Abstract
The Nhe enterotoxin from Bacillus cereus is known to induce cytotoxicity on Vero and CaCo-2 cells by ordered binding of its single components NheA, NheB, and NheC. This study aimed to elucidate functional sites on NheB by identifying the epitopes of the neutralizing monoclonal antibodies 1E11 and 2B11. The binding regions of both antibodies were determined by using recombinant NheB fragments and synthetic peptides. The antigenic site of antibody 1E11 was located within the amino acids 321 to 341 of NheB, whereas reactivity of antibody 2B11 was dependent on the presence of amino acids 122 to 150 and on conformation. Both antibodies were able to bind simultaneously to NheB and did not interfere with target cell binding as shown by immunofluorescence microscopy. A set of neutralization assays revealed that antibody 2B11 most likely interfered with the interaction between NheB and NheC both on the epithelium cell surface and in solution. In contrast, antibody 1E11 inhibited association between NheA and cell-bound NheB in a competitive manner, and effectively neutralized Nhe cytotoxicity on a variety of human cell lines. This distinct mechanism further supports that NheA is the key component during the Nhe mode of action and the C-terminal epitope recognized by antibody 1E11 points to an important functional region of NheB.
INTRODUCTION
Bacillus cereus is a major food-borne pathogen known to produce a range of cytotoxins (for reviews, see references 27 and 28). There are four major toxins involved in food poisoning cases, namely, the emetic toxin (cereulide), a dodecadepsipeptide (1), and the three-component diarrheal toxins hemolysin BL (Hbl) (4, 5) and nonhemolytic enterotoxin (Nhe) (23). In addition, a single-component protein toxin (cytotoxin K) causing severe necrotic enteritis was identified in a rare B. cereus strain (22), for which the name “Bacillus cytotoxis” has been proposed (19). Studies on the prevalence of the nhe and hbl genes (10, 15, 16, 26, 31) in B. cereus indicate that all strains of B. cereus possess the genes of at least one of the diarrheal enterotoxins, and Nhe is the most prevalent enterotoxin harbored by B. cereus. In addition, the overall B. cereus-associated cytotoxic activity is correlated with the Nhe expression level (24).
Nhe was first identified in strain NVH 0075/95, which was isolated following a large food-poisoning outbreak in Norway (23). It is a three-component toxin and consists of the exoproteins NheA (41.0 kDa), NheB (39.8 kDa), and NheC (36.5 kDa) (14). Studies using cell-based tests to assay Nhe-specific cytotoxicity demonstrated toxic effects in Vero, GH4, and CaCo-2 cells (17, 20, 21). The susceptibility of other cell lines has not yet been tested. It is known that Nhe has intrinsic pore-forming capacity (11) and that maximum toxicity will be reached when the ratio of the individual components is 10:10:1 for NheA, NheB, and NheC, respectively (20). NheB and NheC are mostly α-helical molecules with a predicted β-tongue, showing structural similarities to ClyA (11). The region of the predicted β-tongue in NheC is necessary for cell binding but not for interaction with NheB in solution (21). Binding between NheB and NheC could be demonstrated in solution, and both components are able to bind to cell membranes. In contrast, NheA does not bind to cells per se, nor does it seem to interact with NheB and NheC in solution. The presence of NheA is, however, mandatory in the final step of the Nhe mode of action in order to trigger toxicity, indicating a specific binding order of the individual components (21).
In these fundamental studies monoclonal antibodies (MAbs) against NheB have been used to neutralize Nhe toxicity in Vero and Caco-2 cells. Therefore, we thought that identification of the binding regions of these antibodies on NheB could lead to significant functional implications.
MATERIALS AND METHODS
B. cereus strains, culture medium, and culture conditions.
B. cereus strains used in the present study were as follows: NVH 0075/95 (fully cytotoxic), MHI1672 (producing NheA and NheB, low cytotoxic), and MHI1761 (producing NheB and NheC, not cytotoxic). The latter food isolates bear a preliminary stop codon in the 5′ end of the nheC or nheA gene, respectively, as published earlier (21). Cells were grown in CGY medium supplemented with 1% glucose for toxin production, exactly as described previously (21). All strains lacked both hbl and cytK, as demonstrated by PCR, immunoassay, and cell culture assay (31).
Cloning of recombinant full-length Nhe components and NheB deletion mutants.
The full-length NheA gene was amplified and cloned into pBAD102 Directional TOPO Expression system (Invitrogen). Recombinant NheA was then expressed in Escherichia coli (LMG-194). Expression and purification of NheC was performed as described elsewhere (20). Concentration of recombinant protein preparations was determined by in-house enzyme immunoassay (EIA) using MAb 1A8 for NheA and polyclonal rabbit serum for NheC (8).
Truncated NheB genes were PCR amplified, cloned into the pBAD102 directional TOPO expression system and expressed in E. coli (LMG-194) according to the manufacturer's (Invitrogen) recommendations. Corresponding recombinant proteins showed N-terminal deletions of 30, 60, 92, 121, and 151 amino acids (for additional information, see Table 1). The reactivity of deletion mutants was assayed with MAbs 2B11 and 1E11 by EIA and Western blotting. For further epitope mapping of MAb 1E11, three peptide fragments comprising the C-terminal sequence of NheB (amino acids 205 to 372; see Table 1) were generated in the same way.
Table 1.
