021(pYA3681) vector control, χ11021(pYA4890), χ11021(pYA4891), χ11021(pYA4892), χ11021(pYA4893), and χ11021(pYA4894) were grown at 37°C to an OD600 of 0.8 in LB broth supplemented with 0.2% arabinose and 0.2% mannose. The cultures were centrifuged at room temperature (4,000 × g for 15 min), and the pellet was resuspended in 1 ml of buffered saline containing 0.01% gelatin (BSG) (19). Dilutions of the vaccine strains were plated onto LB agar plates (0.2% arabinose, 0.2% mannose) to determine bacterial titers. Groups of 12 mice were orally inoculated with 20 μl of BSG containing 1× 109 CFU of the individual RASVs on days 0, 7, and 49, and another group of 12 mice received 20 μl of BSG on the same days. Water and food were returned to the mice 30 min after immunization. Blood samples were obtained by submandibular vein puncture 2 days before vaccination and at days 21 and 65 after the first vaccination with recombinant strains χ9879(pYA3620) and χ9879(pYA3941) or at day 77 after the first vaccination with recombinant strain χ11021 transformed independently with each Asd+/MurA+ lysis vector derivative. Blood was centrifuged at 4,000 × g for 5 min, and the serum was removed and stored at −70°C until use.
Aerosol challenge with virulent M. tuberculosis.
To assess the protective efficacy of the recombinant Salmonella vaccine strains against M. tuberculosis infection, groups of 5 to 6 immunized mice or those vaccinated with the M. bovis BCG strain or with the control group fed BSG were infected at 4 weeks after the last immunization (day 77) with an estimated inhaled dose of 100 CFU of M. tuberculosis H37Rv per lung, delivered by aerosol in an inhalation exposure system (Glas-Col LLC, Terre Haute, IN). The mice were euthanized at 6 weeks after challenge, and the lungs and the spleen were aseptically collected. Bacterial load in the lungs and spleens was determined by serial dilutions of individual whole-organ homogenates in sterile PBS. Serial dilutions of the samples were plated in duplicate on Middlebrook 7H11 agar supplemented with 10% oleic acid-albumin-dextrose-catalase enrichment. Colonies were counted after 4 weeks of incubation at 37°C. In this study, protection is defined as a bacterial tissue load that is statistically significantly lower than the bacterial load in the BSG control group.
ELISA.
Total IgG, IgG2b, and IgG1 antibody titers against Ag85A and IgG responses to ESAT-6, CFP-10, and SOMPs from vaccinated mice and controls were determined by ELISA. Nunc Immunoplate Maxisorb F96 plates (Nalge Nunc, Rochester, NY) were coated with purified Ag85A at 0.5 μg/well, ESAT-6 or CFP-10 at 1 μg/well, or SOMPs at 0.5 μg/well suspended in 0.05 M carbonate-bicarbonate buffer, pH 9.6. Sera obtained from the same experimental group were pooled and serially diluted by 2-fold dilutions from an initial dilution of 1:200 in PBS. ELISAs were performed in triplicate, as described previously (42). Endpoint titers were expressed as the last sample dilution with an absorbance of 0.1 OD unit above that for the negative controls after 1 h of incubation.
Evaluation of cytokine-secreting cell numbers in the spleen.
The ELISPOT assay was performed using the ELISPOT assay kits (mouse IFN-γ, TNF-α, interleukin-2 [IL-2], and IL-4 ELISPOT assay sets; eBioscience) according to the manufacturer's instructions, to enumerate the IFN-γ,TNF-α, IL-2, and IL-4 cytokine-secreting cells in the spleens of immunized and naïve mice. ELISPOT assays were conducted 3 weeks after the last immunization with the pool of spleens from three mice from the same group; these assays were also done in triplicate. Spleen cells from all groups of mice were incubated with the recombinant antigen at 1 μg/well or culture medium at 37°C in a humidified (5% CO2-in-air) incubator. Splenocytes from mice immunized with Salmonella strains χ9879(pYA3620) and χ9879(pYA3941) were incubated for 24 h (for IFN-γ- and TNF-α-secreting cells) or 48 h (for IL-2- and IL-14-secreting cells), while the spleen cells from mice immunized with the Salmonella χ11021 strains harboring independently each of the Asd+/MurA+ lysis vector derivatives were incubated for 40 h (for IFN-γ- and TNF-α-secreting cells) or 66 h (for IL-2- and IL-14-secreting cells). The spots were counted using an automated ELISPOT assay plate reader (CTL analyzers; Cellular Technology Ltd., Cleveland, OH).
Statistical analysis.
Statistical analysis was performed using GraphPad Prism software (GraphPad Software, San Diego, CA). Differences in antibody responses, cytokine secretion levels measured by ELISPOT assay, and bacterial loads in the lungs and spleen between groups were determined by one-way analysis of variance (ANOVA), followed by Tukey's multiple-comparison test. Differences with P values of <0.05 were considered significant at the 95% confidence interval.
RESULTS
Synthesis and secretion of Ag85A294 by RASV strain χ9879(pYA3941).
RASV strain χ9879 displays regulated delayed attenuation. The construction of this strain was described previously (42). Strain χ9879 contains the ΔPphoPQ176:: TT araC PBAD phoPQ deletion-insertion mutation for the induction of phoPQ expression in the presence of arabinose. Cultures of χ9879 transformed with either pYA3620 (control plasmid) or pYA3941 (synthesizing BlaSS-Ag85A294-BlaCT, referred to as Ag85A294 throughout this study) were grown at 37°C to an OD600 of 0.8 in LB medium with 0.05% arabinose. The protein profile of this RASV strain showed that Ag85A294 had an unprocessed form of 38 kDa and a mature form of 36 kDa (the expected sizes for BlaSS-Ag85A294-BlaCT) that reacted specifically with anti-Ag85A polyclonal antibody in whole-cell lysates assayed by immunoblotting (Fig. 2). Only the 36-kDa protein band was observed in the culture supernatant fraction (Fig. 2), indicating that the signal sequence and carboxy-terminal region of β-lactamase allowed efficient secretion of the Ag85A294 protein.
Fig 2.
Synthesis of Ag85A294 in RASV strain χ9879. Immunoblot of whole-cell lysates (total extract) and supernatant fraction (20× concentrated) of χ9879(pYA3941) synthesizing M. tuberculosis Ag85A294 protein. RASV strains were grown as described in Materials and Methods. The total extract and supernatant fractions were separated by SDS-PAGE and immunoblotted with rabbit polyclonal anti-Ag85A antibody to detect Ag85A294. The numbers at the left of the blot are molecular masses. The unprocessed form of Ag85A (BlaSS-Ag85A294-BlaCT) is indicated by the arrow, and the mature form (Ag85A294) is shown by the asterisk; these forms have the expected molecular masses of 38 kDa and 36 kDa, respectively. Lanes: 1, molecular mass markers (MM); 2, χ9879(pYA3941).
Asd+/MurA+ lysis vectors for synthesis and delivery of M. tuberculosis antigens.
Two groups of Asd+/MurA+ recombinant lysis vectors were constructed to determine the effects of plasmid copy number on the synthesis, secretion, and release by lysis of M. tuberculosis antigens in RASV strains and to determine the correlation between antigen dose and protective immunity against virulent M. tuberculosis aerosol challenge. The first group of plasmids, pYA4890 (pBR ori), pYA4891 (p15A ori), and pYA4892 (pSC101 ori), carried the DNA construct that encodes the chimeric protein ESAT-6–ESAT-6–CFP-10 (E2C) fused to Salmonella SopE protein aa 1 to 80 (SopENt80) (SopENt80-E2C), so that it can be secreted and translocated into the host cell cytoplasm via the Salmonella T3SS. The second group of plasmids, pYA4893 (pBR ori) and pYA4894 (p15A ori), was constructed to synthesize the fusion protein OmpC signal sequence (ompCSS) fused to ESAT-6–ESAT-6–CFP-10 (OmpCSS-E2C). The OmpC signal sequence facilitates the transport of the recombinant proteins to the bacterial periplasmic space via the type II secretion system (T2SS) and, subsequently, into the extracellular milieu. Each recombinant plasmid also contained the blaSS-fbpA294-blaCT nucleotide sequences to produce the recombinant fusion protein BlaSS-Ag85A294-BlaCT that is exported to the periplasmic space of the bacterium due to the β-lactamase signal sequence (type II secretion). This fusion was placed downstream of the ESAT-6 and CFP-10 fusion constructs described above and in Materials and Methods.
Synthesis and secretion of recombinant chimeric proteins SopENt80-E2C, OmpCSS-E2C, and Ag85A294 by Salmonella strain χ11021.
S. enterica serovar Typhimurium χ11021 was designed to display regulated delayed antigen synthesis and regulated delayed lysis in vivo in combination with Asd+/MurA+ lysis expression vectors. Cultures of χ11021 strains independently containing each of the Asd+/MurA+ lysis plasmids were grown and processed for detection of proteins by immunoblotting. Cell extracts of strains containing the pBR ori vector pYA4890 or pYA4893 produced two proteins with approximate molecular masses of 38 kDa and 36 kDa, corresponding to the unprocessed and mature forms of Ag85A294 protein, respectively, in the whole-cell lysates (Fig. 3A, lanes 3 and 6) and as a single 36-kDa protein in the supernatant (data not shown). However, Ag85A294 was detected only as a 36-kDa protein in the whole-cell lysates of χ11021 containing the low-copy-number plasmids pYA4891 (p15A ori), pYA4892 (pSC101 ori), and pYA4894 (p15A ori) (Fig. 3A, lanes 4, 5, and 7). When the χ11021 cells were grown with 1 mM IPTG in the medium, the Ag85A294 protein was detected as two bands of 38 kDa and 36 kDa in whole-cell lysates (Fig. 3B, lanes 3 to 7) and as a band of 36 kDa in the supernatants (Fig. 3C, lanes 3 to 7). These results indicated that the copy number of the recombinant lysis vectors governed the amount of recombinant Ag85A294 produced in the vaccine strain. Smaller amounts of protein were observed in cells grown in LB medium with 0.2% arabinose, and the higher levels of the Ag85A294 protein were observed when induced with 1 mM IPTG.