Peptide fragments used in epitope-mapping experiments and reactivity of the MAbsa
| Fragment (aa start-end) | Primer (5′-3′ sequence) |
EIA titerb |
||
|---|---|---|---|---|
| Forward | Reverse | MAb 2B11 | MAb 1E11 | |
| N1 (31-372) | N1for (CACCAAAGATGCAATGGAAAGAAC) | Bflrev (TTATGCTTTTTTCGTATCTACTTTAATAT) | 640 | >640 |
| N2 (61-372) | N2for (CACCAATGTATCGTCTGTTGATG) | Bflrev (TTATGCTTTTTTCGTATCTACTTTAATAT) | 320 | >640 |
| N3 (93-372) | N3for (CACCCCACAGCTTATTTCAACG) | Bflrev (TTATGCTTTTTTCGTATCTACTTTAATAT) | 320 | >640 |
| N4 (122-372) | N4for (CACCGCAAAGGATAAAGCAACT) | Bflrev (TTATGCTTTTTTCGTATCTACTTTAATAT) | 160 | >640 |
| N5 (152-372) | N5for (CACCGACCTGAAGAAATTCCG) | Bflrev (TTATGCTTTTTTCGTATCTACTTTAATAT) | – | >640 |
| C1 (205-258) | C1for (CACCATTATTATCGGTTCATC) | C1rev (CTTGCGTAATACGATTCCAG) | – | – |
| C2 (251-324) | C2for (CACCACAGCTGGAATCGTATTA) | C2rev (AAGTAAAGAATTGTATTTTGATCC) | – | – |
| C3 (314-372) | C3for (CACCTACACAATGGGATCA) | C3rev (TGCTTTTTTCGTATCTACTAC) | – | >640 |
Amino acid (aa) numbering according to Granum et al. (14), without signal peptide. Gray shading in the forward primer sequences indicates additional nucleotides needed for directional cloning approach.
Reciprocal dilution. –, Negative at the lowest dilution tested (1:5).
Synthetic peptides.
All peptides (P1 to P6) used in epitope mapping experiments (Fig. 1) were synthesized by PSL GmbH (Germany) and N-terminally linked to ovalbumin for use in EIA and sodium dodecyl sulfate (SDS)-immunoblotting.
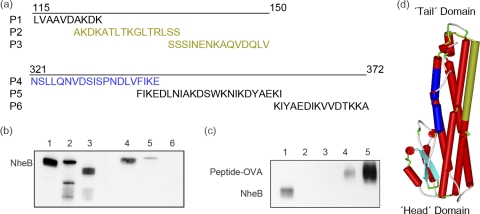
Fig 1.
MAbs 1E11 and 2B11 against NheB react with different epitopes. Immunoblot reactivity of the anti-NheB MAbs 1E11 and 2B11 was tested with synthetic peptides (a) and recombinant NheB fragments (see Table 1). (b) An SDS-immunoblot showing reactivity of MAb 1E11 (lanes 1 to 3) and 2B11 (lanes 4 to 6) with wild-type NheB (lanes 1 and 4), N4 (lanes 2 and 5), and N5 (lanes 3 and 6), respectively. (c) An SDS-immunoblot showing reactivity of MAb 1E11 with wild-type NheB (lane 1), P6 (lane 2), P5 (lane 3), and P4 (lanes 4 and 5). The peptides were coupled to ovalbumin (OVA) and conjugates were used at a concentration of 20 μg/ml (lanes 2 to 4) and 50 μg/ml (lane 5), respectively. (d) Homology model of NheB created by Swiss-Model (2, 6) using the Hbl B crystal structure (PDB ID: 2nrj) as a template. The first 29 and the last 26 residues of the mature sequence of NheB were not present in the model obtained. The protein structure is shown schematically, with the β-hairpin in light blue, the position of P4 in dark blue, and P2 and P3 in brown color (drawn using Accelerys Discovery Studio 3.0 Visualizer).
Purification of wild-type Nhe components.
NheB was purified from 5- to 6-h culture supernatants of B. cereus MHI1672, and the purity was documented by SDS-PAGE (8).
MAbs.
The basic characteristics of the MAbs 1E11 and 2B11 against NheB (no cross-reactivity with NheA and NheC) have been described (8). For immunofluorescence, MAbs were labeled with Alexa Fluor dyes (Alexa Fluor 488 for 1E11 and Alexa Fluor 555 for 2B11) according to the manufacturer's instructions (Invitrogen).
EIAs.
The reactivity of MAbs 1E11 and 2B11 with cell-free culture supernatants of B. cereus strains or recombinant NheB fragments was assayed by indirect EIAs as described previously (8). Antigen titers were defined as the reciprocal of the highest dilution of Nhe preparations that gave an absorbance value of ≥1.0.
SDS-PAGE and immunoblotting.
SDS-PAGE analysis was performed on a PhastGel gradient (10 to 15%) minigel system (GE Healthcare). After PAGE, separated proteins were blotted on a PVDF-P membrane (Millipore), blocked in 3% casein-phosphate-buffered saline (PBS) containing 0.025% Tween 20, and incubated with MAbs 1E11 and 2B11 (1 μg ml−1) for 1 h at room temperature. After three rounds of washing with PBS-Tween 20 rabbit anti-mouse-horseradish peroxidase conjugate (Dako) diluted 1:3,000 was added for 1 h. The membranes were washed three times in PBS-Tween and twice in PBS. Chemiluminescence signals were recorded on a Kodak imager (Eastman Kodak Company) after the application of Super Signal Western Femto (Pierce).
Mammalian cell lines and culture conditions.
Vero and HEp-2 cells were obtained from the European Collection of Cell Cultures. A549, A204, CaCo-2, and Hep-G2 cells were from the German collection of microorganisms and cell cultures (DSMZ). Primary human umbilical vein endothelial cells (HUVEC) were obtained from Cell Systems. The cell lines were cultured in media recommended by the supplier. Media were supplemented with fetal bovine serum and, if requested, with 2 mM l-glutamine or 1 mM sodium pyruvate (Biochrom AG, Berlin, Germany). The cells were maintained in a humidified incubator at 37°C in an atmosphere of 7% CO2.
Immunofluorescence microscopy.
Vero, A549, and CaCo-2 cells cultivated in 8-well Lab-Tek chamber slides (Nunc) were treated with cell-free B. cereus supernatants from MHI 1761 containing NheB and NheC for 2 h at 37°C. Cell labeling protocol for immunofluorescence-microscopy was as follows: the cells were fixed with ice-cold methanol for 10 min, permeabilized with 0.5% Triton X-100, and blocked with 5% inactivated goat serum for 60 min. Then, Alexa fluor dye-labeled MAb 1E11 and 2B11 against NheB were added at a concentration of 4 μg per well, followed by incubation for 1 h. For some experiments, unlabeled MAb 1E11 and 2B11 were used at the same concentration and detected by addition of Alexa Fluor 488-labeled secondary antibody (Invitrogen, 2 μg per well). For all immunoreagents, PBS containing 1% bovine serum albumin was used as a diluent. Finally, nuclei were counterstained with DAPI (4′,6′-diamidino-2-phenylindole), and micrographs were taken on a BZ-8000 fluorescence microscope (Keyence).
Cytotoxicity assays.
Cleavage of WST-1 (water-soluble tetrazolium salt; Roche Diagnostics) to formazan by cellular mitochondrial dehydrogenases served as a final readout in all cytotoxicity assays. The optical density at 450 nm was determined on a Tecan photometer. Titers were defined as the highest dilution of Nhe preparations, which inhibited mitochondrial activity by >50%, as calculated by linear interpolation. The cytotoxic activity of Nhe preparations and B. cereus culture supernatants was determined as an endpoint titer under simultaneous incubation conditions using Vero cells as previously described (7, 21).
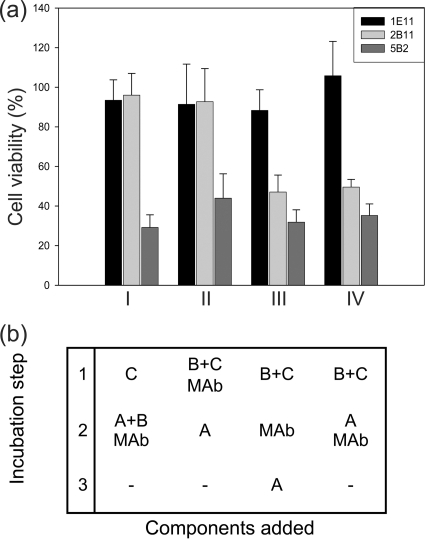
To differentiate neutralization effects of MAbs 1E11 and 2B11, a Vero cell assay was modified to a consecutive testing order resulting in four different experimental setups as depicted in Fig. 4. The amount of neutralizing MAb used was 10 μg/well, while an irrelevant MAb (5B2; 10 μg/well) served as control. NheB and NheC were used at the optimum ratio of 10:1 (approximately 250 and 25 ng/ml), and NheA was used in slight excess (400 to 500 ng/ml). To remove unbound toxin components or MAbs between the incubation steps, the cells were washed four times with cell culture medium after each step. To further elucidate the neutralizing mechanism of MAb 1E11, different molar ratios of NheA and the respective antibodies were tested on Vero cells primed with NheB and NheC.
Fig 4.
MAbs 1E11 and 2B11 exhibit different neutralization mechanisms. (a) In consecutive incubation experiments, MAb 2B11 (light gray bars) is only effective when added together with NheB in the second incubation step (I) or during priming with NheB and NheC (II). MAb 1E11 (black bars) shows complete neutralization capacity in all experimental setups (I to IV). An unrelated MAb (5B2, dark gray bars) was used as a control. (b) Different combinations of components and the order of incubation steps used. Unbound Nhe components and antibodies were washed off after each incubation step. All data represent the means and standard deviations of six experiments.
In order to check the cytotoxic activity of Nhe and the neutralization capacity of the antibody 1E11 in human cell lines, serial dilutions of toxin supernatants were placed in 96-well plates (0.1 ml per well) and, except for HUVEC, cell suspensions (0.1 ml) were added immediately afterward. The optimal cell densities had been determined for each cell line in preliminary experiments (1 × 104 cells per well for A204 and Vero cells and 2 × 104 cells per well for A549, CaCo-2, Hep-G2, and HEp-2 cells). HUVEC were seeded at low cell densities (1.7 × 103 per well) to avoid cell dedifferentiation and cultured for three to 4 days to reach confluence before adding toxin dilutions. For neutralization assays, 10 μg of the purified MAb 1E11 (1 mg/ml in PBS) was added additionally to each well, and cytotoxicity titers were determined after 24 h.
RESULTS
Epitope mapping of NheB specific MAbs 1E11 and 2B11.