Fig 3.
Synthesis of Ag85A294 in RASV strain χ11021. Immunoblot of whole-cell lysates and supernatant fractions (20× concentrated) of χ11021 strains containing plasmids producing Ag85A294. (A) Strains were grown in LB broth with 0.2% arabinose, to repress chimeric protein synthesis. (B and C) Strains were grown in LB broth with 1 mM IPTG, to induce a maximal chimeric protein synthesis. Total extracts and supernatant fractions were separated by SDS-PAGE and immunoblotted with rabbit polyclonal anti-Ag85A antibody. The numbers at the left of the blots are molecular masses. The unprocessed protein is indicated by the arrows, and the mature form is shown by the asterisks; these forms have expected molecular masses of 38 kDa and 36 kDa, respectively. Lanes: 1, molecular mass markers (MM); 2, RASV χ11021(pYA3681) vector control; 3, χ11021(pYA4890 [SopENt80-E2C/BlaSS-Ag85A294-BlaCT, pBR ori]); 4, χ11021(pYA4891 [SopENt80-E2C/BlaSS-Ag85A294-BlaCT, p15A ori]); 5, χ11021(pYA4892 [SopENt80-E2C/BlaSS-Ag85A294-BlaCT, pSC101 ori]); 6, χ11021(pYA4893 [OmpCSS-E2C/BlaSS-Ag85A294-BlaCT, pBR ori]); 7, χ11021(pYA4894 [OmpCSS-E2C/BlaSS-Ag85A294-BlaCT, p15A ori]).
Synthesis of the SopENt80-E2C fusion proteins (T3SS dependent) from pYA4890, pYA4891, and pYA4892 was detected by immunoblotting as a breakdown product of approximately 32 kDa that reacted specifically with anti-ESAT-6 polyclonal antibody in the whole-cell lysates from cultures grown in LB medium with 0.2% arabinose (Fig. 4A, lanes 3 to 5) and was also detected as a band of 40.6 kDa, along with its hydrolysis products of 35 kDa and 32 kDa, in whole-cell lysates (Fig. 4B, lanes 3 to 5) and supernatants (Fig. 4C, lanes 3 to 5) of cultures grown in the presence of 1 mM IPTG.
Fig 4.
Synthesis of SopENt80-E2C or OmpCSS-E2C in RASV strain χ11021. Immunoblot of whole-cell lysates and supernatant fractions of χ11021 strains harboring Asd+ lysis plasmids and producing SopE-E2C or OmpC-E2C. Strains were grown as described in the legend to Fig. 2A to C. Total extracts and supernatant fractions were separated by SDS-PAGE and immunoblotted using rabbit polyclonal anti-ESAT-6 antibody to detect SopENt80-E2C or OmpCSS-E2C. pBR ori is high copy number, p15A ori is low copy number, and pSC101 ori is very low copy number. The numbers at the left of the blot are molecular masses. Squares, SopE80-E2C chimeric protein (expected molecular mass, 34 kDa); circles, SopE80-E2C chimeric protein breakdown products (expected molecular masses, 35 kDa and 32 kDa, respectively); arrows, unprocessed OmpCSS-E2C (expected molecular mass, 34 kDa); asterisks, the mature form of OmpCSS-E2C (expected molecular mass 32 kDa). Lanes: 1, molecular mass markers (MM); 2, RASV χ11021(pYA3681) vector control; 3, χ11021(pYA4890 [SopENt80-E2C/BlaSS-Ag85A294-BlaCT, pBR ori]); 4, χ11021(pYA4891 [SopENt80-E2C/BlaSS-Ag85A294-BlaCT, p15A ori]); 5, χ11021(pYA4892 [SopENt80-E2C/BlaSS-Ag85A294-BlaCT, pSC101 ori]); 6, χ11021(pYA4893 [OmpCSS-E2C/BlaSS-Ag85A294-BlaCT, pBR ori]); 7, χ11021(pYA4894 [OmpCSS-E2C/BlaSS-Ag85A294-BlaCT, p15A ori]).
Synthesis of the OmpCSS-E2C fusion protein (T2SS dependent) from pYA4893 and pYA4894 was confirmed by the presence of bands of approximately 34 kDa and 32 kDa (detected using anti-ESAT-6 polyclonal antibody) that correspond to the unprocessed and mature forms of the protein in the whole-cell lysates, respectively (Fig. 4A and B, lanes 6 and 7), and in the supernatants as a band of 32 kDa (Fig. 4C, lanes 6 and 7) from cultures grown in LB medium with 0.2% arabinose or 1 mM IPTG. The amounts of both chimeric proteins, SopENt80-E2C and OmpCSS-E2C, were reduced in LB medium with 0.2% arabinose and elevated after induction with 1 mM IPTG, as observed with the recombinant Ag85A294 grown under similar conditions. These results showed that synthesis of the recombinant antigens was efficiently regulated in Salmonella χ11021 harboring the Asd+/MurA+ lysis vectors during growth in broth cultures.
Plasmid stability.
All plasmids were 100% stable in χ9879 and χ11021 throughout 50 generations of growth under both selective and nonselective conditions (data not shown).
Serum IgG titers in mice immunized with recombinant S. Typhimurium vaccines.
Sera from groups of C57BL/6 mice orally immunized with Salmonella strain χ9879 carrying pYA3941 (producing Ag85A294) or control plasmid pYA3620 or with buffered saline (BSG control) were obtained at days 21 and 65 after the first immunization to analyze serum IgG responses by ELISA. Sera from groups of mice orally immunized with Salmonella strains χ11021 carrying pYA4890, pYA4891, or pYA4892 (producing SopENt80-E2C/Ag85294), pYA4893 or pYA4894 (producing OmpCSS-E2C/Ag85A294), control plasmid pYA3681, or BSG were obtained at day 77 after the first immunization to analyze serum IgG responses.
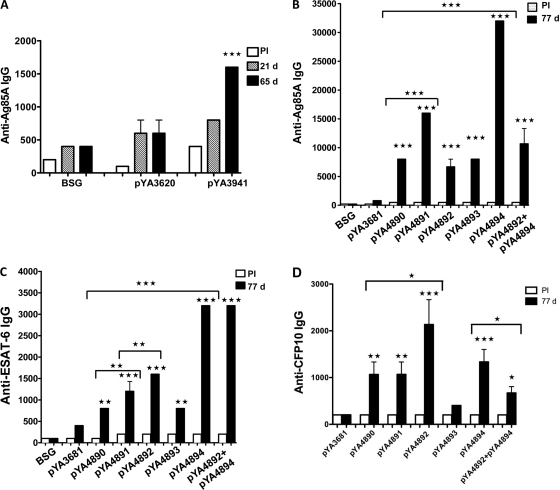
IgG antibody responses to Ag85A.
Slightly higher serum IgG titers against Ag85A294 were detected in mice vaccinated with χ9879(pYA3941) at 21 days and significantly higher titers were detected at 65 days (P < 0.001) compared to the titers in mice receiving BSG alone or the control strain, χ9879(pYA3620) (Fig. 5A). All mice immunized with the χ11021 strains harboring Asd+/MurA+ lysis plasmids synthesizing Ag85A294 produced significantly higher anti-Ag85A IgG responses at day 77 than either mice immunized with the χ11021(pYA3681) vector control or mice receiving BSG (P < 0.001) (Fig. 5B). Mice immunized with χ11021(pYA4894 [p15A ori]) synthesizing OmpCSS-E2C/Ag85A294 proteins elicited the highest level of IgG anti-Ag85A (endpoint titer, 33,000) compared to those elicited by mice immunized with either χ11021(pYA4893 [pBR ori]) (endpoint titer, 7,000) or χ11021 synthesizing SopENt80-E2C/Ag85A294 from pYA4890, pYA4891, and pYA4892 (endpoint titers, between 7,000 and 16,000) (P < 0.001).
Fig 5.