A set of recombinant NheB fragments comprising the N-terminal and C-terminal parts of the protein (Table 1) was generated and used in EIA analyses to determine the relative positions of each epitope and to establish broad regions onto which the epitopes mapped. The EIA data presented in Table 1 revealed a distinct reactivity pattern for each antibody. It became evident that the C terminus of the B component includes the epitope for MAb 1E11. MAb 1E11 bound only to the C-terminal fragment C3, containing amino acids 314 to 372 of NheB, thus indicating a putative epitope in the C-terminal part of the B component. Finally, three overlapping peptides (P4 to P6) covering the C3 sequence (Fig. 1a) were synthesized, and peptide P4 (NSLLQNVDSISPNDLVFIKE) conjugated to ovalbumin was recognized by MAb 1E11 (Fig. 1c). In addition, only the free peptide P4 (added in a 1,000-fold molar excess) inhibited binding of MAb 1E11 to wild-type NheB in EIA (data not shown).
Antibody 2B11 showed good affinity in EIA but lower reactivity in immunoblotting experiments than 1E11 (Fig. 1b) and did not bind any of the C-terminal fragments or N5 (Table 1). Binding, however, occurred with all fragments, which contained amino acids 122 to 151. Three synthetic peptides (P1 to P3; Fig. 1a) covering this region were, however, not recognized by MAb 2B11. Thus, a conformation-dependent and/or discontinuous epitope seemed likely, which was not further characterized.
Antibodies to NheB do not interfere with the cell binding of NheB.
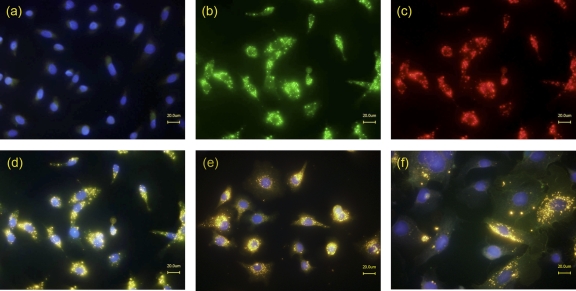
Figure 2 shows Vero cells, treated with culture supernatants of B. cereus MHI 1761 lacking NheA expression and stained simultaneously with the fluorescence-labeled antibodies 1E11 (green, Fig. 2b) and 2B11 (red, Fig. 2c). Superimposed images showed a color shift to yellow, indicating that MAbs 1E11 and 2B11 are able to attach simultaneously to NheB (Fig. 2d) and suggesting that epitopes recognized by the antibodies are not involved in the binding of NheB to the cell. To prove that the antibodies do not interfere with cell binding of NheB, the culture supernatants were preincubated with the antibodies, and a labeled secondary antibody was added after fixation and blocking of the cells. Cell-associated fluorescence intensity did not change significantly regardless if the antibodies were added before or after cell binding (Fig. 3). In addition, similar staining patterns (superimposed images) were obtained using A549 or CaCo-2 cells (Fig. 2e and f).
Fig 2.
MAbs 1E11 and 2B11 react with cell-bound NheB. Vero cells, untreated (a) and treated (b to d) with culture supernatants of B. cereus strain MHI1761 (producing NheB and NheC), were simultaneously stained with Alexa Fluor 488-labeled anti-NheB MAb 1E11 (b) and Alexa Fluor 555-labeled anti-NheB MAb 2B11 (c). (d) Superimposed images (merge). (e and f) Staining of A549 and CaCo-2 cells, respectively (merged images).
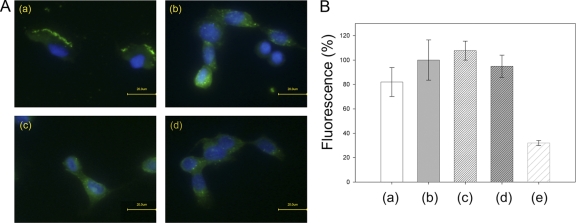
Fig 3.
MAbs 1E11 and 2B11 do not inhibit binding of NheB to Vero cells. (A) Vero cells were treated with culture supernatants of B. cereus strain MHI1761 (producing NheB and NheC), which had been preincubated for 30 min with antibody 1E11 (a), 2B11 (c), or buffer (b and d). After fixation and blocking of the cells, Alexa Fluor 488-labeled secondary antibody (green fluorescence) was added in panels a and c. Antibodies 1E11 and 2B11, followed by Alexa Fluor 488-labeled secondary, antibody were added in panels b and d, respectively. Also, DAPI counterstaining (blue fluorescence) was performed. Negative controls, performed without primary antibodies, showed only DAPI staining. (B) Images recorded by immunofluorescence microscopy were analyzed by the means of BZ Analyzer Software (Keyence) using the “Area Measure” tool. Fluorescence intensities of equal surface areas of six to eight cells for each of the experimental setups (columns a to d) were recorded. The average fluorescence intensity of experiment b was set to 100%. Differences in cell-associated fluorescence intensity (columns a to d) were not significant (Student t test, P > 0.1). The average background fluorescence is also shown (column e).
Antibodies to NheB inhibit association of Nhe components in solution (2B11) and on the cell surface (1E11).