Anti-Ag85A and anti-ESAT-6 serum IgG in mice. C57BL/6 mice were orally immunized at days 0, 7, and 49 with 1 × 109 CFU of RASV strain χ9879 harboring either pYA3620 (vector control) or its derivative, pYA3941 (synthesizing the BlaSS-Ag85A294-BlaCT protein), or RASV strain χ11021 independently harboring Asd+/MurA+ plasmids: pYA3681 (vector control) or the isogenic pBR, p15A, and pSC101 ori plasmids pYA4890, pYA4891, and pYA4892, respectively (synthesizing the SopENt80-E2C/BlaSS-Ag85A294-BlaCT protein), or harboring the isogenic pBR or p15A ori plasmids pYA4893 and pYA4894, respectively (synthesizing OmpCSS-E2C/BlaSS-Ag85A294-BlaCT), or were immunized simultaneously with both χ11021(pYA4892) and χ11021(pYA4894). The anti-Ag85A IgG titers in preimmune serum (PI) and in serum from immunized mice at 21 and 65 days (d) or at 77 days after the first immunization were measured by ELISA. (A) Serum IgG total response to Ag85A. ***, P < 0.001 for comparison with mice immunized with vector control strain χ11021(pYA3620), BSG-dosed mice, or preimmune serum. (B) Serum IgG total response to Ag85A. ***, P < 0.001 for comparison with mice immunized with vector control strain χ11021(pYA3681), BSG-dosed mice, or preimmune serum; P < 0.001 for comparison of mice immunized with χ11021(pYA4894) versus mice immunized with χ11021(pYA4890), χ11021(pYA491), χ11021(pYA4892), and χ11021(pYA4893); and P < 0.001 for comparison of mice immunized with χ11021(pYA4891) versus mice immunized with χ11021(pYA4890). (C) Serum IgG total response to ESAT-6. ***, P < 0.001, and **, P < 0.01, for comparison of mice immunized with the vector control strain, BSG-dosed mice, or preimmune serum; **, P < 0.01 for comparison of mice immunized with χ11021(pYA4892) versus χ11021(pYA4891) and P < 0.01 for comparison of mice immunized with χ11021(pYA4891) versus χ11021(pYA4890); ***, P < 0.001 for comparison of mice immunized with χ11021(pYA4894) versus mice immunized with χ11021(pYA4890), χ11021(pYA4891), χ11021(pYA4892), and χ11021(pYA4893). D Serum IgG total response to CFP-10. ***, P < 0.001; **, P < 0.01; *, P < 0.05 for comparison of mice immunized with the vector control strains. *, P < 0.05 for comparison of mice immunized with χ11021(pYA4892) versus χ11021(pYA4890) or χ11021(pYA4891); *, P < 0.05 for comparison of mice immunized with χ11021(pYA4894) versus χ11021(pYA4892 + pYA4894). The data represent endpoints of antibodies in pooled sera from 6 mice immunized at the indicated time after immunization. Error bars represent variations between duplicate wells. The statistical significance was calculated by one-way ANOVA and Tukey's posttest.
When the effect of plasmid copy number was compared among χ11021 strains synthesizing SopENt80-E2C/Ag85A294, mice immunized with χ11021(pYA4891 [p15A ori]) elicited a significantly higher IgG response to Ag85A (P < 0.001) than mice immunized with either χ11021(pYA4890 [pBR ori]) or χ11021(pYA4892 [pSC101 ori]). Interestingly, in mice vaccinated simultaneously with two strains, χ11021(pYA4892 [pSC101 ori]) synthesizing SopENt80-E2C/Ag85A294 and χ11021(pYA4894 [p15A ori]) synthesizing OmpCSS-2EC/Ag85A294, the serum anti-Ag85A IgG titers decreased significantly compared to the levels in mice immunized with χ11021(pYA4894) alone (P < 0.001) (Fig. 5B). No such difference was observed in the case of ESAT-6 (Fig. 5C). These results suggested that higher anti-Ag85A IgG titers were induced in mice immunized with the regulated delayed lysis strain χ11021 carrying Ag85A294 Asd+/MurA+ lysis plasmids (pYA4890 to pYA4894), which is designed to deliver the antigen by both protein secretion and lysis of the vaccine strain, compared to the levels induced by χ9879(pYA3941) displaying regulated delayed attenuation, which delivers the antigen only by secretion. These results demonstrated that χ11021 carrying either Ag85A294 plasmid pYA4890 or pYA4892, with high-copy-number pBR ori and low-copy-number pSC101 ori, respectively, induced lower humoral immune responses to Ag85A in the immunized mice than mice immunized with χ11021 carrying the p15A ori plasmids pYA4891 and pYA4894.
IgG antibody responses to ESAT-6.
Sera from the first group of mice immunized with χ11021 producing the recombinant proteins SopENt80-E2C/Ag85A294 were analyzed on day 77. Anti-ESAT-6 total IgG titers in mice immunized with χ11021(pYA4890), χ11021(pYA4891), or χ11021(pYA4892) were significantly higher than those measured in mice vaccinated with χ11021(pYA3681) harboring the vector alone as a control or given BSG (P < 0.01 for pYA4890; P < 0.001 for pYA4891 and pYA4892) (Fig. 5C).
The effect of antigen dose (due to differences in plasmid copy number) was different for ESAT-6 than for Ag85A. Anti-ESAT-6 IgG titers in sera of mice immunized with χ11021(pYA4892 [pSC101 ori]) were significantly higher than the titers observed in mice immunized with χ11021(pYA4890 [pBR ori]) or χ11021(pYA4891 [p15A ori]) (pYA4892 < pYA4891 [P < 0.01]; pYA4891 < pYA4890 [P < 0.01]) (Fig. 5C). In the second group of mice immunized with χ11021(pYA4893) or χ11021(pYA4894) producing the recombinant proteins OmpCSS-E2C/Ag85A294 and analyzed similarly, both vaccine strains induced significantly higher IgG titers than the χ11021(pYA3681) vector control or BSG alone (pYA4893, P < 0.01; pYA4894, P < 0.001). The levels of serum anti-ESAT-6 IgG in mice immunized with χ11021(pYA4894 [p15A ori]) or mice immunized simultaneously with the two strains χ11021(pYA4892) and χ11021(pYA4894) were highest compared to the levels observed in mice immunized with χ11021 harboring any of the other Asd+/MurA+ lysis vectors (P < 0.001) (Fig. 5C). As observed with Ag85A294, χ11021(pYA4894 [p15A ori]) induced the highest levels of anti-ESAT-6 IgG in vaccinated mice (Fig. 5C).
IgG antibody responses to CFP-10.
Mice immunized with χ11021(pYA4892 [pSC101 ori]) elicited the highest levels of anti-CFP-10 IgG compared to mice immunized with the χ11021(pYA3681) vector control or preimmune serum (P < 0.001; Fig. 5D). The effect of antigen dose (due to differences in plasmid copy number) for CFP-10 was similar to that observed with ESAT-6. The anti-CFP-10 IgG titers observed in sera of mice immunized with χ11021(pYA4892) were significantly higher than the titers observed in mice immunized with χ11021(pYA4890 [pBR ori]) or χ11021(pYA4891 [p15A ori]) (P < 0.05), although mice immunized with the last two RASVs produced similar anti-CFP-10 titers (Fig. 5D). In the second group of mice immunized with χ11021(pYA4893 [pBR ori]) or χ11021(pYA4894 [p15A ori]), producing the recombinant protein OmpCSS-E2C/Ag85A294, only χ11021(pYA4894) induced significantly higher anti-CFP-10 titers than the vector control χ11021(pYA3681) or preimmune serum (P < 0.001). The level of anti-CFP-10 IgG was significantly higher in mice immunized with χ11021(pYA4894) than mice immunized simultaneously with χ11021(pYA4892) and χ11021(pYA4894) (P < 0.01; Fig. 5D).
IgG immune responses to SOMPs.
Serum IgG titers against SOMPs were detected in all mice vaccinated with either χ9879(pYA3941) or χ11021 carrying chimeric protein Asd+/MurA+ lysis plasmids or the control plasmids (pYA3620 [Asd+] or pYA3681 [Asd+/MurA+ lysis]) at 21 days (after the first vaccination) and increased by days 65 and 77 (Fig. 6A and B). The anti-SOMP IgG levels in mice immunized with the χ9879(pYA3620) vector control at 21 and 65 days were higher than the levels observed in the χ9879(pYA3941)-immunized mice (P < 0.05 and P < 0.01, respectively), which could be due to the metabolic burden imposed by the high level of Ag85A294 synthesis in χ9879(pYA3941). No such difference was observed in mice immunized with χ11021 harboring the Asd+/MurA+ lysis vector derivatives (Fig. 6B). These data suggest that RASV strain χ11021 complemented with the Asd+/MurA+ lysis plasmids is able to invade and survive in the lymphoid tissues of the vaccinated mice for a time sufficient to stimulate a robust immune response before undergoing lysis, since its immunogenic capabilities were superior to those observed with χ9879(pYA3941).
Fig 6.
Anti-SOMP total serum IgG and anti-Ag85A IgG2b and IgG1 in serum in mice. C57BL/6 mice were orally immunized at days 0, 7, and 49 with 1 × 109 CFU of χ9879 harboring either pYA3620 (control) or pYA3941 (specifying the BlaSS-Ag85A294-BlaCT protein) or with 1 × 109 CFU of χ11021 harboring either pYA3681 (lysis vector control) or the isogenic lysis plasmids pYA4890 (pBR ori), pYA4891 (p15A ori), and pYA4892 (pSC101 ori), each specifying SopENt80-E2C/BlaSS-Ag85A294-BlaCT, as well as pYA4893 (pBR ori) and pYA4894 (p15A ori), each synthesizing OmpCSS-E2C/BlaSS-Ag85A294-BlaCT, or were immunized simultaneously with both χ11021(pYA4892) and χ11021(pYA4894). The anti-Ag85A IgG2b and IgG1 titers and anti-SOMP titers in preimmune serum (PI) and in immunized mice at 21, 65, and 77 days after the first immunization were measured by ELISA. (A and B) Total serum IgG responses to SOMPs. ***, P < 0.001, **, P < 0.01, and *, P < 0.05, for comparison with mice immunized with the vector control strain, BSG-dosed mice, or preimmune serum. (C and D) Subclasses IgG2 and IgG1 in serum against Ag85A. The data represent endpoints of antibodies in pooled sera from 6 mice immunized at the indicated time after immunization. Error bars represent variations between duplicate wells. The statistical significance was calculated by one-way ANOVA and Tukey's posttest.