To examine the mechanism by which the MAbs against NheB neutralize toxicity, we conducted a set of experiments where cells were primed with recombinant NheC or supernatants of strain MHI1761 (producing NheB and NheC). After a washing step, recombinant NheA or the supernatant of strain MHI1672 (producing NheA and NheB) was added. These combinations were tested with or without adding the respective antibodies. The results presented in Fig. 4 clearly show that MAb 1E11 neutralized toxicity in any combination between the Nhe components. Antibody 2B11 neutralized toxicity when added together with NheB after priming with NheC. Together with the finding that neither of the antibodies interfered with cell binding of the NheB (Fig. 2 and 3), this result indicates that MAb 2B11 prevents association of NheB to cell bound NheC. Also, MAb 2B11 neutralized toxicity when added during priming with NheB and NheC. Considering the fact that in solution almost all NheC will bind to NheB (21), the reduced toxicity observed in experiment II (Fig. 4) indicates that formation of NheB/NheC complexes in solution is hampered as well by MAb 2B11. In contrast, MAb 1E11 was also efficient when added after priming cells with NheB and NheC, suggesting that it most likely prevents association of NheA.
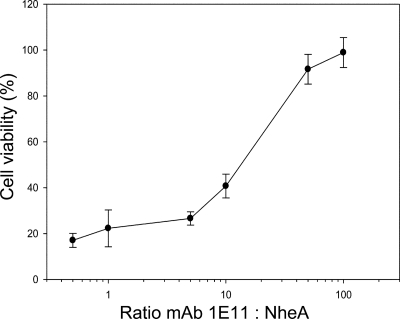
To support the latter mechanism, we conducted a competitive assay, where solutions containing different molar ratios of MAb 1E11 and NheA were added simultaneously to cells primed with NheB and NheC. The resulting dose-response curve (Fig. 5) indicated a competition of NheA and MAb 1E11 for cell-bound NheB. In addition, the data demonstrate a high apparent affinity of NheA to cell-bound NheB, and a 100-fold excess of MAb 1E11 was needed for full neutralization.
Fig 5.
NheA and MAb 1E11 compete for binding to cell-bound NheB. Cytotoxicity assays using NheA and MAb 1E1 at different molar ratios after priming cells with NheB and NheC yielded a classical dose-response curve. A 100-fold molar excess of 1E11 leads to complete neutralization of cytotoxicity. All data represent the means and standard deviations of six experiments.
Antibodies to NheB differ in their neutralization capacity.
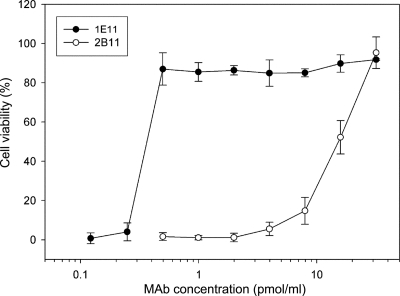
When added in equal concentrations to Vero cells during a simultaneous incubation experiment, the neutralization of MAb 1E11 was clearly more effective than that of MAb 2B11 (Fig. 6). In these experiments, simultaneous incubation conditions were applied in which MAb 1E11 reacts primarily with NheB in solution and does not compete with NheA for cell-bound NheB. As a result, a substantially higher neutralization capacity of the MAb was observed, i.e., ∼0.5 pmol of MAb 1E11/ml were able to neutralize the cytotoxic effect of 2 pmol of NheB/ml.
Fig 6.
MAbs 1E11 and 2B11 exhibit different neutralization capacities. In microtiter plates, Vero cells were simultaneously incubated for 2 h with culture supernatant of NVH 0075/95 (diluted 1:50; corresponds to approximately 2 pmol of NheA, 2 pmol of NheB, and 0.2 pmol of NheC/ml) and decreasing amounts of the respective MAb. Residual cytotoxic activity was determined by a WST-1 assay. All data represent the means and standard deviations of three experiments.
Nhe toxicity is neutralized by MAb 1E11 in human epithelial and endothelial cell lines.
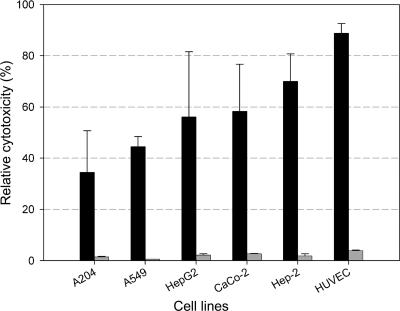
To test the neutralization capacity of MAb 1E11, different human cell lines were challenged with Nhe and compared to the effect on Vero cells. A cell-free supernatant of the B. cereus strain NVH 0075/95, containing all three Nhe components (but not Hbl or cytotoxin K), was used as (wild-type) toxin source. The NheB concentration of the supernatant was 10.0 ± 2 μg/ml, as measured by EIA (24). The reciprocal titers obtained under these conditions for Vero cells ranged from 780 to 860, with a mean value of 820, and was used as a 100% value in Fig. 7. Treatment of the human cell lines gave distinct toxic effects, with HUVEC being the most sensitive (mean titer, 1:728; 89% in Fig. 7) human cell type. In other words, the concentration of NheB necessary to cause 50% loss of mitochondrial activity was ∼14 ng/ml for HUVEC. When MAb 1E11 was added, the reciprocal titers were between 10 and 32 corresponding to a marginal residual toxicity of 1.2 and 3.9% (Fig. 7). In summary, the data prove that human epithelial cells and the primary endothelial cell line (HUVEC) were highly susceptible to Nhe.
Fig 7.
MAb 1E11 neutralizes Nhe cytotoxicity in human epithelial and endothelial cell lines. Different human cell lines were exposed for 24 h to serial dilutions of cell free supernatants of B. cereus NVH 0075/95 expressing all Nhe components. For comparison, cytotoxic activity of Nhe on Vero cells were determined in parallel by the WST-1 bioassay, and the mean reciprocal titer of 820 obtained under these conditions was used as the 100% value. Black bars indicate Nhe activity; gray bars show residual toxicity after neutralization by MAb 1E11 (10 μg/well). All data represent the means and standard deviations of three experiments.
DISCUSSION
Nhe, which was involved in food-poisoning outbreaks, is a three-component enterotoxin produced by most B. cereus strains and other members of the B. cereus group and is highly toxic to Vero and Caco-2 cells. Despite its high prevalence and toxic potential, we are still at the beginning to understand the Nhe mode of action. The current model (21) proposes that the first step in the mode of action of Nhe is associated with binding of NheB and NheC to the cell surface. This step must be accompanied by conformational changes in NheB, which allow subsequent binding of NheA. These events lead finally to cell lysis. There is a high probability that the predicted β-tongues in NheB and NheC are necessary for cell binding. It is, however, not known to what extent NheA, NheB, and NheC interact and which regions of the individual components mediate interaction. Based on the observation that MAbs against the B-component of Nhe neutralized the toxic activity (8), we hypothesized that the epitopes of these antibodies could be part of such structural and functional important sites on NheB. This idea prompted us to define the binding region of MAb 1E11, together with that of MAb 2B11, which both performed well in a sandwich-type EIA (24). In SDS-immunoblotting analyses, however, the reactivity of MAb 2B11 was clearly lower than that of MAb 1E11 (Fig. 1b). Therefore, we assumed that MAb 1E11 recognizes a linear epitope, whereas a conformation-dependent epitope seemed likely for MAb 2B11. In EIA, MAb 2B11 bound to the NheB fragments N1 to N4 (Table 1) but not to N5. Therefore, we concluded that some of these amino acids (121 to 150) must contribute to the 2B11 epitope. The finding that MAb 2B11 did not recognize the synthetic peptides (P1 to P3) covering this area, however, indicated a conformation-dependent and/or discontinuous epitope. On the other hand, MAb 1E11 was highly reactive with wild-type NheB, recombinant fragments containing the C-terminal part of NheB (N1 to N5 and C3) and the synthetic peptide P4 in EIA, as well as in SDS-immunoblot. The 1E11 epitope is therefore located within amino acids 321 to 341 (Fig. 1). Comparing the results of the present study with the structural model for NheB (Fig. 1d), it could be predicted that (i) both epitopes are located on the surface of the model protein, thus allowing access of the antibodies; (ii) the epitopes are not located in the head region and show sufficient distance to the putative transmembrane region containing the beta-hairpin in order not to inhibit binding of NheB to the cell surface; and (iii) the epitopes are sterically located far enough from each other to allow simultaneous binding of both antibodies.
Considering the binding order of the three Nhe components, we tried to address the mechanism by which the antibodies inhibit the action of Nhe. Both antibodies are able to neutralize the cytotoxic activity of Nhe, albeit with a different efficacy (Fig. 6), but do not interfere with binding of NheB to the cell surface (Fig. 2 and 3). We therefore hypothesized that this was probably due to blocking of the binding site of either NheA or NheC on NheB or inhibition of oligomerization of NheB molecules. Prohibiting the formation of an active toxin complex on the cell surface by inhibition of conformational changes in NheB is also a possible explanation.
The epitope of MAb 2B11 is located in the “tail” region of NheB, and the results shown in Fig. 4 indicate that most likely, the antibody inhibits binding of NheC to NheB. This assumption is supported by the finding that MAb 2B11 was effective only when added together with NheB after cell priming with NheC or if added together with NheB and NheC during the priming step. After association of NheB and NheC on the cell surface, MAb 2B11 was still able to bind to NheB (Fig. 2) but no longer neutralized Nhe-induced cytotoxicity. This means that the epitope of MAb 2B11 was still accessible after this step and therefore probably not involved in interaction between NheB molecules. Most interestingly, MAb 2B11 seemed to neither block association of NheA to cell-bound NheB nor inhibit conformational changes in NheB necessary for binding of NheA. This final step in the Nhe mode of action was, however, prohibited by MAb 1E11. Clearly, the results presented in Fig. 4 indicate that this antibody neutralized toxicity when added before or together with NheA after cells have been primed with NheB and NheC. If MAb 1E11 was added together with NheA and the molar ratio between antibody and NheA was varied, a dose-response curve was obtained. This is expected, if NheA and MAb 1E11 were competing for a common binding site (Fig. 5). From the fact that MAb 1E11 was able to inhibit cytotoxicity even if NheB and NheC had time to rearrange properly on the cell surface, we concluded that steric hindrance of NheA association by MAb 1E11 was more likely than inhibition of conformational changes in NheB. Since peptide P4 did not inhibit cytotoxicity (results not shown), the binding of NheA probably does not occur exactly at the epitope of MAb 1E11 but could be simply within the surface area blocked by the antibody. The importance of the C-terminal part of NheB for toxicity is also supported by the observation that a nontoxic nheBC mutant expressing only NheA and a truncated form of NheB (last 80 amino acids are absent [11]) showed no cytotoxicity when supplemented with active NheC (results not shown). From our results two important conclusions can be drawn: (i) steric hindrance of association of NheA to the NheB/C complex on the cell surface by MAb 1E11 is very likely and (ii) NheA exhibits a high binding affinity for cell-bound NheB, whereas binding of NheA to NheB in solution could not be demonstrated.