IgG antibody subclass responses to Ag85A.
The immune responses induced by χ9879 synthesizing Ag85A294 from pYA3941 and χ11021 synthesizing Ag85A294 from the Asd+/MurA+ lysis plasmids were further examined by measuring the levels of IgG isotype subclasses IgG1 and IgG2b both in preimmune serum and at days 21, 65, or 77 after the first vaccination. Serum IgG2b titers to Ag85A were higher than the IgG1 titers at 21, 65, or 77 days (Fig. 6C and D). These data indicate that χ11021 synthesizing Ag85A294 predominantly induced Th1-type immune responses in immunized mice. Anti-Ag85A IgG1 and IgG2b antibodies were barely detected in sera obtained from mice vaccinated with control strains or in preimmune serum (data not shown), confirming that the immune responses were stimulated by the antigen and not by the delivery vector.
Cytokine production in mice orally immunized with S. Typhimurium χ9879(pYA3941).
Three weeks after the last immunization, lymphocytes were isolated from spleens from each group of vaccinated mice as well as control groups of mice, to compare the stimulation of the proinflammatory Th1 cytokines IFN-γ, TNF-α, and IL-2 and the anti-inflammatory Th2 cytokine IL-4 (65). The lymphocytes were stimulated with 1 μg/well of recombinant Ag85A or medium as described in Materials and Methods. Significantly higher numbers of Ag85A-specific IFN-γ, TNF-α, and IL-2 spot-forming units (SFU) were detected from mice immunized with S. Typhimurium χ9879(pYA3941) synthesizing Ag85A294 (P < 0.001) than mice that received BSG (control) (Fig. 7A to C). Only the TNF-α and IL-4 levels were significantly higher in the immunized mice (P < 0.05), although we do not consider these to be biologically significant. This splenic lymphocyte activity observed in mice immunized with χ9879(pYA3941) is a consequence of the ability of live Salmonella vaccine strains to induce cell-mediated immunity with IFN-γ-dominant immune responses, in addition to humoral immunity (52).
Fig 7.
Antigen-specific stimulation of cytokine responses in lymphocytes from mice vaccinated with Salmonella χ9879(pYA3620) or χ9879pYA3941. Antigen-specific IFN-γ (A), TNF-α (B), IL-2 (C), and IL-4 (D) cytokine-forming lymphocytes were determined by ELISPOT assay. C57BL/6 mice were orally immunized with χ9879(pYA3941) specifying BlaSS-Ag85A294-BlaCT or the χ9879(pYA3620) vector control or were BSG dosed at days 0, 21, and 49. Three weeks after the last immunization, spleen cells from three mice per group were harvested and pooled. The pool of spleen cells from the same group of mice was analyzed in triplicate. Cells were restimulated for 24 h (for IFN-γ- and TNF-α-secreting cells) or 48 h (for IL-2- and IL-14-secreting cells) with 1 μg/well of recombinant Ag85A or medium for ELISPOT assays. The results are presented as number of ELISPOTs per million lymphocytes minus background number of ELISPOTs from unpulsed mock controls. ***, P < 0.001 for comparison of mice immunized with χ9879(pYA3941) or with χ9879(pYA3620) and BSG group for Ag85A-specific IFN-γ-, TNF-α-, and IL-2-secreting cells; *, P < 0.05 for comparison of the mice immunized with χ9879(pYA3941) and mice immunized with χ9879(pYA3620) for Ag85A-specific TNF-α-secreting cells and P < 0.05 for comparison of mice immunized with χ9879(pYA3941) and BSG group for Ag85A-specific IL-4-secreting cells. Error bars represent variations between triplicate wells. The statistical significance was calculated by one-way ANOVA and Tukey's posttest.
Cytokine production in mice orally immunized with S. Typhimurium χ11021 synthesizing ESAT-6–CFP-10–Ag85A chimeric proteins.
Splenic lymphocytes from mice immunized with χ11021 harboring independently each of the lysis vectors synthesizing either recombinant SopENt80-E2C/Ag85A294 or OmpCSS-2EC/Ag85A294 produced significantly larger amounts of Ag85A-specific IFN-γ-secreting cells than mice that received BSG (control) (P < 0.001 for χ11021 carrying pYA4890; P < 0.01 for pYA4891; and P < 0.05 for pYA4892, pYA4893, or pYA4894) (Fig. 8A). Lymphocytes from mice immunized with χ11021(pYA4890) or χ11021(pYA4891) or mice immunized simultaneously with both χ11021(pYA4892) and χ11021(pYA4894) produced significantly larger amounts of Ag85A-specific IFN-γ-secreting cells than lymphocytes from mice vaccinated with the χ11021(pYA3681) vector control (P < 0.05 for χ11021 carrying pYA4890; P < 0.05 for pYA4891; and P < 0.001 for pYA4892 and pYA4894) (Fig. 8A).
Fig 8.
Antigen-specific stimulation of cytokine responses in spleen cells from mice vaccinated with RASV χ11021 harboring either Asd+/MurA+ lysis plasmid pYA3681 (vector control) or plasmids pYA4890, pYA4891, and pYA4892 (specifying synthesis of SopENt80-E2C/BlaSS-Ag85A294-BlaCT) or harboring plasmids pYA4893 and pYA4894 (specifying synthesis of OmpCSS-E2C/BlaSS-Ag85A294-BlaCT), or were immunized simultaneously with both χ11021(pYA4892) and χ11021(pYA4894). Ag85A-specific IFN-γ (A), TNF-α (B), IL-2 (C), and IL-4 (D) cytokine-forming lymphocytes were detected by ELISPOT assay. C57BL/6 mice were orally immunized with the Salmonella vaccine strains or BSG dosed at days 0, 21, and 49. Three weeks after the last immunization, spleen cells from three mice per group were harvested and pooled. Cells were restimulated for 40 h (for IFN-γ- and TNF-α-secreting cells) or 66 h (for IL-2- and IL-14-secreting cells) with 1 μg/well of recombinant Ag85A or medium for ELISPOT assays. The results are presented as the number of ELISPOTs per million lymphocytes minus background number of ELISPOTs from unpulsed mock controls. *, P < 0.05, and ***, P < 0.001, for comparison of BSG-dosed mice. (A) For Ag85A-specific IFN-γ secreting cells, *, P < 0.05 for comparison of mice immunized with either χ11021(pYA4890) or χ11021(pYA4891) and mice immunized with χ11021(pYA3681); **, P < 0.01 for comparison of mice immunized with both χ11021(pYA4892) and χ11021(pYA4894) versus mice immunized with χ11021(pYA4894); ***, P < 0.001 for comparison of mice immunized with both strains χ11021(pYA4892) and χ11021(pYA4894) versus mice immunized with χ11021(pYA3681). (B) For Ag85A-specific TNF-α-secreting cells; *, P < 0.05 for comparison of mice immunized with χ11021(pYA4890) and mice immunized with χ11021(pYA4892) or χ11021(pYA3681); **, P < 0.01 for comparison of mice immunized with both χ11021(pYA4892) and χ11021(pYA4894) versus mice immunized with χ11021(pYA4894) or χ11021(pYA3681). (C) For Ag85A-specific IL-2-secreting cells, **, P < 0.01 for comparison of mice immunized with both χ11021(pYA4892) and χ11021(pYA4894) versus mice immunized with χ11021(pYA4894). (D) For Ag85A-specific IL-4-secreting cells; *, P < 0.05 for comparison of mice immunized with χ11021(pYA3681) and mice immunized with χ11021(pYA4890) and P < 0.05 for comparison of mice immunized with χ11021(pYA4890) and mice immunized with χ11021(pYA4891); ***, P < 0.001 for comparison of mice immunized with both strains χ11021(pYA4892) and χ11021(pYA4894) versus mice immunized with χ1102(pYA4894). Error bars represent variations between triplicate wells. The statistical significance was calculated by one-way ANOVA and Tukey's posttest.
In the case of Ag85A-specific TNF-α- and IL-2-secreting cells, the numbers were significantly higher in mice immunized with χ11021 harboring independently each lysis plasmid than in the mice that received BSG (control) (P < 0.001) (Fig. 8B and C). However, only the splenocytes from mice vaccinated with either χ11021(pYA4890) or simultaneously with both χ11021(pYA4892) and χ11021(pYA4894) produced significantly larger amounts of Ag85A-specific TNF-α-secreting cells than splenocytes from mice vaccinated with the χ11021(pYA3681) vector control (P < 0.05 for χ11021 harboring pYA4890 and P < 0.01 for pYA4892 and pYA4894; Fig. 8B). The number of Ag85A-specific IFN-γ, TNF-α, and IL-2 SFU from splenocytes from mice vaccinated with either χ11021(pYA4893 [pBR ori]) or χ11021(pYA4894 [p15A ori]) synthesizing OmpCSS-E2C/Ag85A294 was significantly higher than the number of SFU from cells producing these cytokines from mice that received BSG (control) (P < 0.05 for IFN-γ SFU; P < 0.001 for TNF-α SFU; and P < 0.001 for IL-2 SFU). The number of Ag85A-specific IFN-γ, TNF-α, and IL-2 SFU from splenocytes of mice vaccinated with χ11021(pYA4894) was slightly higher than the number from cells producing these cytokines from mice vaccinated with χ11021(pYA4893). The number of Ag85A-specific IFN-γ, TNF-α, and IL-2 SFU from lymphocytes of mice vaccinated simultaneously with χ11021(pYA4892) and χ11021(pYA4894) was significantly higher than the number of cells producing these cytokines from mice vaccinated with either χ11021(pYA4892) or χ11021(pYA4894) alone (P < 0.01 for IFN-γ; P < 0.01 for TNF-α; and P < 0.01 for IL-2). These data suggested a synergistic effect of both vaccine strains in the induction of the T-cell immune responses.