Interestingly, the epitope of MAb 1E11 is located on the predicted C-terminal α helix of NheB, which shows a high degree of similarity with the αG helix of ClyA, located in the tail region of the ClyA monomer and found to be on the outer side of the dodecameric transmembrane pore assembly formed after a series of conformational changes (25). In addition, previous studies (3, 30) stated a key role of αG in ClyA function and chemical immobilization of the αG helix lead to inhibition of ClyA activity in a late stage of pore formation, after membrane association and oligomerization. Accordingly, after removing 12 or more amino acids from the C terminus of ClyA hemolytic activity was lost (3). Taken together, there is a high probability that the C-terminal amino acids of NheB are essential for Nhe toxicity.
The results presented above were obtained on Vero cells using mutant strains producing either NheB and NheC or NheA and NheB in combination with recombinant proteins (NheA and NheC). In addition, the “natural” mode of Nhe action was dissected into consecutive steps. These experimental modifications are likely to influence the neutralization capacity of the MAbs. Therefore, we tested the potential of the antibodies to neutralize wild-type toxicity in different human cell lines in comparison to Vero cells. Since neutralization capacity of MAb 2B11 was ∼100-fold lower than that of MAb 1E11 (Fig. 6), all further experiments were done using the MAb 1E11 to neutralize cytotoxicity of unpurified culture supernatants of the enteropathogenic strain NVH 0075/95. Only in the first dilutions of the supernatant was a residual toxicity of below 5% observed, which could reflect non-Nhe-related toxicity (Fig. 7). These results underline that inhibition of association of NheA to cell-bound NheB is an efficient mechanism to neutralize toxicity of Nhe and human epithelial cells were found to be highly sensitive to Nhe. This finding indicates that the expression of only Nhe may be sufficient to induce diarrhea in humans, although in vivo studies demonstrating epithelial damage in the intestine attributable to Nhe are still missing. In addition, the primary human endothelial cell line (HUVEC) was severely affected by Nhe. Interestingly, it has been known for a long time that cell-free supernatants of B. cereus strains increase the vascular permeability in animal models (12, 13, 29), an event that has been associated with enterotoxic activity. The high susceptibility of HUVEC, indicating the potential of Nhe to destroy the endothelial barrier, could at least partly explain this former observation. In addition, epithelial cell lines representing different tissues were affected by Nhe, thus suggesting a possible role as a virulence factor in nongastrointestinal infections (9, 18). Currently, however, it is unknown whether the binding and cytotoxic action of Nhe depend on cellular parameters or are mediated by a cell surface receptor.
In conclusion, we have been able to reveal important mechanisms during the mode of action of Nhe by defining the binding regions of two MAbs. The epitopes of antibodies 2B11 and 1E11 are involved in the interaction of NheB with NheC and NheA, respectively. Under natural conditions, interaction between NheB and NheC seems to occur mainly in solution, whereas interaction between NheB and NheA can only take place on the cell surface. The epitope of MAb 1E11, located at the C-terminal part of NheB, identifies a region of NheB, which plays a pivotal role in toxicity and inhibition of attachment of NheA by antibody 1E11 is an efficient mechanism to protect a broad spectrum of human cell lines. This finding further supports the idea that NheA is the key component during the Nhe action by triggering pore formation after association with cell bound NheB and particularly antibody 1E11 could be a versatile tool to clarify the mechanisms underlying the complex mode of action of Nhe.
ACKNOWLEDGMENTS
We thank Brunhilde Minich, Franziska Witzko, and Diana Hermann for excellent technical assistance.
Footnotes
Published ahead of print 11 November 2011
REFERENCES
- 1. Agata N, Ohta M, Mori M, Isobe M. 1995. A novel dodecadepsipeptide, cereulide, is an emetic toxin of Bacillus cereus. FEMS Microbiol. Lett. 129:17–19 [DOI] [PubMed] [Google Scholar]
- 2. Arnold K, Bordoli L, Kopp J, Schwede T. 2006. The Swiss-Model workspace: a web-based environment for protein structure homology modeling. Bioinformatics 22:195–201 [DOI] [PubMed] [Google Scholar]
- 3. Atkins A, et al. 2000. Structure-function relationships of a novel bacterial toxin, hemolysin E. J. Biol. Chem. 275:41150–41155 [DOI] [PubMed] [Google Scholar]
- 4. Beecher DJ, Schoeni JL, Wong ACL. 1995. Enterotoxic activity of hemolysin BL from Bacillus cereus. Infect. Immun. 63:4423–4428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beecher DJ, Wong ACL. 1994. Improved purification and characterization of hemolysin BL, a hemolytic dermonecrotic vascular permeability factor from Bacillus cereus. Infect. Immun. 62:980–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bordoli L, et al. 2009. Protein structure homology modeling using SWISS-MODEL workspace. Nat. Protoc. 4:1–13 [DOI] [PubMed] [Google Scholar]