The numbers of Ag85A-specific IL-4 SFU from splenocytes of mice vaccinated with the χ11021(pYA3681) vector control, χ11021(pYA4890), or χ11021(pYA4892) or simultaneously with χ11021(pYA4892) and χ11021(pYA4894) were significantly higher than the numbers from cells producing IL-4 from mice that received BSG (control) (P < 0.001; Fig. 8D), while the numbers of Ag85A-specific IL-4 SFU from splenocytes of mice vaccinated with either χ11021(pYA4891) or χ11021(pYA4894) were only slightly higher than those observed in mice that received BSG (control) (P < 0.05; Fig. 8D). Together, these results indicated that a dominant Th1 immune response, characterized by the secretion of IFN-γ, TNF-α, and IL-2, was induced in most of the mice immunized with χ11021 harboring any of the Asd+/MurA+ lysis plasmids, while the anti-inflammatory Th2 cytokine IL-4 was also stimulated in mice immunized with the χ11021(pYA3681) vector control, χ11021(pYA4892 [pSC101 ori]), and χ11021(pYA4890 [pBR ori]).
In contrast to the stimulation of cytokines by splenocytes from mice immunized with χ11021 harboring any Asd+/MurA+ lysis expression plasmid, the SopENt80-E2C or OmpCSS-E2C chimeric proteins induced barely detectable ESAT-6-specific or CFP-10-specific IFN-γ-, TNF-α-, IL-2-, or IL-4 secreting cells (data not shown).
Evaluation of protective immunity.
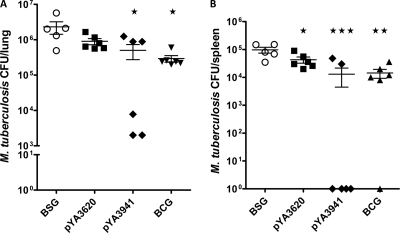
To examine the protective efficacy of Salmonella vaccines against M. tuberculosis infection, groups of orally immunized C57BL/6 mice were challenged with virulent M. tuberculosis H37Rv as indicated in Materials and Methods. Six mice per group were euthanized 6 weeks after challenge, and the protective efficacy of the vaccines was measured by the enumeration of M. tuberculosis CFU in the lungs and spleens. The mice immunized with the regulated delayed attenuation strain χ9879(pYA3941) synthesizing Ag85A294 showed a significant reduction in the number of CFU of M. tuberculosis in lungs and spleen in comparison with the BSG-dosed mice (P < 0.05 and P < 0.001, respectively). The number of CFU in the lungs and spleens of mice immunized with χ9879(pYA3941) were nearly at the same level as those observed in mice immunized with M. bovis BCG (Fig. 9A and B). The group of mice immunized with the χ9879(pYA3620) vector control also showed a moderate reduction in the number of CFU in lungs and spleens in comparison with the BSG-dosed mice.
Fig 9.
RASV χ9879(pYA3941) confers significant protection against mycobacterial infection. C57BL/6 mice were orally immunized with χ9879(pYA3941), specifying synthesis of BlaSS-Ag85A294-BlaCT, the χ9879(pYA3620) vector control, or BSG at days 0, 21, and 49. One group of mice was immunized subcutaneously with M. bovis BCG at day 0. All mice were challenged with M. tuberculosis by aerosol 4 weeks after the last immunization and euthanized 6 weeks later, to determine the bacterial loads in the lungs (A) and spleens (B) in each group of mice. *, P < 0.05, **, P < 0.01, and ***, P < 0.001, for significance of vaccinated groups compared with mice that received BSG as a control.
In the groups of mice immunized with the regulated delayed lysis strain χ11021 independently harboring each of the Asd+/MurA+ lysis plasmids (pYA4890, pYA4891, and pYA4892) synthesizing the chimeric proteins SopENt80-E2C/Ag85A294, a significant reduction in the number of M. tuberculosis CFU in lungs was observed with χ11021(pYA4891 [p15A ori]) (P < 0.001) and χ11021(pYA4890 [pBR ori]) (P < 0.01) compared to the BSG-dosed mice (Fig. 10A). The number of CFU in the spleens was also significantly reduced in mice immunized with χ11021(pYA4891) and χ11021(pYA4890) compared to the BSG-dosed group (P < 0.05). No protection was observed in either the lungs or spleens of mice immunized with χ11021(pYA4892 [pSC101 ori]) (Fig. 10A and B).
Fig 10.
RASV χ11021 harboring the Asd+/MurA+ lysis plasmids confers significant protection against mycobacterial infection. C57BL/6 mice were orally immunized with the χ11021(pYA3681) vector control or with χ11021 harboring each of isogenic plasmids pYA4891, pYA4892, and pYA4893 (synthesizing SopENt80-E2C/BlaSS-Ag85A294-BlaCT) as well as either pYA4890 or pYA4894 (synthesizing OmpCSS-E2C/BlaSS-Ag85A294-BlaCT); were immunized simultaneously with both χ11021(pYA4892) and χ11021(pYA4894); or were BSG dosed at days 0, 7, and 49. One group of mice was immunized subcutaneously with M. bovis BCG at day 0. All mice were challenged with M. tuberculosis by aerosol 4 weeks after the last immunization and euthanized 6 weeks later, to determine M. tuberculosis loads in the lungs (A) and spleens (B) of each group of mice. *, P < 0.05, **, P < 0.01, and ***, P < 0.001, for significance in numbers of CFU between vaccinated and BSG-dosed mice.
The groups of mice immunized with χ11021(pYA4893 [pBR ori]) and χ11021(pYA4894 [p15A ori]) synthesizing OmpCSS-E2C/Ag85A294 showed a significant reduction in the number of CFU in the lungs compared to the BSG-dosed group (P < 0.001). In the spleens, a moderate reduction in the number of CFU was observed in mice immunized with χ11021(pYA4893) and a significant reduction was obtained in mice vaccinated with χ11021(pYA4894) compared to the BSG-dosed mice (P < 0.05). The mice immunized simultaneously with both strains χ11021(pYA4892) and χ11021(pYA4894) showed a significant reduction in the number of CFU in the lungs and spleens compared to the BSG-dosed mice (P < 0.01 for lungs and P < 0.05 for spleens). However, the protection against the virulent M. tuberculosis observed in these mice immunized with both strains was not higher than the protection conferred in mice vaccinated only with strain χ11021(pYA4894).
The protection generated in the lungs of mice immunized with χ11021(pYA4891), χ11021(pYA4894), and χ11021(pYA4893) was better than the protection conferred in mice immunized with M. bovis BCG, while the protection generated in the lungs of mice immunized with either χ11021(pYA4890) alone or simultaneously with χ11021(pYA4892) and χ11021(pYA4894) was approximately same as that in mice immunized with M. bovis BCG, suggesting that our first-generation RASV-based M. tuberculosis vaccines delivering only 3 different protective antigens were highly effective.
Mice immunized with χ11021(pYA4890), χ11021(pYA4891), or χ11021(pYA4894) or simultaneously with χ11021(pYA4892) and χ11021(pYA4894) showed approximately the same level of protection in spleens as those immunized with M. bovis BCG (Fig. 10).
DISCUSSION
Tuberculosis is an infectious disease that is treatable using the available chemotherapeutic agents. However, the drugs are sometimes not effective due to patient noncompliance with the recommended treatment regimen or because disease is caused by antibiotic-resistant strains of M. tuberculosis. Use of the M. bovis BCG vaccine to prevent TB is controversial since its protective efficacy is variable, immunity is not long lasting (effective only in childhood), and the vaccine is not routinely used in some countries (for example, the United States). Several strategies have been developed to improve the efficacy of the BCG vaccine or to generate new vaccines that induce protective humoral and cell-mediated immunity against M. tuberculosis infection, including the use of live attenuated bacteria such as Salmonella for delivery of protective M. tuberculosis antigens. Delivery of recombinant Salmonella vaccines by oral, intranasal, intravenous, and intraperitoneal routes stimulates mucosal immunity with production of secretory IgA in all mucosal tissues and all secretory glands, primarily due to the cross-communication within the mucosal immune system (37). The antibody responses induced in mice immunized with Salmonella vaccines may contribute to the control of M. tuberculosis infection since there are IgG and IgA antibodies present in the mucosal secretions of the lower respiratory tract (10). Although M. tuberculosis is primarily an intracellular pathogen, there is also an extracellular phase in its infectious cycle. There are studies that show the importance of antibody responses against M. tuberculosis in controlling the infection (33, 35, 70). Therefore, oral immunization with Salmonella vaccine vectors may be effective in protecting mucosal surfaces such as those of the lungs. In addition, live recombinant Salmonella vaccines induce Th-1 cytokines IFN-γ and TNF-α, which are important in controlling infections and preventing diseases caused by intracellular pathogens such as M. tuberculosis (17, 30, 68).
Kong et al. have developed an Asd+/MurA+ balanced-lethal host-vector lysis system for complementation of the lethal chromosomal deletion of both the asd and murA genes in RASV strains (46). This system ensures the stability of plasmid vectors producing protective antigens in vivo after immunization of animal hosts and eliminates the use of drug resistance markers. Furthermore, RASV strains have been constructed that are phenotypically similar to wild-type Salmonella at the time of oral vaccination but display (i) regulated delayed attenuation (22), (ii) regulated delayed synthesis of recombinant antigens (71), and (iii) regulated delayed lysis to release protective antigens and confer complete biological containment, after colonizing the host (46).
In this study, we evaluated the protective efficacy of two RASV strains that, in vivo, exhibit regulated delayed attenuation (χ9879) and regulated delayed lysis (χ11021). Each of these strains produces secreted M. tuberculosis proteins such as Ag85A294 alone or Ag85A294 produced with either SopENt80-E2C or OmpCSS-E2C (in χ11021) and delivers these protective antigens to the host immune system to elicit protective immunity against M. tuberculosis infection. Antigens Ag85A, ESAT-6, and CFP-10 are effective as subunit vaccines (3, 39), and vaccines expressing these antigens alone or together afford protection against M. tuberculosis infection (38, 45, 54).
Secreted antigenic proteins are generally more effective in inducing protective immunity against intracellular pathogens than proteins that remain in the cell cytosol. Therefore, we constructed RASVs producing chimeric proteins such as Bla-Ag85A294 and OmpCSS-E2C that are efficiently exported to the periplasm and subsequently to the outside of the bacterial cell via the T2SS, while the chimeric protein SopENt80-E2C is efficiently secreted to the supernatant and translocated to eukaryotic cell cytoplasm via the T3SS (42; this study).
Moreover, the level of recombinant antigen synthesis directly influences the quality of the immune response induced (14) since high expression of heterologous genes on plasmids can generate a metabolic burden that overattenuates the Salmonella vaccine strains, resulting in impaired colonization and either a lack of or decreased immunogenicity. Therefore, we controlled the amount of heterologous antigen synthesis in RASV by two ways: first, using balanced-lethal Asd+/MurA+ plasmids that carry the mycobacterial genes fused to T2SS or T3SS effector sequences, with their transcription under the control of the Ptrc promoter, whose activity is in turn controlled by the chromosomal arabinose-regulated lacI gene in the Salmonella vaccine strain χ11021. The second control is through the use of isogenic Asd+/MurA+ expression plasmids with different replication origins, such as pBR, p15A, and pSC101 for high-, low-, and very-low-copy-number plasmids, respectively, to carry the nucleotide sequences encoding the mycobacterial chimeric proteins. The Asd+ pYA3941 plasmid and all of the Asd+/MurA+ lysis plasmid derivatives constructed in this study are stable (100%) in RASV strains for over 50 generations of growth in the presence of DAP, independent of their copy number.
We further extended our study to determine the ability of our RASV strains to induce both humoral and cellular immune responses. We detected anti-Ag85A IgG titers in mice orally vaccinated with RASV strain χ9879(pYA3941) or χ11021 independently harboring each constructed Asd+/MurA+ lysis vector producing and delivering Ag85A294. Higher anti-Ag85A IgG titers were obtained in mice immunized with the χ11021 strains harboring the Asd+/MurA+ lysis vectors producing Ag85A294 than in mice immunized with χ9879(pYA3941). These data suggest that Salmonella vaccines displaying regulated delayed lysis were superior in inducing IgG antibody production in vaccinated mice compared to RASVs that had only attenuating mutations. Interestingly, among the vaccine strains harboring isogenic plasmids with different replication origins, we observed that the anti-Ag58A IgG titers were influenced by the copy number of the plasmids, obtaining the highest titers with plasmids containing the p15A ori (χ11021 harboring pYA4894 or pYA4891).
Anti-ESAT-6 IgG titers were detected in all mice vaccinated with χ11021 harboring the Asd+/MurA+ lysis vector derivatives expressing and delivering either the SopENt80-E2C or OmpCSS-E2C chimeric proteins. Similar to the pattern of humoral responses to Ag85A, the antibody response against ESAT-6 was inversely related to the copy number of the plasmids, suggesting that a metabolic burden was imposed on the RASV by the synthesis of the protective antigens. A similar observation has been described previously, where the immune response to antigens delivered by Salmonella Typhi vaccines was improved when the ClyA-PA83 antigen was expressed from low-copy-number plasmids (pSC101 ori), which induced the highest antibody responses (32). In this study, the highest anti-ESAT-6 IgG titers were observed in the mice vaccinated with χ11021(pYA4894 [p15A ori]) alone or in mice vaccinated simultaneously with both χ11021(pYA4894) and χ11021(pYA4892 [pSC101 ori]). In addition to the copy number of the plasmid, the OmpC signal peptide also positively influenced the humoral response, since the OmpCSS-E2C chimeric proteins were more effective in eliciting higher antibody titers than the SopENt80-E2C chimeric proteins.
The IgG responses to CFP-10 in immunized mice were similar to the patterns observed with ESAT-6. Induction of anti-CFP-10 IgG was also inversely related to the copy number of the plasmid, although the highest titers were seen in mice vaccinated with χ11021(pYA4892 [pSC101 ori]). Mice immunized with χ11021(pYA4894 [p15A ori]) had the second highest titer of anti-CFP IgG, suggesting that the OmpC signal sequence in the OmpCSS-E2C chimeric protein produced by χ11021(pYA4894) did not increase the humoral response to CFP-10 as it appeared to do for both ESAT-6 and Ag85A (Fig. 5B and C). It is unclear why this difference occurred.
Analysis of the IgG subclasses showed higher levels of anti-Ag85A IgG2b antibodies than IgG1 titers, which is typical for a Th1 response. The switch between secretion of IgG2b or IgG1 is determined by the differential production of cytokines; thus, the stimulation of IgG2b production is dependent on IFN-γ production. Th1-type immune responses were observed in all mice immunized with either strain χ9879 or χ11021 delivering M. tuberculosis chimeric proteins. These results are in agreement with the T-cell response that was characterized by Ag85A-specific secretion of IFN-γ, TNF-α, and IL-2, which are more favorable for a protective immune response against M. tuberculosis. The secretion of Ag85A294-specific cytokines was higher in the mice vaccinated with Salmonella vaccine strain χ11021 harboring Asd+/MurA+ lysis plasmids delivering Ag85A294 and displaying regulated delayed lysis than in the mice vaccinated with Salmonella strain χ9879 harboring the Asd+ pYA3941 plasmid. Interestingly, the induction of Ag85A-specific secretion of IFN-γ, TNF-α, and IL-2 in vaccinated mice was directly related to the copy number of the plasmids producing SopENt80-E2C/Ag85A294 chimeric proteins (pYA4890, pYA4891, and pYA4892) but not those pro-ducing OmpCSS-E2C/Ag85A294 chimeric proteins (pYA4893 and pYA4894), where a slightly greater cytokine production was observed in mice vaccinated with χ11021(pYA4894) than mice vaccinated with χ11021(pYA4893). These results suggest that, in mice orally vaccinated with Salmonella vaccines secreting the combined chimeric proteins Ag85A294 and SopENt80-E2C, there is better stimulation of the Th1-associated cytokines. The highest induction of IFN-γ production was observed in mice vaccinated with both RASV strains χ11021(pYA4892 [p15A ori]) synthesizing SopENt80-E2C/Ag85A294 and χ11021(pYA4894 [p15A ori]) synthesizing OmpCSS-E2C/Ag85A294, indicating a synergistic effect of both vaccines in the enhancement of the T-cell immune response.
Significant increases in production of the IL-4 cytokine and low or moderate levels of IFN-γ were detected in mice vaccinated with the χ11021(pYA3681) vector control and with χ11021(pYA4892). It has been reported that significant production of the Th2-associated IL-4 cytokine is related to failure to control M. tuberculosis infections (58, 62). Consistent with these reports, mice immunized with the RASV χ11021(pYA4892) strain showed no protection against M. tuberculosis challenge.
In mice orally vaccinated with the RASV strain χ9879(pYA4257) synthesizing SopENt80-E2C and displaying regulated delayed attenuation, we previously detected induction of a significant increase in the number of ESAT-6-specific IFN-γ- and TNF-α-secreting T cells in mouse spleens 1 week after the last immunization (42). However, here, neither ESAT-6-specific nor CFP-10-specific T-lymphocyte activity was observed in mice orally vaccinated with χ11021 delivering either SopENt80-E2C/Ag85A294 or OmpCSS-E2C/Ag85A294 chimeric proteins. In this study, the ESAT-6-specific and CFP-10-specific stimulation of T-cell responses was analyzed at 3 weeks after the last immunization. If the stimulation of ESAT-6- and CFP-10-specific T-lymphocyte activity occurs at 1 week postboost and rapidly declines, as has been reported (4), this needs to be further investigated. Other studies have determined that vaccination with the ESAT-6 protein results in low immunogenicity and requires a strong adjuvant to prime specific immune responses (11). Moreover, secretion of ESAT-6 alone or as a fusion protein with Ag85B delivered by recombinant BCG induces weakened T-cell responses or completely diminishes T-cell responses, respectively (59). If the absence of ESAT-6-specific and CFP-10-specific stimulation of T-cell responses (or dominant Ag85A immune responses) is the result of competition between both Ag85A294 and SopENt80-E2C or OmpCSS-E2C, resulting in a dominant Ag85A immune response over the response to ESAT-6 and/or CFP-10, remains to be determined.