- 7. Dietrich R, Fella C, Strich S, Märtlbauer E. 1999. Production and characterization of monoclonal antibodies against the hemolysin BL enterotoxin complex produced by Bacillus cereus. Appl. Environ. Microbiol. 65:4470–4474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dietrich R, Moravek M, Bürk C, Granum PE, Märtlbauer E. 2005. Production and characterization of antibodies against each of the three subunits of the Bacillus cereus nonhemolytic enterotoxin complex. Appl. Environ. Microbiol. 71:8214–8220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Drobniewski FA. 1993. Bacillus cereus and related species. Clin. Microbiol. Rev. 6:324–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ehling-Schulz M, et al. 2005. Emetic toxin formation of Bacillus cereus is restricted to a single evolutionary lineage of closely related strains. Microbiology 151:183–197 [DOI] [PubMed] [Google Scholar]
- 11. Fagerlund A, Lindback T, Storset AK, Granum PE, Hardy SP. 2008. Bacillus cereus Nhe is a pore-forming toxin with structural and functional properties similar to the ClyA (HIyE, SheA) family of haemolysins, able to induce osmotic lysis in epithelia. Microbiology 154:693–704 [DOI] [PubMed] [Google Scholar]
- 12. Glatz BA, Goepfert JM. 1973. Extracellular factor synthesized by Bacillus cereus which evokes a dermal reaction in guinea pigs. Infect. Immun. 8:25–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Glatz BA, Spira WM, Goepfert JM. 1974. Alteration of vascular permeability in rabbits by culture filtrates of Bacillus cereus and related species. Infect. Immun. 10:229–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Granum PE, O'Sullivan K, Lund T. 1999. The sequence of the non-haemolytic enterotoxin operon from Bacillus cereus. FEMS Microbiol. Lett. 177:225–229 [DOI] [PubMed] [Google Scholar]
- 15. Guinebretiere MH, Broussolle V, Nguyen-The C. 2002. Enterotoxigenic profiles of food-poisoning and food-borne Bacillus cereus strains. J. Clin. Microbiol. 40:3053–3056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hansen BM, Hendriksen NB. 2001. Detection of enterotoxic Bacillus cereus and Bacillus thuringiensis strains by PCR analysis. Appl. Environ. Microbiol. 67:185–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haug TM, et al. Formation of very large conductance channels by Bacillus cereus Nhe in Vero and GH4 cells identifies NheA plus B as the inherent pore-forming structure. J. Membr. Biol. 237:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kotiranta A, Lounatmaa K, Haapasalo M. 2000. Epidemiology and pathogenesis of Bacillus cereus infections. Microbes Infect. 2:189–198 [DOI] [PubMed] [Google Scholar]
- 19. Lapidus A, et al. 2008. Extending the Bacillus cereus group genomics to putative food-borne pathogens of different toxicity. Chem. Biol. Interact. 171:236–249 [DOI] [PubMed] [Google Scholar]
- 20. Lindbäck T, Fagerlund A, Rodland MS, Granum PE. 2004. Characterization of the Bacillus cereus Nhe enterotoxin. Microbiology 150:3959–3967 [DOI] [PubMed] [Google Scholar]
- 21. Lindbäck T, et al. 2010. Cytotoxicity of the Bacillus cereus Nhe enterotoxin requires specific binding order of its three exoprotein components. Infect. Immun. 78:3813–3821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lund T, De Buyser ML, Granum PE. 2000. A new cytotoxin from Bacillus cereus that may cause necrotic enteritis. Mol. Microbiol. 38:254–261 [DOI] [PubMed] [Google Scholar]
- 23. Lund T, Granum PE. 1996. Characterisation of a non-hemolytic enterotoxin complex from Bacillus cereus isolated after a food-borne outbreak. FEMS Microbiol. Lett. 141:151–156 [DOI] [PubMed] [Google Scholar]
- 24. Moravek M, et al. 2006. Determination of the toxic potential of Bacillus cereus isolates by quantitative enterotoxin analyses. FEMS Microbiol. Lett. 257:293–298 [DOI] [PubMed] [Google Scholar]
- 25. Mueller M, Grauschopf U, Maier T, Glockshuber R, Ban N. 2009. The structure of a cytolytic alpha-helical toxin pore reveals its assembly mechanism. Nature 459:726–731 [DOI] [PubMed] [Google Scholar]
- 26. Prüss BM, Dietrich R, Nibler B, Märtlbauer E, Scherer S. 1999. The hemolytic enterotoxin HBL is broadly distributed among species of the Bacillus cereus group. Appl. Environ. Microbiol. 65:5436–5442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schoeni JL, Wong ACL. 2005. Bacillus cereus food poisoning and its toxins. J. Food Prot. 68:636–648 [DOI] [PubMed] [Google Scholar]
- 28. Stenfors Arnesen LP, Fagerlund A, Granum PE. 2008. From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol. Rev. 32:579–606 [DOI] [PubMed] [Google Scholar]
- 29. Turnbull PCB, Jorgensen K, Kramer JM, Gilbert RJ, Parry JM. 1979. Severe clinical conditions associated with Bacillus cereus and the apparent involvement of exotoxins. J. Clin. Pathol. 32:289–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wallace AJ, et al. 2000. Escherichia coli hemolysin E (HlyE, ClyA, SheA): X-ray crystal structure of the toxin and observation of membrane pores by electron microscopy. Cell 100:265–276 [DOI] [PubMed] [Google Scholar]
- 31. Wehrle E, et al. 2009. Comparison of multiplex PCR, enzyme immunoassay and cell culture methods for the detection of enterotoxinogenic Bacillus cereus. J. Microbiol. Methods 78:265–270 [DOI] [PubMed] [Google Scholar]