In spite of the lack of ESAT-6- and CFP-10-specific T-cell activity observed in mice vaccinated with the Salmonella vaccines synthesizing SopENt80-E2C/Ag85A294 or OmpCSS-E2C/Ag85A294 at the specific times assessed, we cannot rule out the possibility that the antibody responses against ESAT-6, CFP-10, and Ag85A may also play a role in the induction of T-cell activity. In addition to the role of serum antibodies in classical opsonization, phagocytosis, and killing of pathogens, there exist an interdependence and synergy between humoral and cell-mediated immunity (1). B cells are necessary for rapid T-cell activation via Fc receptors (FcR) by an FcR-dependent antibody-enhanced uptake, processing, and presentation of pathogen-derived antigens by FcR-bearing antigen-presenting cells (36, 40, 56). The enhanced uptake of BCG coated with antimycobacterial antibodies by dendritic cells and the subsequent processing of these bacteria, resulting in the proliferation of mycobacterium-specific CD4+ and CD8+ T cells secreting IFN-γ, support the role of specific antimycobacterial antibodies in the uptake of bacteria via Fc receptors by professional antigen-presenting cells for the activation of T cells (27).
The mycobacterial loads were significantly reduced in both the lungs and spleens of mice vaccinated with the monovalent Salmonella strain χ9879(pYA3941) and with the trivalent Salmonella vaccine χ11021 harboring the Asd+/MurA+ lysis plasmids and displaying the regulated delayed lysis phenotype. Among the trivalent vaccines, those harboring pYA4891, pYA4893, and pYA4894 were the most effective in conferring protection in the lungs against aerosol challenge with M. tuberculosis, and this level of protection was slightly better than that afforded by BCG. On the other hand, mice immunized with strain χ11021(pYA4890) or with both χ11021(pYA4892) and χ11021(pYA4894) achieved a protection in the lungs comparable to that generated with BCG. In contrast, in the spleens of vaccinated mice, the reduction in number of CFU was similar to that observed with BCG, with the exception of the reduction caused by strains χ11021(pYA4893) and χ11021(pYA4892), which did not afford significant protection.
All the observations made in the present study indicate that there is a requirement for an optimum level of synthesis of the protective antigens to induce a robust immune response and protection against M. tuberculosis, without impairing the capability of the live Salmonella vaccine to colonize the host lymphoid tissues. In this work, this was achieved by employing low-copy-number Asd+/MurA+ lysis vectors containing the p15A ori to produce secreted recombinant antigens. Thus, the trivalent Salmonella vaccine harboring either of the Asd+/MurA+ lysis plasmids with the p15A ori and expressing SopENt80-E2C/Ag85A294 or OmpCSS-E2C/Ag85A294, pYA4891 and pYA4894, respectively, afforded the most effective protection against M. tuberculosis challenge in orally immunized mice.
Our results show the efficacy of the Salmonella/Asd+/MurA+ lysis system for delivery of mycobacterial antigens to confer protection against M. tuberculosis infection and encourage further development of improved vaccines based on the Salmonella/Asd+/MurA+ lysis system to prevent M. tuberculosis infection. Since BCG is used in many countries to immunize infants, it is likely that an RASV-M. tuberculosis vaccine would be used as a boosting immunization in BCG-vaccinated individuals. Thus, in future experiments, we will explore the use of improved RASV-M. tuberculosis vaccines in prime-boost immunization regimens with BCG as the priming vaccine. In such experiments, antigens other than ESAT-6 and CFP-10 will be included, since the genes encoding these antigens are absent from the BCG genome. Alternatively, prime-boost vaccination strategies using RASV-M. tuberculosis vaccines with subunit vaccines with the same antigens will also be assessed.
ACKNOWLEDGMENTS
We thank Ascención Torres-Escobar for valuable suggestions and critical review of the manuscript.
This research was supported by National Institutes of Health grant AI056289.
Footnotes
Published ahead of print 5 December 2011
REFERENCES
- 1. Abebe F, Bjune GG. 2009. The protective role of antibody responses during Mycobacterium tuberculosis infection. Clin. Exp. Immunol. 157:235–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Amann E, Brosius J, Ptashne M. 1983. Vectors bearing a hybrid trp-lac promoter useful for regulated expression of cloned genes in Escherichia coli. Gene 25:167–178 [DOI] [PubMed] [Google Scholar]
- 3. Andersen P. 1994. Effective vaccination of mice against Mycobacterium tuberculosis infection with a soluble mixture of secreted mycobacterial proteins. Infect. Immun. 62:2536–2544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Badell E, et al. 2009. Protection against tuberculosis induced by oral prime with Mycobacterium bovis BCG and intranasal subunit boost based on the vaccine candidate Ag85B-ESAT-6 does not correlate with circulating IFN-gamma producing T-cells. Vaccine 27:28–37 [DOI] [PubMed] [Google Scholar]
- 5. Bald D, Koul A. 2010. Respiratory ATP synthesis: the new generation of mycobacterial drug targets? FEMS Microbiol. Lett. 308:1–7 [DOI] [PubMed] [Google Scholar]
- 6. Bastos RG, Borsuk S, Seixas FK, Dellagostin OA. 2009. Recombinant Mycobacterium bovis BCG. Vaccine 27:6495–6503 [DOI] [PubMed] [Google Scholar]
- 7. Bertani G. 1951. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 62:293–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Black S, Wright NG. 1955. Aspartic β-semialdehyde dehydrogenase and aspartic β-semialdehyde. J. Biol. Chem. 213:39–50 [PubMed] [Google Scholar]
- 9. Bolivar F, et al. 1977. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene 2:95–113 [PubMed] [Google Scholar]
- 10. Boyton RJ, Openshaw PJ. 2002. Pulmonary defenses to acute respiratory infection. Br. Med. Bull. 61:1–12 [DOI] [PubMed] [Google Scholar]
- 11. Brandt L, Elhay M, Rosenkrands I, Lindblad EB, Andersen P. 2000. ESAT-6 subunit vaccination against Mycobacterium tuberculosis. Infect. Immun. 68:791–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Branger CG, et al. 2009. Oral vaccination with LcrV from Yersinia pestis KIM delivered by live attenuated Salmonella enterica serovar Typhimurium elicits a protective immune response against challenge with Yersinia pseudotuberculosis and Yersinia enterocolitica. Vaccine 27:5363–5370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brown ED, Vivas EI, Walsh CT, Kolter R. 1995. MurA (MurZ), the enzyme that catalyzes the first committed step in peptidoglycan biosynthesis, is essential in Escherichia coli. J. Bacteriol. 177:4194–4197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bumann D. 2001. Regulated antigen expression in live recombinant Salmonella enterica serovar Typhimurium strongly affects colonization capabilities and specific CD4(+)-T-cell responses. Infect. Immun. 69:7493–7500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cárdenas L, Clements JD. 1992. Oral immunization using live attenuated Salmonella spp. as carriers of foreign antigens. Clin. Microbiol. Rev. 5:328–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cohen SN, Miller CA. 1970. Non-chromosomal antibiotic resistance in bacteria. II. Molecular nature of R-factors isolated from Proteus mirabilis and Escherichia coli. J. Mol. Biol. 50:671–687 [DOI] [PubMed] [Google Scholar]
- 17. Cooper AM, et al. 1993. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 178:2243–2247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cozzarelli NR, Kelly RB, Kornberg A. 1968. A minute circular DNA from Escherichia coli 15. Proc. Natl. Acad. Sci. U. S. A. 60:992–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Curtiss R., III 1965. Chromosomal aberrations associated with mutations to bacteriophage resistance in Escherichia coli. J. Bacteriol. 89:28–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Curtiss R, III, Kong W. June 2006. Regulated bacterial lysis for gene vaccine vector delivery and antigen release. US patent 2006/0140975. [Google Scholar]
- 21. Curtiss R, III, et al. April 2009. Recombinant bacterium capable of eliciting an immune response against enteric pathogens. International patent WO/2009/046449. [Google Scholar]
- 22. Curtiss R, III, et al. 2009. Salmonella enterica serovar Typhimurium strains with regulated delayed attenuation in vivo. Infect. Immun. 77:1071–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Curtiss R, III, Wang S, Wanda SY, Kong W. May 2010. Regulated expression of antigen and/or regulated attenuation to enhance vaccine immunogenicity and/or safety. US patent 2010/0124558. [Google Scholar]
- 24. Curtiss R, III, et al. 2010. New technologies in using recombinant attenuated Salmonella vaccine vectors. Crit. Rev. Immunol. 30:255–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Curtiss R, III, et al. 2007. Induction of host immune responses using Salmonella-vectored vaccines, p 297–313 In Brogden KA, et al. (ed), Virulence mechanisms of bacterial pathogens, 4th ed. ASM Press, Washington, DC [Google Scholar]
- 26. Denis M. 1991. Involvement of cytokines in determining resistance and acquired immunity in murine tuberculosis. J. Leukoc. Biol. 50:495–501 [DOI] [PubMed] [Google Scholar]
- 27. de Vallière S, Abate G, Blazevic A, Heuertz RM, Hoft DF. 2005. Enhancement of cell-mediated immunity by antimycobacterial antibodies. Infect. Immun. 73:6711–6720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. D'Souza S, et al. 2003. Mapping of murine Th1 helper T-cell epitopes of mycolyl transferases Ag85A, Ag85B, and Ag85C from Mycobacterium tuberculosis. Infect. Immun. 71:483–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fine PE. 1995. Variation in protection by BCG: implications of and for heterologous immunity. Lancet 346:1339–1345 [DOI] [PubMed] [Google Scholar]
- 30. Flynn JL, et al. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249–2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Galán JE, Curtiss R., III 1990. Expression of Salmonella typhimurium genes required for invasion is regulated by changes in DNA supercoiling. Infect. Immun. 58:1879–1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Galen JE, et al. 2010. A new generation of stable, nonantibiotic, low-copy-number plasmids improves immune responses to foreign antigens in Salmonella enterica serovar Typhi live vectors. Infect. Immun. 78:337–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guirado E, et al. 2006. Passive serum therapy with polyclonal antibodies against Mycobacterium tuberculosis protects against post-chemotherapy relapse of tuberculosis infection in SCID mice. Microbes Infect. 8:1252–1259 [DOI] [PubMed] [Google Scholar]
- 34. Gunn BM, Wanda SY, Burshell D, Wang C, Curtiss R., III 2010. Construction of recombinant attenuated Salmonella enterica Typhimurium vaccine (RASV) vector strains for safety in newborn and infant mice. Clin. Vaccine Immunol. 17:354–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hamasur B, et al. 2004. A mycobacterial lipoarabinomannan specific monoclonal antibody and its F(ab′) fragment prolong survival of mice infected with Mycobacterium tuberculosis. Clin. Exp. Immunol. 138:30–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hoehlig K, et al. 2008. Immune regulation by B cells and antibodies: a view towards the clinic. Adv. Immunol. 98:1–38 [DOI] [PubMed] [Google Scholar]
- 37. Hopkins S, et al. 1995. A recombinant Salmonella Typhimurium vaccine induces local immunity by four different routes of immunization. Infect. Immun. 63:3279–3286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Horwitz MA, Harth G, Dillon BJ, Maslesa-Galic' S. 2000. Recombinant bacille Calmette-Guérin (BCG) vaccines expressing Mycobacterium tuberculosis 30 kDa major secreting protein induce greater protective immunity against tuberculosis than conventional BCG vaccines in a highly susceptible animal model. Proc. Natl. Acad. Sci. U. S. A. 97:13853–13858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Huygen K, et al. 1996. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat. Med. 2:893–898 [DOI] [PubMed] [Google Scholar]
- 40. Igietseme JU, Eko FO, He Q, Black CM. 2004. Antibody regulation of T cell immunity: implications for vaccine strategies against intracellular pathogens. Expert Rev. Vaccines 3:23–34 [DOI] [PubMed] [Google Scholar]
- 41. Jain R, et al. 2008. Enhanced and enduring protection against tuberculosis by recombinant BCG-Ag85C and its association with modulation of cytokine profile in lung. PLoS One 3:e3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Juá rez-Rodríguez MD, Arteaga-Cortés LT, Kader R, Curtiss R, III, Clark-Curtiss JE. 2012. Live attenuated Salmonella vaccines against Mycobacterium tuberculosis with antigen delivery via the type III secretion system. Infect. Immun. 80:798–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kang HY, Srinivasan J, Curtiss R., III 2002. Immune responses to recombinant pneumococcal PspA antigen delivered by live attenuated Salmonella enterica serovar Typhimurium vaccine. Infect. Immun. 70:1739–1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kang HY, Curtiss R., III 2003. Immune responses dependent on antigen location in recombinant attenuated Salmonella Typhimurium vaccines following oral immunization. FEMS Immunol. Med. Microbiol. 37:99–104 [DOI] [PubMed] [Google Scholar]
- 45. Klucar P, et al. 2009. Vaccination strategies to enhance local immunity and protection against Mycobacterium tuberculosis. Vaccine 27:1816–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kong W, et al. 2008. Regulated programmed lysis of recombinant Salmonella in host tissues to release protective antigens and confer biological containment. Proc. Natl. Acad. Sci. U. S. A. 105:9361–9366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Koshland D, Botstein D. 1980. Secretion of beta-lactamase requires the carboxy end of the protein. Cell 20:749–760 [DOI] [PubMed] [Google Scholar]
- 48. Launois P, et al. 1994. T-cell-epitope mapping of the major secreted mycobacterial antigen Ag85A in tuberculosis and leprosy. Infect. Immun. 62:3679–3687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Li Y, et al. 2009. Evaluation of new generation Salmonella enterica serovar Typhimurium vaccines with regulated delayed attenuation to induce immune responses against PspA. Proc. Natl. Acad. Sci. U. S. A. 106:593–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liu T, Chopra AK. 2010. An enteric pathogen Salmonella enterica serovar Typhimurium suppresses tumor growth by downregulating CD44high and CD4T regulatory (Treg) cell expression in mice: the critical role of lipopolysaccharide and Braun lipoprotein in modulating tumor growth. Cancer Gene Ther. 17:97–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lozes E, et al. 1997. Immunogenicity and efficacy of a tuberculosis DNA vaccine encoding the components of the secreted antigen 85 complex. Vaccine 15:830–833 [DOI] [PubMed] [Google Scholar]
- 52. Mastroeni P, Villarreal-Ramos B, Hormaeche CE. 1992. Role of T cells, TNF alpha and IFN gamma in recall of immunity to oral challenge with virulent Salmonellae in mice vaccinated with live attenuated aro− Salmonella vaccines. Microb. Pathog. 13:477–491 [DOI] [PubMed] [Google Scholar]
- 53. Matsuyama S, Inokuchi K, Mizushima S. 1984. Promoter exchange between ompF and ompC, genes for osmoregulated major outer membrane proteins of Escherichia coli K-12. J. Bacteriol. 158:1041–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mir FA, Kaufmann SH, Eddine AN. 2009. A multicistronic DNA vaccine induces significant protection against tuberculosis in mice and offers flexibility in the expressed antigen repertoire. Clin. Vaccine Immunol. 16:1467–1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mollenkopf HJ, Groine-Triebkorn D, Andersen P, Hess J, Kaufmann SH. 2001. Protective efficacy against tuberculosis of ESAT-6 secreted by a live Salmonella Typhimurium vaccine carrier strain and expressed by naked DNA. Vaccine 19:4028–4035 [DOI] [PubMed] [Google Scholar]
- 56. Moore T, et al. 2003. Fc receptor-mediated antibody regulation of T cell immunity against intracellular pathogens. J. Infect. Dis. 188:617–624 [DOI] [PubMed] [Google Scholar]
- 57. Nakayama K, Kelly SM, Curtiss R., III 1988. Construction of an ASD+ expression-cloning vector: stable maintenance and high level expression of cloned genes in a Salmonella vaccine strain. Biotechnology (NY) 6:693–697 [Google Scholar]
- 58. Ordway DJ, et al. 2004. Increased interleukin-4 production by CD8 and gamma/delta T cells in health-care workers is associated with the subsequent development of active tuberculosis. J. Infect. Dis. 190:756–766 [DOI] [PubMed] [Google Scholar]
- 59. Palendira U, Spratt JM, Britton WJ, Triccas JA. 2005. Expanding the antigenic repertoire of BCG improves protective efficacy against aerosol Mycobacterium tuberculosis infection. Vaccine 23:1680–1685 [DOI] [PubMed] [Google Scholar]
- 60. Pym AS, et al. 2003. Recombinant BCG exporting ESAT-6 confers enhanced protection against tuberculosis. Nat. Med. 9:533–539 [DOI] [PubMed] [Google Scholar]
- 61. Ravn P, et al. 1999. Human T cell responses to the ESAT-6 antigen from Mycobacterium tuberculosis. J. Infect. Dis. 179:637–645 [DOI] [PubMed] [Google Scholar]
- 62. Rook GAW, Hernandez-Pando R, Dheda K, Seah GT. 2004. IL-4 in tuberculosis: implications for vaccine design. Trends Immunol. 9:483–488 [DOI] [PubMed] [Google Scholar]
- 63. Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 64. Santos A, et al. 2010. Severe axillary lymphadenitis after BCG vaccination: alert for primary immunodeficiencies. J. Microbiol. Immunol. Infect. 43:530–537 [DOI] [PubMed] [Google Scholar]
- 65. Schmidt-Weber CB, Alexander SI, Henault LE, James L, Lichtman AH. 1999. IL-4 enhances IL-10 gene expression in murine Th2 cells in the absence of TCR engagement. J. Immunol. 162:238–244 [PubMed] [Google Scholar]
- 66. Schumann W, Lindenblatt E, Bade EG. 1976. Bacteriophage-specific DNA-binding proteins in P22-lysogenic and in P22-infected Salmonella Typhimurium. J. Virol. 20:334–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Torres-Escobar A, et al. 2010. Fine-tuning synthesis of Yersinia pestis LcrV from runaway-like replication balanced-lethal plasmid in a Salmonella enterica serovar Typhimurium vaccine induces protection against a lethal Y. pestis challenge in mice. Infect. Immun. 78:2529–2543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Toussirot E, Wendling D. 2004. The use of TNF-alpha blocking agents in rheumatoid arthritis: an overview. Expert Opin. Pharmacother. 5:581–594 [DOI] [PubMed] [Google Scholar]
- 69. Trunz BB, Fine P, Dye C. 2006. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet 367:1173–1180 [DOI] [PubMed] [Google Scholar]
- 70. Vordermeier HM, Venkataprasad N, Harris DP, Ivanyi J. 1996. Increase of tuberculous infection in the organs of B cell-deficient mice. Clin. Exp. Immunol. 106:312–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wang S, et al. 2010. Salmonella vaccine vectors displaying delayed antigen synthesis in vivo to enhance immunogenicity. Infect. Immun. 78:3969–3980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. World Health Organization 2010. Global tuberculosis control surveillance, planning, financing: WHO report 2010. Document WHO/HTM/TB/2010.104. World Health Organization, Geneva, Switzerland [Google Scholar]